Introduction
Vitamin C, also termed ascorbic acid, has been
widely used, orally and parenterally, in clinical settings and is
vital to human health. Vitamin C typically functions in the
prevention and treatment of the common cold, hypersensitivity and
viral infection, and is anti-aging (1–3).
Recently, evidence has emerged to suggest that vitamin C has
additional functions other than its anti-oxidant ability. Vitamin C
activates the Jak-Stat2 signaling pathway and enhances Nanog
expression (4), which has been
demonstrated to be a direct target of TLR4 (5) and is associated with stem cell
properties (6,7). Vitamin C induces the generation of
induced pluripotent stem cells (iPSCs) (8) and the transition from pre-iPSCs to
iPSCs (9). Vitamin C also inhibits
the retinoid acid-induced differentiation of mouse embryonic stem
cells (4).
In addition to its use as an auxiliary treatment for
patients with cancer, attempts have been made to utilize vitamin C
as a more substantial therapeutic agent for patients with cancer.
Although vitamin C is an unorthodox therapeutic agent for cancer,
previous studies have supported the hypothesis that vitamin C
restricts metastatic spread via collagen deposition and
hyaluronidase inhibition (10,11).
Intravenous ascorbate has been reported to be effective at
prolonging patient survival times in terminal human cancer
(12). A recent report has shown
that high-dose parenteral ascorbate is able to enhance
chemosensitivity of carboplatin and paclitaxel in an ovarian cancer
model (13). Vitamin C may represent
an attractive and innovative therapeutic strategy for patients with
cancer. At present, the majority of preclinical studies and
clinical trials in this field are investigating the oral or
parenteral administration of vitamin C; however, no previous
studies have reported local delivery of vitamin C into the tumor
microenvironment.
In the present study, the direct tumoricidal effect
of vitamin C in murine cancer was assessed. The present study
provides a rationale for reexamining vitamin C treatment in
patients with cancer.
Materials and methods
Cell lines and reagents
Murine 4T1 breast cancer cells and CT26 colon cancer
cells (both American Type Culture Collection, Manassas, VA, USA)
were maintained in RPMI-1640 (Gibco-BRL, Carlsbad, CA, USA),
supplemented with 10% fetal bovine serum (both Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) in a humidified atmosphere
containing 5% CO at 37°C. Cisplatin was obtained from the West
China Hospital, Sichuan University (Chengdu, China). NAC and
vitamin C and were purchased from Sigma-Aldrich (Merck Millipore,
Darmstadt, Germany).
MTT assay
A total of 3,000 tumor cells/well were seeded in
96-well plates. Tumor cells were subsequently exposed to 100 and
200 µg/ml vitamin C the following day. At 0, 24, 48 and 72 h, MTT
reagent (Sigma-Aldrich; Merck Millipore) was added to each well and
incubated for 4 h at 37°C. Cell supernatant was subsequently
removed and formazan precipitates were dissolved in 150 µl dimethyl
sulfoxide. Optical density 570 nm was detected and a growth curve
was plotted.
Flow cytometry
CT26 tumor cells (5×104) were exposed to
200, 500 and 1,000 µg/ml vitamin C. Following 24 h, tumor cells
were stained using an Annexin-V-fluorescein isothiocyanate (FITC)
and propidium iodide (PI) kit (Roche Diagnostics GmbH, Mannheim,
Germany; cat. no. 11988549001), according to the manufacturer's
protocol. For flow cytometry analysis, cells were analyzed with a
FACSCalibur flow cytometer and CellQuest software (version 6.0)
(both BD Biosciences, San Jose, CA, USA). To assess the synergistic
effect of vitamin C and chemotherapy, CT26 tumor cells were exposed
to 1 mg/ml cisplatin and 200 µg/ml vitamin C for 48 h. Apoptotic
cells were examined by flow cytometry.
Blockade assay
CT26 tumor cells were treated with 200, 500 and
1,000 µg/ml vitamin C for 24 h. To antagonize the cytotoxicity of
vitamin C, tumor cells were simultaneously exposed to 2 mM
N-acetyl-cysteine. Apoptotic cells were assessed by flow cytometry
(BD Biosciences).
Animal models
Animal experiments were approved by the Ethics
Committee of Sichuan University (Chengdu, China). Female BALB/c
mice (8–10 weeks old; 20±3 g; n=33) were purchased from Vital River
Laboratories, Co., Ltd. (Beijing, China). Mice were maintained
under pathogen-free conditions at between 21 and 27°C, a humidity
of between 40 and 60%, a light/dark cycle of 12 h and ad libitum
access to food and water. For subcutaneous tumor models,
1×106 4T1 cells were subcutaneously inoculated into the
right flanks of BALB/c mice (n=15). CT26 tumor cells were
subcutaneously inoculated into the right flanks of BALB/c mice
(n=18). Eight days post-inoculation, when palpable tumors reached
~90 mm3, vitamin C was delivered intratumorally into
tumors. Tumor-bearing mice were randomly divided into three groups:
Control, 1ow-dose and high-dose groups (4T1, n=5 for each group;
CT26, n=6 for each group). Tumor growth was monitored by measuring
i) large and ii) short length with a sliding caliper. Tumor volume
was calculated as: Tumor volume = a × b2 × 0.52.
Statistical analysis
Statistical analysis was performed using Student's
t-test between two groups, and one-way analysis of variance between
more than two groups. Results were expressed as the mean ± standard
deviation. P<0.05 was considered to indicate a statistically
significant difference.
Results
Vitamin C inhibits tumor cells
proliferation
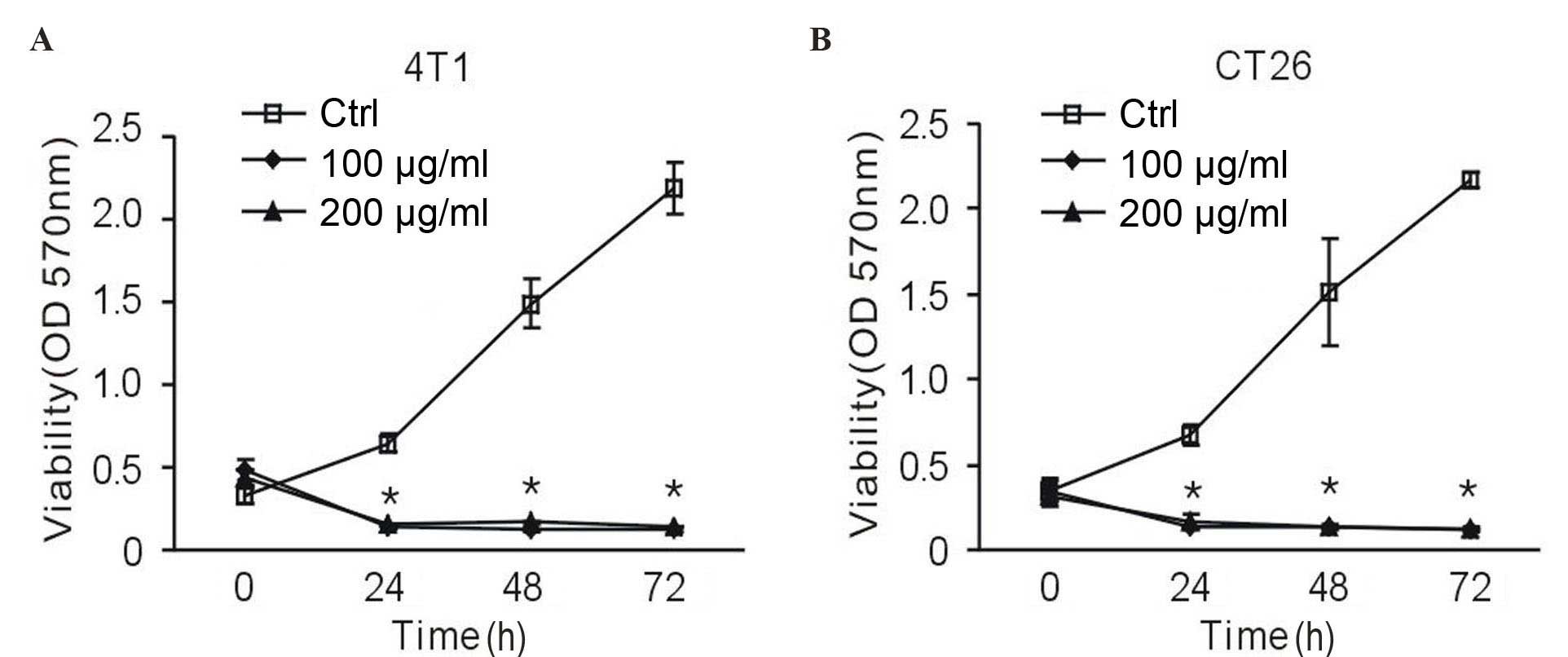
To investigate whether vitamin C influenced the
proliferation of tumor cells, an MTT assay was performed. In the
presence of vitamin C, 4T1 murine breast cancer cells exhibited a
reduced proliferation rate; however, there was no dose-dependent
effect. A significant suppressive effect began at 24 h, and
persisted over the course of treatment (P<0.01 vs. the control
group; Fig. 1A). To demonstrate that
vitamin C is universally cytotoxic or cytostatic, it was assessed
against additional cells lines. Notably, 100 and 200 µg/ml vitamin
C exhibited a significantly suppressive effect on CT26 murine colon
cancer cells at 24, 48 and 72 h (P<0.01 vs. the control group;
Fig. 1B). This data suggests that
high-dose vitamin C inhibits tumor cell proliferation.
Vitamin C induces apoptosis in
vitro
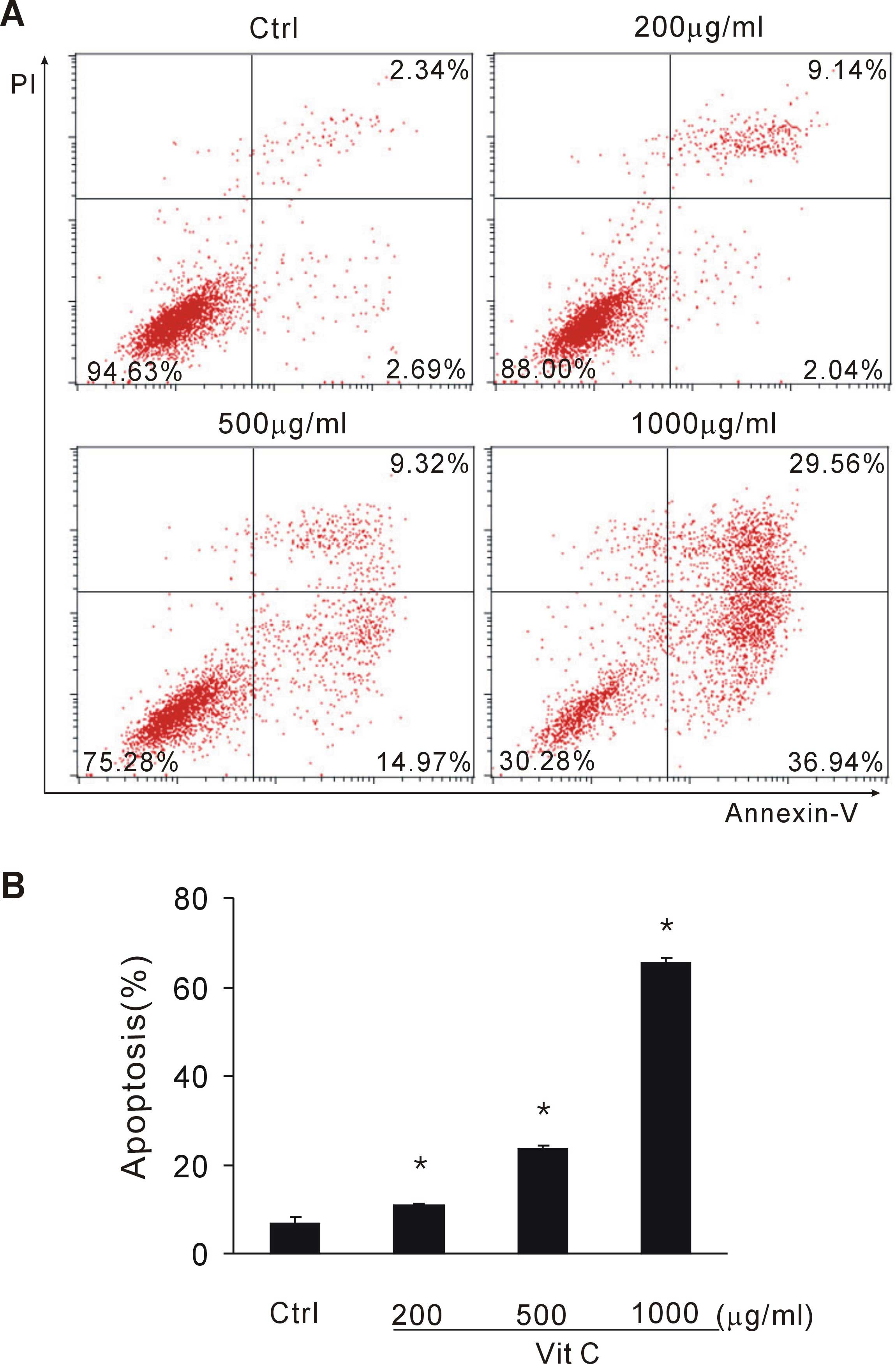
To investigate whether vitamin C induced apoptosis
of tumor cells, CT26 tumor cells exposed to vitamin C were stained
with Annexin-V and PI. Early and late apoptotic cell fractions were
determined by flow cytometry. When exposed to >200 µg/ml vitamin
C, tumor cells underwent apparent apoptosis. In the present
experiment, 200, 500 and 1,000 µg/ml vitamin C significantly
induced tumor cell apoptosis in a dose-dependent manner (P<0.05
vs. the control group; Fig. 2A and
B). This data suggests that a large dose of vitamin C induces
tumor cell apoptosis.
NAC partially antagonizes the
tumoricidal effect of vitamin C
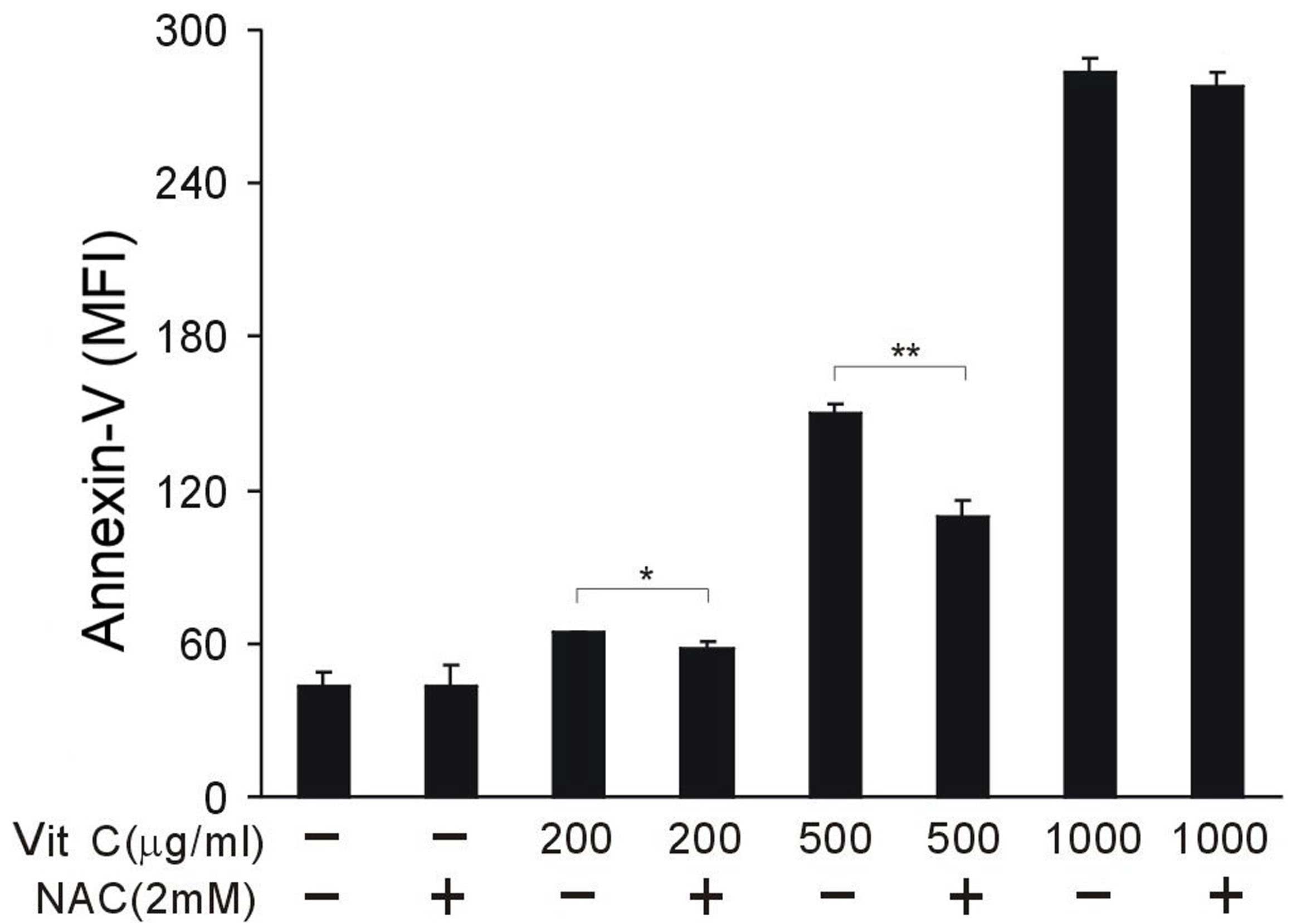
To investigate the key mechanism of vitamin C, NAC
was used to block the tumoricidal effect of vitamin C. A total of 2
mM NAC was used per sample. NAC did not cause observable toxicity
to CT26 cancer cells. NAC was able to partially reverse the effect
of vitamin C and protected tumor cells from cell death when vitamin
C was administered at 200 and 500 µg/ml; however, NAC was not able
to block the cytotoxicity of 1,000 µg/ml vitamin C (P<0.05;
Fig. 3). These results indicate that
vitamin C function, in this context, may be unrelated to its
antioxidant activity, and inversely, oxidative stress suppression
may partially antagonize the tumoricidal effect of a relatively low
dose of vitamin C.
Vitamin C enhances the anti-tumor
effect of cisplatin
Various chemotherapeutical agents, such as
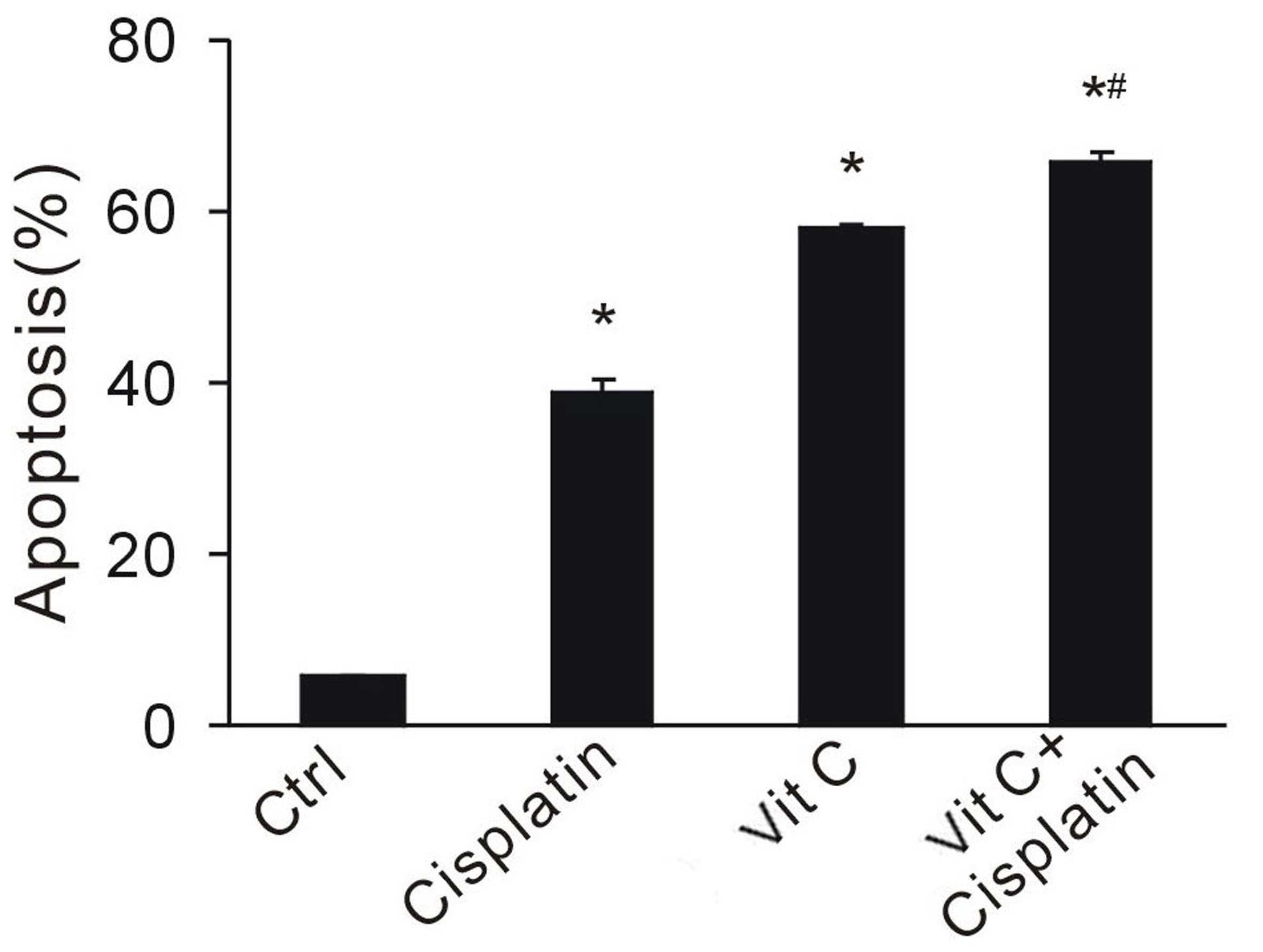
cisplatin, rely on the redox system to kill cancer cells. To
investigate whether vitamin C enhances the anti-tumor effect of
chemotherapy, a large dose of vitamin C was administered in
combination with cisplatin. Apoptotic cell fractions were
determined by flow cytometry. Vitamin C and cisplatin significantly
increased cell apoptosis (P<0.05 vs the control group; Fig. 4). CT26 cancer cells exposed to both
drugs exhibited the highest apoptotic rates, indicating the
synergistic effect of combination treatment (Fig. 4). This data suggests that vitamin C
enhances the effect of chemotherapy, and may provide a rationale
for combination therapy.
Local delivery of vitamin C is
effective for cancer treatment
To investigate the anti-tumoral effect of vitamin C
in vivo, 4T1 and CT26 tumor-bearing mice were treated
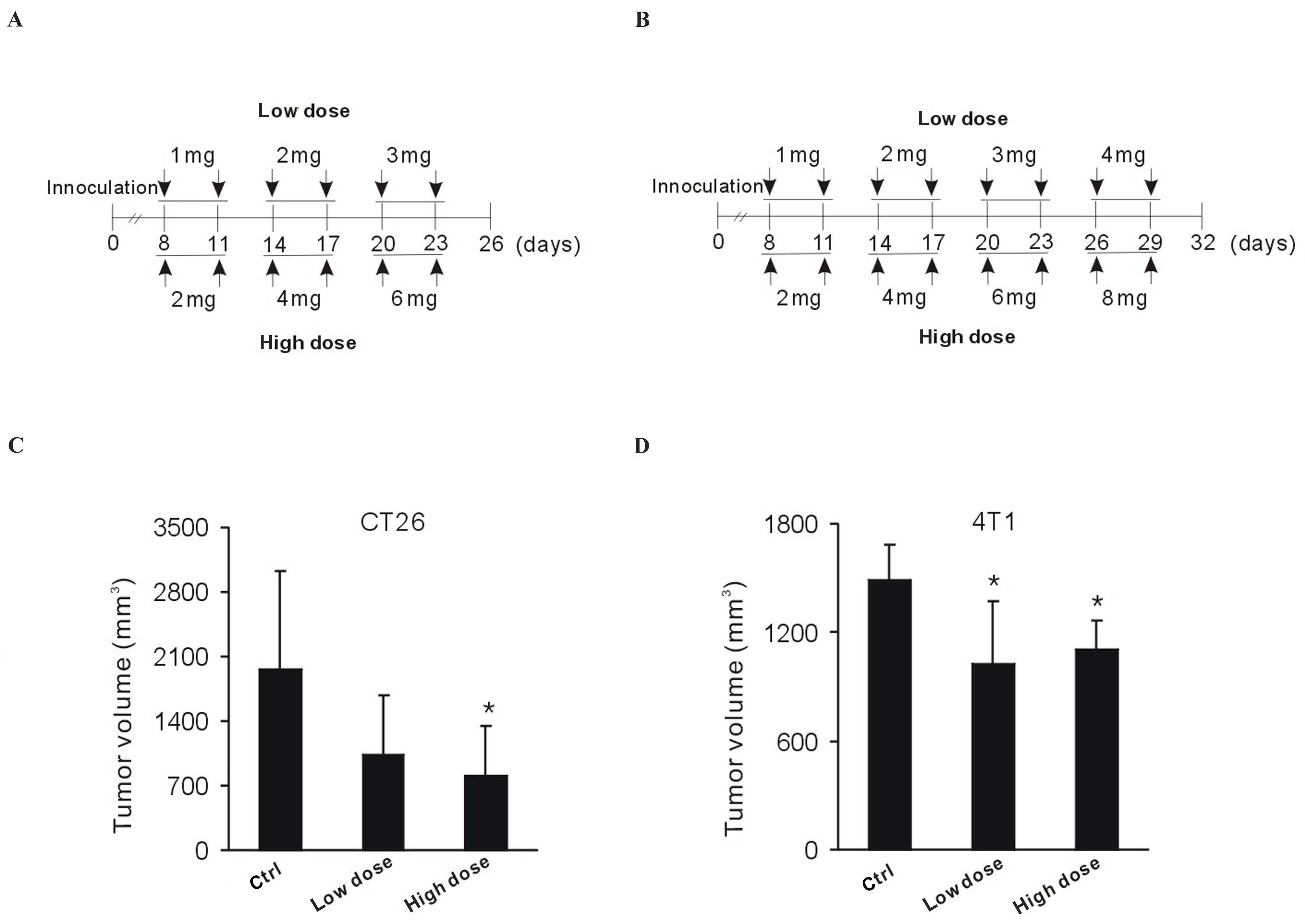
intratumorally with dose-escalating vitamin C. Drug schedules were
as indicated in Fig. 5A and B. Over
the course of treatment, CT26 tumor-bearing BALB/c mice displayed a
marked response to vitamin C (Fig.
5C). Tumor volume markedly decreased in the low-dose group,
whereas a significant decrease was detected in the high-dose group,
indicating a dose-dependent effect (P<0.05 vs. the control
group; Fig. 5C). Significant
diminution of 4T1 tumor growth was observed following low– and
high-dose vitamin C treatment (P<0.05 vs. the control group;
Fig. 5D). Therefore, the ability of
vitamin C to restrain tumor growth was not limited by the type of
tumors being examined. This data indicates that local delivery of
vitamin C may be effective for tumor growth retardation.
Discussion
The present study investigated the biological
effects of large doses of vitamin C on murine tumor cells in
vitro and in vivo. This anti-tumoral agent, with a high
safety profile, was effective whether used alone or in combination
with cisplatin chemotherapy in the present study. In addition,
intratumoral delivery of vitamin C significantly retarded tumor
growth in murine solid tumor models.
Vitamin C has previously been used as an unorthodox
therapy for patients with cancer (14); however, in most clinical settings,
vitamin C is used as an auxillary treatment and the dosage is low.
Low-dose vitamin C (25–50 µg/ml) does not induce apoptosis;
conversely, it is associated with stem cell proliferation and
maintenance (4,8). Previous studies have shown that
high-dose vitamin C (10 g/day) is effective at treating cancer
(12). In the present study, murine
CT26 colon cancer cells were used. Low-dose vitamin C (25–50 µg/ml)
induced minimal apoptosis. However, the larger dose had a
cytotoxic/cytostatic effect and induced marked cell apoptosis. In
addition, dose-escalating vitamin C yielded a measurable and
significant anti-tumor effect in vivo. These findings
provide a rationale for further investigation of this phenomenon to
elucidate the association between vitamin C and cancer stem
cells.
The mechanism of action of vitamin C for cancer
treatment remains unclear. Several hypotheses have been suggested.
Vitamin C acts as a cofactor in various reactions driven by
dioxygenases, including histone demethylases, hypoxia-inducible
factor prolyl hydroxylases and collagen prolyl hydroxylases
(15). Vitamin C inhibits the
enzymatic activity of hyaluronidase, which otherwise destroys
collagen (11); thus through
strengthening the collagen structure, vitamin C restrains tumor
cell metastasis. In a recent paper, Ma et al (13) suggest that the anti-tumor effect of
vitamin C is due to pro-oxidative properties, which activate
ATM/AMPK and inhibit the mTOR pathway in ovarian cancer cells.
Vitamin C, within pharmacological concentrations, forms ascorbate
radicals which produce hydrogen peroxide in extracellular fluid
that are cytotoxic to various cancer cells (16). In the present study, NAC, a
well-known anti-oxidant agent (17),
was demonstrated to antagonize the anti-tumor effect of a
relatively low dose of vitamin C (200 and 500 µg/ml). However, NAC
was not able to block the cytotoxicity of 1,000 µg/ml vitamin C.
Additional studies are required to fully explore the mechanism of
vitamin C against cancer cells.
Delivery route influences the effect of vitamin C.
Intravenous vitamin C and orally administered vitamin C were
demonstrated to induce apoptosis in tumor cells; however, it has
previously been demonstrated that the same dose of vitamin C was
ineffective when administered orally (18). Furthermore, a previous study has
determined that orally administered and intravenous vitamin C have
different pharmacokinetics (19).
When administered orally, plasma and tissue concentrations of
vitamin C are influenced by absorption, tissue transport and renal
excretion processes (20); whereas
intravenous vitamin C bypasses the absorption process, thus high
plasma concentrations are easily maintained. Although intravenous
doses as high as 1.5 g/kg vitamin C are tolerated by patients with
normal renal function and glucose-6-phosphate dehydrogenase (G6PD)
activity (21), the clinical use of
vitamin C should be carefully monitored, as G6PD is not commonly
detected by clinical tests. Considering these defects, a local
delivery strategy may prove most beneficial to the treatment of
patients with cancer. In vitro experiments showed the
cytotoxic effect of vitamin C; therefore, vitamin C was injected
directly into solid tumors. This routine was demonstrated to be
safe and effective in tumor-bearing mice.
In conclusion, the present study demonstrated the
cytotoxic effect of large dose vitamin C. Considering its low
toxicity and high availability, intratumoral delivery of vitamin C
may provide a therapeutic option for those with advanced
tumors.
Acknowledgements
This work was supported by the National Natural
Science Foundation of China (grant no. 81501609) and the Chinese
Postdoctoral Science Foundation (grant no. 81301980).
References
|
1
|
Klenner FR: The treatment of poliomyelitis
and other virus diseases with vitamin C. South Med Surg.
111:209–214. 1949.PubMed/NCBI
|
|
2
|
Bansal P, Saw S, Govindaraj D and Arora N:
Intranasal administration of a combination of choline chloride,
vitamin C, and selenium attenuates the allergic effect in a mouse
model of airway disease. Free Radic Biol Med. 73:358–365. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Padayatty SJ, Sun AY, Chen Q, Espey MG,
Drisko J and Levine M: Vitamin C: Intravenous use by complementary
and alternative medicine practitioners and adverse effects. PLoS
One. 5:e114142010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wu H, Wu Y, Ai Z, Yang L, Gao Y, Du J, Guo
Z and Zhang Y: Vitamin C enhances Nanog expression via activation
of the JAK/STAT signaling pathway. Stem Cells. 32:166–176. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chen CL, Tsukamoto H, Liu JC, Kashiwabara
C, Feldman D, Sher L, Dooley S, French SW, Mishra L, Petrovic L, et
al: Reciprocal regulation by TLR4 and TGF-β in tumor-initiating
stem-like cells. J Clin Invest. 123:2832–2849. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cavaleri F and Schöler HR: Nanog: A new
recruit to the embryonic stem cell orchestra. Cell. 113:551–552.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Suzuki A, Raya A, Kawakami Y, Morita M,
Matsui T, Nakashima K, Gage FH, Rodríguez-Esteban C and Izpisúa
Belmonte JC: Nanog binds to Smad1 and blocks bone morphogenetic
protein-induced differentiation of embryonic stem cells. Proc Natl
Acad Sci USA. 103:10294–10299. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Esteban MA, Wang T, Qin B, Yang J, Qin D,
Cai J, Li W, Weng Z, Chen J, Ni S, et al: Vitamin C enhances the
generation of mouse and human induced pluripotent stem cells. Cell
Stem Cell. 6:71–79. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Silva J, Barrandon O, Nichols J, Kawaguchi
J, Theunissen TW and Smith A: Promotion of reprogramming to ground
state pluripotency by signal inhibition. PLoS Biol. 6:e2532008.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
McCormick WJ: Cancer: The preconditioning
factor in pathogenesis; a new etiologic approach. Arch Pediatr.
71:313–322. 1954.PubMed/NCBI
|
|
11
|
Cameron E and Rotman D: Ascorbic acid,
cell proliferation, and cancer. Lancet. 1:5421972. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cameron E and Pauling L: Supplemental
ascorbate in the supportive treatment of cancer: Reevaluation of
prolongation of survival times in terminal human cancer. Proc Natl
Acad Sci USA. 75:4538–4542. 1978. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ma Y, Chapman J, Levine M, Polireddy K,
Drisko J and Chen Q: High-dose parenteral ascorbate enhanced
chemosensitivity of ovarian cancer and reduced toxicity of
chemotherapy. Sci Transl Med. 6:222ra182014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
González MJ, Miranda-Massari JR, Mora EM,
Guzmán A, Riordan NH, Riordan HD, Casciari JJ, Jackson JA and
Román-Franco A: Orthomolecular oncology review: Ascorbic acid and
cancer 25 years later. Integr Cancer Ther. 4:32–44. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Shi Y: Histone lysine demethylases:
Emerging roles in development, physiology and disease. Nat Rev
Genet. 8:829–833. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chen Q, Espey MG, Sun AY, Lee JH, Krishna
MC, Shacter E, Choyke PL, Pooput C, Kirk KL, Buettner GR and Levine
M: Ascorbate in pharmacologic concentrations selectively generates
ascorbate radical and hydrogen peroxide in extracellular fluid in
vivo. Proc Natl Acad Sci USA. 104:8749–8754. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Supabphol A, Muangman V, Chavasiri W,
Supabphol R and Gritsanapan W: N-acetylcysteine inhibits
proliferation, adhesion, migration and invasion of human bladder
cancer cells. J Med Assoc Thai. 92:1171–1177. 2009.PubMed/NCBI
|
|
18
|
Creagan ET, Moertel CG, O'Fallon JR,
Schutt AJ, O'Connell MJ, Rubin J and Frytak S: Failure of high-dose
vitamin C (ascorbic acid) therapy to benefit patients with advanced
cancer. A controlled trial. N Engl J Med. 301:687–690. 1979.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Padayatty SJ, Sun H, Wang Y, Riordan HD,
Hewitt SM, Katz A, Wesley RA and Levine M: Vitamin C
pharmacokinetics: Implications for oral and intravenous use. Ann
Intern Med. 140:533–537. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Levine M, Conry-Cantilena C, Wang Y, Welch
RW, Washko PW, Dhariwal KR, Park JB, Lazarev A, Graumlich JF, King
J and Cantilena LR: Vitamin C pharmacokinetics in healthy
volunteers: Evidence for a recommended dietary allowance. Proc Natl
Acad Sci USA. 93:3704–3709. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hoffer LJ, Levine M, Assouline S,
Melnychuk D, Padayatty SJ, Rosadiuk K, Rousseau C, Robitaille L and
Miller WH Jr: Phase I clinical trial of i.v. ascorbic acid in
advanced malignancy. Ann Oncol. 19:1969–1974. 2008. View Article : Google Scholar : PubMed/NCBI
|