Introduction
Epileptogenic focus resection is the primary
treatment option for refractory frontal lobe epilepsy; however, it
is less effective than epileptogenic focus resection for temporal
epilepsy (1). Patients with
postoperative recurrence of frontal lobe epilepsy or a high risk of
postoperative recurrence, patients with epileptogenic foci
primarily in one frontal lobe, or patients with epileptogenic foci
in one frontal lobe in which the precise area of foci cannot be
determined, often undergo prefrontal lobe resection with
preservation of the motor area. However, removal of large areas of
brain tissue may result in substantial postoperative complications,
and thus prolonged hospitalization and high costs (2).
Previous outcomes following hemispheric and
posterior quadrantic disconnection surgery demonstrate that
disconnection surgery results in the preservation of the majority
of the biologically active brain tissues in the epileptogenic zone,
and the isolation of the epileptogenic zone from other cerebral
cortical regions and central structures (3,4). These
procedures have similar rates of seizure control to the
corresponding anatomical resection procedures, but are associated
with reduced surgical injury and fewer postoperative complications.
Disconnection surgery is currently used with increasing frequency
for the treatment of intractable epilepsy when the epileptogenic
foci are in one hemisphere, or in the posterior part of a
hemisphere (5–8). The principle of ‘more incision, less
excision’ is becoming increasingly accepted by surgeons.
Numerous studies of disconnection surgery in
patients with unilateral temporal lobe or
temporal-parietal-occipital lobe epilepsy have been reported
(5–7,9–13). However, to the best of our knowledge,
no studies of disconnection surgery for frontal lobe epilepsy have
been reported. Based on the successful implementation of
hemispheric disconnection and posterior quadrantic disconnection
surgery for epileptogenic foci in other regions (11,13), the
concepts of ‘epileptogenic focus isolation’ and ‘more incision,
less excision’ were applied in the current case report. The report
describes the treatment of a patient with frontal lobe epilepsy
with recurrence following resection of epileptogenic foci.
Following complete frontal lobe isolation surgery, the patient had
no further seizures during the 16 months of the follow-up period.
In the present study, the surgical anatomy, surgical technique and
indications for frontal lobe isolation surgery are discussed.
Case report
A 17-year-old female was hospitalized on March 11,
2013 (Department of Neurosurgery, Tianjin Huanhu Hospital, Tianjin,
China). The patient had a 14-year history of epileptic seizures. At
3 years of age the patient was experiencing 7–8 seizures per day,
which involved right eyelid twitching, associated with fear and
suddenly holding onto people. The patient was diagnosed with
epilepsy secondary to nodular sclerosis at the Department of
Epilepsy, Tianjin Children's Hospital (Tianjin, China) and was
treated orally with topiramate, followed by carbamazepine and
valproate (dose and supplier unknown). Each of these medications
was only effective during the first 20 days of administration. The
present study was approved by the Ethics Committee of Tianjin
Huanhu Hospital. Written informed consent was obtained from the
father of the patient.
In 2008, the patient underwent resection of left
frontal lobe epileptogenic foci at the Epilepsy Center, Yuquan
Hospital (Beijing, China), but this did not reduce the frequency of
the seizures. The patient subsequently developed psychiatric
symptoms and personality changes (the patient became stubborn,
greedy and capricious). Prior to admission to the Department of
Neurosurgery, Tianjin Huanhu Hospital, in March 2013, the patient
was administered oxcarbazepine, levetiracetam and clonazepam
sequentially, but the seizures were not reduced. The patients
seizures were preceded by sudden fear and holding onto people,
followed by absence and secondary tonic-clonic seizures. The
patient experienced psychiatric symptoms, irritability and
personality changes. Physical examination showed normal growth and
development, an unkempt appearance and a bandaged right hand, which
had been burned when it came into contact with a object during a
seizure. A Wechsler Memory Scale (14) was used to evaluate the patient's
memory prior to surgery, and it was found that the patient's memory
was poor (Wechsler score, 78). During the examination, the patient
was uncooperative, unable to concentrate and hyperactive; normal
limb muscle strength and muscle tension was observed, with no
pathological tendon reflexes.
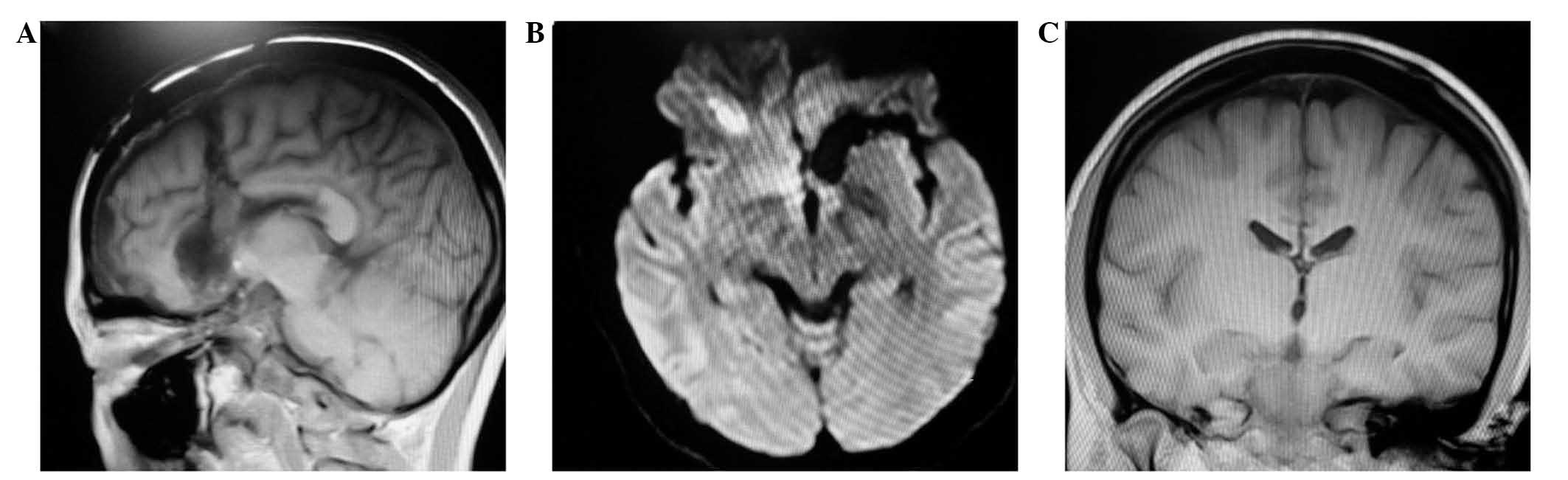
Magnetic resonance imaging (MRI; Magnetom Avanto
1.5T; Siemens Healthcare GmbH, Erlangen, Germany) showed evidence
of the previous left frontal craniotomy, an irregular prefrontal
lobe, expanded subarachnoid space, and abnormal signals in the
cortex and subcortex of the frontal orbital gyrus and gyrus rectus
(Fig. 1A and B).
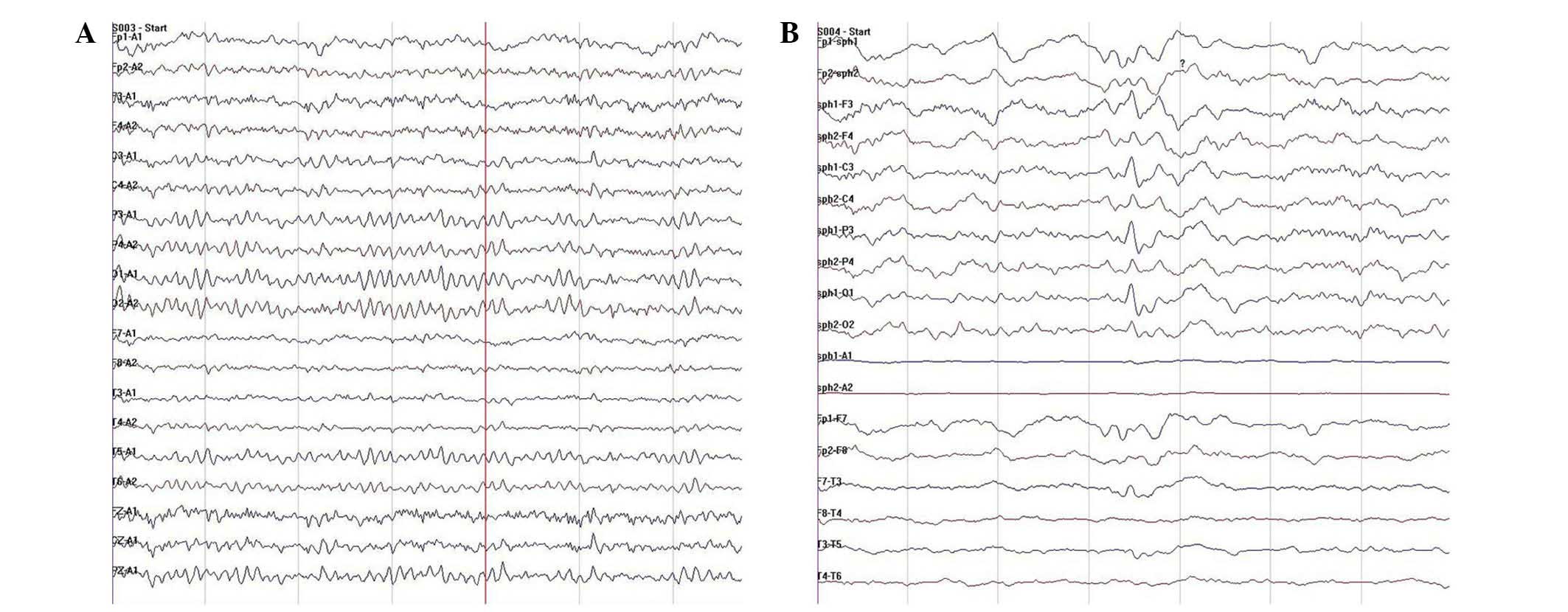
Vigilance-controlled electroencephalogram (EEG; Bio-logic System
Corporation, Lake County, IL, USA) changes were monitored by
long-range video EEG. Ten seizures characterized by excessive
movements were observed during a 24-h period. While resting, the
patient suddenly showed signs of discomfort and reduced blinking,
and did not respond to being called. Her head and body twisted from
the supine position into the prone position, associated with
involuntary pedaling movements of the lower limbs. The longest
episode lasted 80 sec. During this activity, the EEG showed
frequent irregular long-range low/moderate-amplitude slow waves at
2–3 cycles/sec, originating in the left temporal region and
involving all the left-sided leads. There were also isolated
moderate-amplitude sharp waves in the left anterior temporal
region. During seizures, paroxysmal low-amplitude fast waves at 30
cycles/sec with gradually increasing amplitude were observed in all
leads, in particular in the bilateral prefrontal temporal regions.
The wave rate gradually slowed to ~8 cycles/sec. Moderate-amplitude
sharp waves and sharp-slow waves were detected in the posterior
frontal region, in particular on the right side. The left-sided
leads showed irregular paroxysmal moderate/high-amplitude slow
waves at 2–3 cycles/sec. The wave amplitudes were very high in the
left temporal regions. Low/moderate-amplitude alpha activity at 10
cycles/sec was observed in the parieto-occipital leads (Fig. 1C and D). These results suggested a
diagnosis of frontal lobe epilepsy. Originally, it was thought that
epileptogenic focus resection or expanded focus resection would be
unsuccessful. However, complete left prefrontal lobe isolation
surgery was performed on March 18, 2013, including anatomical
incision of the prefrontal lobe, the anterior part of the corpus
callosum and the anterior commissure.
 | Figure 1.Magnetic resonance imagine (MRI) and
electroencephalogram (EEG) changes on admission into hospital. (A
and B) T2-weighted MRI images, showing the previous left frontal
craniotomy, an irregular prefrontal lobe, enlarged subarachnoid
space, abnormal signals in the cortex and subcortex, mixed abnormal
signals in the orbital gyrus and gyrus rectus of the frontal lobe,
and numerous non-enhanced lesions. (C and D) While in a resting
state, the patient suddenly experienced reduced blinking, did not
respond to being called and had increased motor activity. (C)
During seizures, there were paroxysmal low-amplitude fast waves at
30 cycles/sec in all the leads, in particular in the bilateral
prefrontal temporal regions. The amplitude gradually increased and
the wave rate gradually decreased to ~8 cycles/sec. (D) The
interictal EEG showed frequent irregular long-range
low/moderate-amplitude slow waves at 2–3 cycles/sec, originating in
the left temporal region and involving all the left-sided leads.
There were also isolated moderate-amplitude slow sharp waves in the
left anterior temporal region. |
Under general anesthesia using propofol (2.5 mg/kg;
Aztra Zeneca, London, UK) fentanyl citrate (0.01 µg/kg),
cosatracurium (0.6 mg/kg) and Remifentanyl (2.4 mg/ml) (all
purchased from Jiangsu Hengrui Medicine Co., Ltd., Linayungang,
China), the patient was positioned supine, and her head was fixed
with a Mayfield head frame. Craniotomy was performed at the site of
the previous frontotemporal scalp incision, through the left
frontal bone at the midline. The dura was cut in a petal shape, and
the superior frontal gyrus and middle frontal gyrus had areas of
gliosis with a yellow appearance and solid texture. The surgical
procedure was performed as follows: i) White matter at the bottom
of the middle part of the superior frontal sulcus was isolated and
incised. There was a nodule visible beneath the ventricular
ependyma at the anterolateral part of the interventricular foramen
of the left lateral ventricle. The nodule was solid and was colored
yellow and gray, with a poorly defined border with poor vascular
supply. There was visible gliosis at the edge of the nodule. The
abnormal tissue was removed and sent for pathological examination
(4.5 µm tissue size) using formalin-fixing (Tianjin Bodi Cemical
Co., Ltd., Tiajnin, China) and hematoxylin and eosin staining
(Sigma-Aldrich, St. Louis, MO, USA). Arachnoid from the
longitudinal fissure was observed in the medial part of the gray
matter. Arachnoid from the skull base, the A1 segment of the
anterior cerebral artery and the optic chiasm were observed after
the gray matter was incised. ii) An incision was made from the
lateral end of the incision previously described, along the frontal
horn of the lateral ventricle through the ependyma, white matter
and gray matter, to the lateral cleft and the anterior part of the
circular sulcus of the insula. The subependymal incision was
extended downwards until the bone of the anterior skull base was
visible beneath the arachnoid. iii) The middle of the superior
frontal gyrus was incised transversely from the superior frontal
sulcus in the midline to the arachnoid of the longitudinal fissure,
and then directly downwards. The medial parts of the superior
frontal gyrus and cingulate gyrus were incised towards the corpus
callosum. The pericallosal artery was observed, but the arachnoid
was left intact to protect the artery. The fibers of the anterior
part of the corpus callosum were transected anteriorly until this
incision joined the incision made previously in the anterior part
of the interventricular foramen. iv) The cortices of the middle
frontal gyrus and inferior frontal gyrus were incised transversely
outwards, from the incision in the superior frontal sulcus to the
anterior end of the lateral cleft. The incision was made deep to
the left lateral ventricle, end extended through the cortex, white
matter and ependyma. v) The corpus callosum and anterior commissure
were incised. The longitudinal fissure was incised anteriorly to
expose the white corpus callosum. The corpus callosum was incised
strictly along the midline between two pericallosal arteries using
a microscopic suction device (Sonastar-FS-1000-RF; Misonix, Inc.,
Farmingdale, NY, USA). In the deep region, a cavity of the septum
pellucidum was visible. The incision of the corpus callosum was
extended forwards and backwards until 2/3 of the body of the corpus
callosum was divided. Anteriorly, the genu and rostrum of the
corpus callosum were cut. After entering the cavity of the septum
pellucidum, the anterior commissure was divided in the midline
until the superior cistern and optic chiasm were observed. vi) The
cerebral ventricle and surgical cavity were washed with warm
saline. The dura were carefully sutured, and the bone was reset. No
surgical drainages tubes were used. The scalp was sutured closed in
layers.
When performing the surgery, the skull base,
arachnoid of the longitudinal fissure, and arterial and venous
circulation of the frontal lobe was left intact to ensure survival
of the isolated brain tissue.
The patient received intravenous sodium valporate
(0.5 g twice daily; Sanofi Pharmaceutical Co., Ltd., Hangzhou,
China) and dexamethasone (5 mg every 12 h; Wonder Pharmaceutical
Co., Ltd., Shanghai, China) for 3 days after surgery. Postoperative
recovery was uneventful, and the patient was discharged 10 days
after surgery. Pathological examination of the resected nodule
showed a pilocytic astrocytoma. The patient was treated with oral
oxcarbazepine, and no seizures were detected during 16 months of
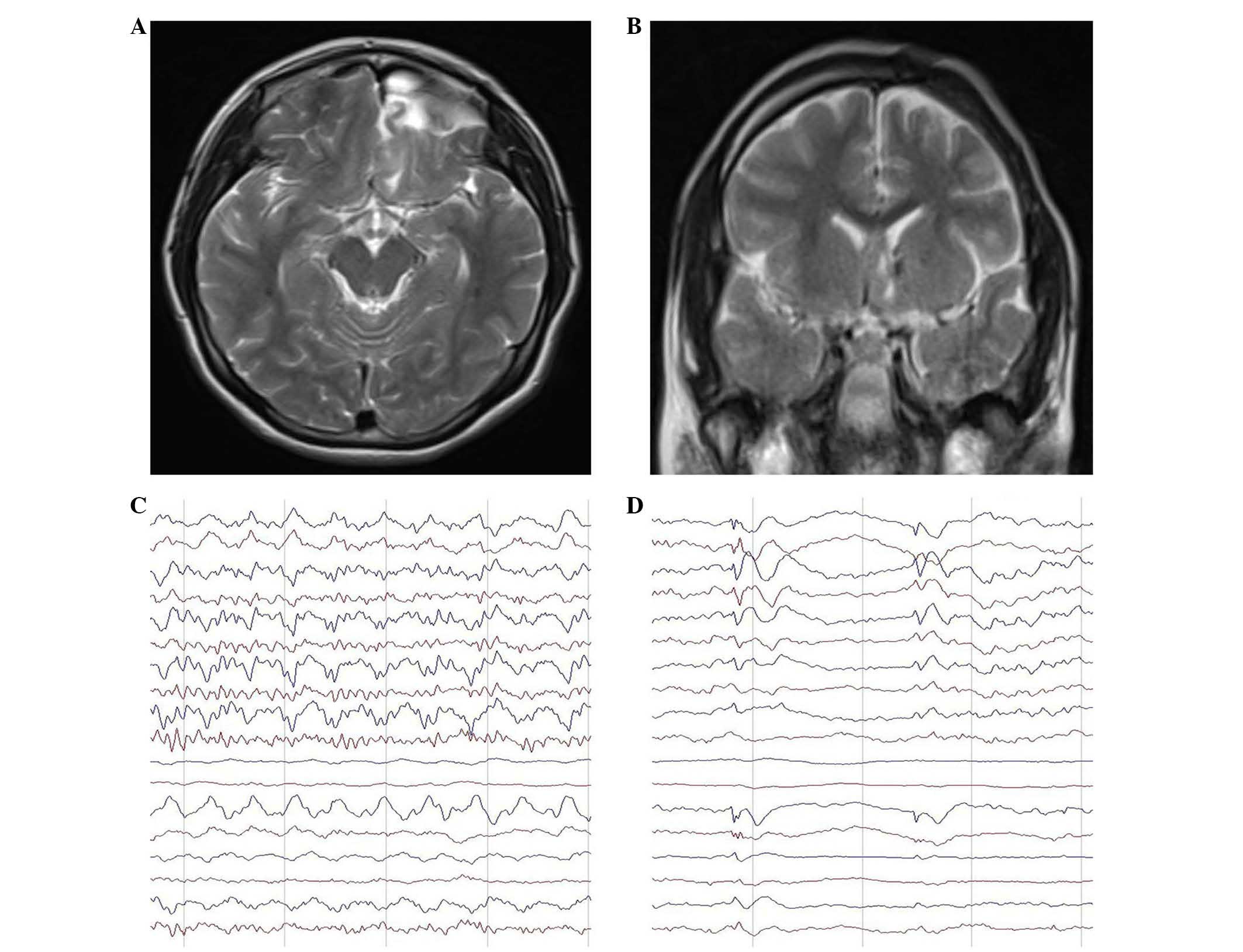
follow-up. Three months after surgery, MRI showed complete
isolation of the left frontal lobe and division of the corpus
callosum and anterior commissure (Fig.
2). A repeat EEG examination showed a number of sharp waves in
the left prefrontal temporal region, with no spread to the right
side. At 1 year after surgery, the patient's Wechsler score
improved to 89.
Discussion
Intractable localized epilepsy originates in the
frontal lobe in ~20% of cases. Frontal lobe epilepsy is less
frequent, but more problematic, than temporal lobe epilepsy, and
patients with frontal lobe epilepsy typically experience several
seizures per day (15). The surgery
for frontal lobe epilepsy has poor outcomes compared with surgery
for temporal lobe epilepsy; therefore, the surgical management of
frontal lobe epilepsy is challenging (16).
Epileptogenic focus resection remains the primary
surgical procedure for the treatment of frontal lobe epilepsy.
However, a previous study demonstrated that the rate of remission
following surgery for frontal lobe epilepsy was 80% in patients
with epileptogenic foci, and 45% in patients without (16). Furthermore, the postoperative
outcomes were improved in patients with lateral frontal lobe
epilepsy compared with those in patients with medial frontal lobe
epilepsy (16). If there are
extensive epileptogenic foci in one frontal lobe, if epileptogenic
foci can be localized to the frontal lobe but the exact location
cannot be determined, or if there is recurrence of seizures
following epileptogenic focus resection, surgeons may perform
prefrontal lobe resection with retention of the motor area. This
procedure may result in the resection of a large quantity of brain
tissue and a relatively high risk of postoperative complications,
resulting in prolonged hospitalization and high costs (2).
In patients with diffuse lesions affecting both
hemispheres, or with epileptogenic foci in functional areas that
cannot be resected, division of the corpus callosum, transection of
multiple areas, or cortical thermal burn therapy may be beneficial
(17). These procedures disrupt the
fiber connections between cortical columns, but not the projection
fibers from the cortex to the subcortical centers (18). However, they are considered
palliative as they may reduce seizure frequency or severity, but do
not stop seizure activity (19). A
previous study observed that hemispheric and posterior quadrantic
disconnection achieved the same rates of seizure control as
epileptogenic focus resection in patients with intractable epilepsy
(20). Disconnection is achieved by
surgical incision to achieve isolation of the epileptogenic foci
(21). Such isolation surgery
disrupts connections within and between hemispheres, and the
projection fibers between the cortex and the subcortical center.
The epileptogenic zone retains its biological activity, but is
electrophysiologically disconnected from other brain regions, so
that the pathways of epileptiform discharges are interrupted
(22).
Hemispheric disconnection or posterior quadrantic
disconnection surgery preserves the biological activity of the
epileptogenic brain tissue, and isolates the epileptogenic zone
from other brain regions, the thalamus and basal ganglia (13). Disconnection surgery is becoming
increasingly popular for the treatment of intractable epilepsy in
patients with extensive epileptogenic foci in one cerebral
hemisphere or in the posterior part of a cerebral hemisphere. The
concept of ‘more incision, less excision’ is gaining increasing
acceptance; the majority of isolation procedures involve
hemispheric disconnection, and increasing numbers of posterior
quadrantic disconnections are being reported (22). Temporal lobe incision and
hypothalamic hamartoma incision have also been reported (8). For frontal lobe epilepsy, surgery that
partially transects the frontal cortex and subcortical white matter
without entering the cerebral ventricles has been reported
(23); this transects the nerve
fibers of the subcortical epileptogenic foci, but does not result
in frontal lobe isolation. To the best of our knowledge, no
previous studies have reported frontal lobe isolation surgery. The
patient in the present study underwent complete prefrontal lobe
isolation surgery, and the surgical methods are described in detail
in order to encourage standardization of surgical techniques to
help with future comparisons of postoperative outcomes.
It is important to completely disconnect the
anatomical and functional (neurophysiological) contact between the
target region (epileptogenic zone) and other regions to completely
isolate the epileptogenic zone, and prevent transmission and
amplification of epileptiform discharges (24). To achieve this, the surgeon must have
the appropriate knowledge and experience of microdissection
techniques. The incision near the interventricular foramen may
easily injure the column of the fornix (Fig. 3A and B), which may cause
postoperative memory impairment; incomplete division may result in
incomplete postoperative seizure control. In the patient in the
current study, a curved incision was made in the ependyma anterior
and lateral to the interventricular foramen, as far as the skull
arachnoid and avoiding the column of the fornix. The anterior part
of the corpus callosum and the anterior commissure were also
divided. This avoided injury to the column of the fornix, and
ensured complete isolation of the frontal lobe.
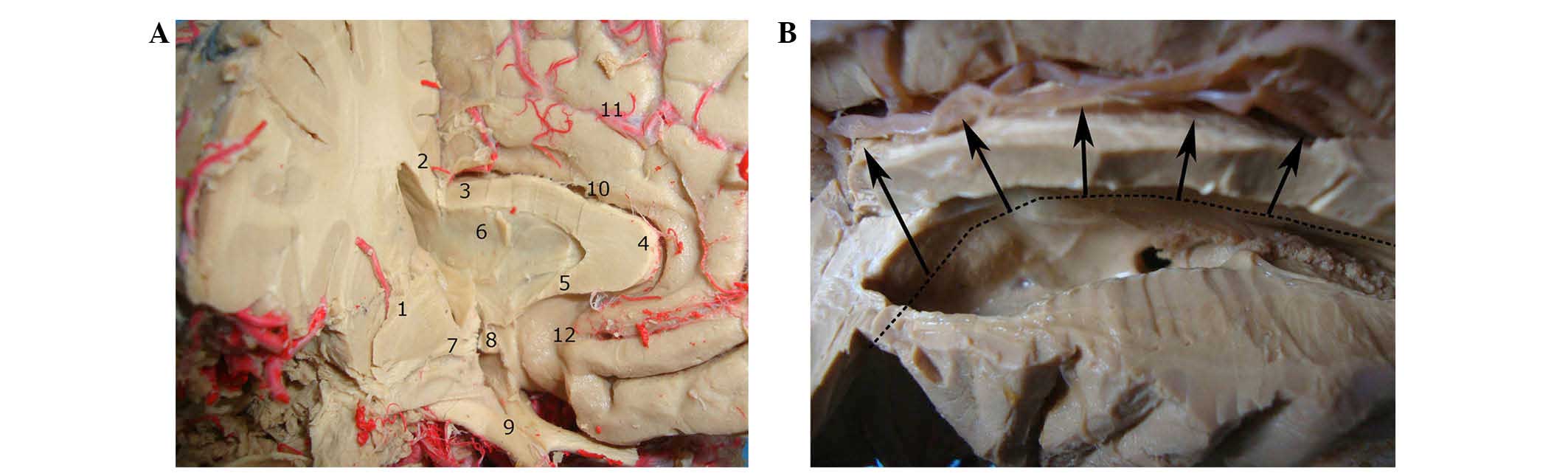 | Figure 3.Surgical views. (A) Associations
between the midline structures around the right third ventricle;
the body, genu and rostrum of the corpus callosum were resected
along the midline. After entering the cavity of the septum
pellucidum, the anterior commissure was observed. It is important
to protect the anterior part of the third ventricle and the optic
chiasm. (B) Incision in the medial and ventral aspects of the left
frontal lobe. The long black line indicated the location of the
cut; the black arrows indicate the direction of the incision. The
anterior incision should be anterolateral to the interventricular
foramen. The bidirectional black arrow indicates the frontal horn
of the cerebral ventricle and the anterior sulcus of the insula. 1,
Basal ganglia; 2, corpus callosum body; 3, corpus callosum body, 4,
corpus callosum knee; 5, corpus callosum mouth; 6, septum
pellucidum; 7, anterior commissure; 8, anterior commissure; 9,
optic chiasma; 10, cingulate gyrus; 11, anterior cerebral artery
branch; 12, endplate pool. |
In the present study, the patient's memory improved
postoperatively. Although a number of sharp waves were detected on
EEG following surgery, the patient's seizures arrested, suggesting
that the epileptogenic zone was fully isolated (Fig. 4B). Similar to posterior quadrantic
disconnection, frontal lobe isolation surgery involves the incision
of the dorsolateral cortex, followed by incision of the
dorsolateral white matter, cerebral ventricle, ventromedial white
matter and ventromedial cortex (22). The dorsolateral cortical incision
extends from the middle and posterior parts of the superior frontal
gyrus, obliquely beneath the surface of the brain to the anterior
part of the lateral cleft, and to the anterior part of the circular
sulcus of the insula (in the dominant hemisphere, the posterior
part of the inferior frontal gyrus should be left intact) (25). The remainder of the procedure is
performed as described above, resulting in complete frontal lobe
isolation. Postoperative MRI showed division of the anterior part
of the corpus callosum and the anterior commissure, with complete
isolation of the frontal lobe.
In conclusion, the current study demonstrated that
prefrontal lobe isolation surgery may be an effective method of
treating frontal lobe epilepsy; however, this conclusion requires
confirmation by further studies. The results suggest that frontal
lobe epilepsy that has recurred following epileptogenic focus
resection, or refractory frontal lobe epilepsy without structural
foci detected by MRI, may benefit from complete prefrontal lobe
isolation surgery.
Acknowledgements
The authors thank Professor Zhang Yuqin, Professor
Yan Xiaoling and Professor Chen Jun for their technical assistance.
The study was supported by the Science and Technology Foundation of
Tianjin Health and Family Planning Commission (grant no.
2014KG116).
References
|
1
|
Mu J, Rampp S, Carrette E, Roessler K,
Sommer B, Schmitt FC, De TX, Hamer H, Boon P, Pauli E, et al:
Clinical relevance of source location in frontal lobe epilepsy and
prediction of postoperative long-term outcome. Seizure. 23:553–559.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Stone JJ, Reynolds MR and Leuthardt EC:
Transient hemispatial neglect after surgical resection of a right
frontal lobe mass. World Neurosurg. 76:361.e7–e10. 2011. View Article : Google Scholar
|
|
3
|
Yin SY, Feng KK, Feng M, Zhang XQ and
Zhang YQ: Posterior quadrantic disconnection maintains the activity
of isolated temporal-parietal-occipital nerve tissue:
Neuroprotective measures in the surgical treatment of epilepsy.
Neural Regen Res. 9:447–448. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lee YJ, Kim EH, Yum MS, Lee JK, Hong S and
Ko TS: Long-term outcomes of hemispheric disconnection in pediatric
patients with intractable epilepsy. J Clin Neurol. 10:101–107.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bulteau C, Otsuki T and Delalande O:
Epilepsy surgery for hemispheric syndromes in infants:
Hemimegalencepahly and hemispheric cortical dysplasia. Brain Dev.
35:742–747. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Dorfer C, Czech T, Dressler A, Gröppel G,
Mühlebner-Fahrngruber A, Novak K, Reinprecht A, Reiter-Fink E,
Traub-Weidinger T and Feucht M: Vertical perithalamic
hemispherotomy: A single-center experience in 40 pediatric patients
with epilepsy. Epilepsia. 54:1905–1912. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kovanda TJ, Rey-Dios R, Travnicek J and
Cohen-Gadol AA: Modified periinsular hemispherotomy: Operative
anatomy and technical nuances. J Neurosurg Pediatr. 13:332–338.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Massager N, Tugendhaft P, Depondt C,
Coppens T, Drogba L, Benmebarek N, De Witte O, Van Bogaert P and
Legros B: Long-term outcome of surgical disconnection of the
epileptic zone as an alternative to resection for nonlesional
mesial temporal epilepsy. J Neurol Neurosurg Psychiatry.
84:1378–1383. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Guan YG, Luan GM and Zhou J:
Hemispherotomy for children with intractable epilepsy. Zhong Hua
Shen Jing Wai Ke Za Zhi. 28:994–997. 2012.(In Chinese).
|
|
10
|
Su CD, Chang PF and Yu L: Clinical
analysis of disconnection surgery of posterior part of the cerebrum
for one case of refractory epilepsy. Li Ti Ding Xiang He Gong Neng
Xing Shen Jing Wai Ke Za Zhi. 25:247–250. 2012.(In Chinese).
|
|
11
|
Sugano H, Nakanishi H, Nakajima M, Higo T,
Iimura Y, Tanaka K, Hosozawa M, Niijima S and Arai H: Posterior
quadrant disconnection surgery for Sturge-Weber syndrome.
Epilepsia. 55:683–689. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yin SY, Feng KK, Yue W, Feng M, Wang SM
and Zhang XQ: Hemisphere disconnection in the treatment of
intractable epilepsy. Zhong Hua Shen Jing Wai Ke Za Zhi.
29:714–718. 2013.(In Chinese).
|
|
13
|
Yin SY, Feng M, Li QY, Zhang XG, Yue W,
Zhao A, Liu QJ and Zhang YG: Posterior quadrantic disconnection for
temporal-parietal-occipital epilepsy in two cases and review of the
literature. Zhong Hua Shen Jing Wai Ke Za Zhi. 29:1235–1236.
2013.(In Chinese).
|
|
14
|
Kan R, Watabe M, Takahashi R, Kaneko Y,
Miyamoto Y and Niwa S: Comparison of IMP-single photon emission
computed tomography findings to Wechsler Intelligence Scale and
Benton Visual Memory Scale. Psychiatry Clin Neurosci. 49:S225–S227.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Fuentes A and Smith ML: Patterns of verbal
learning and memory in children with intractable temporal lobe or
frontal lobe epilepsy. Epilepsy Behav. 53:58–65. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Garcia PA and Laxer KD: Lateral Frontal
Lobe Epilepsies. Bleasel AF: Medial Frontal Lobe EpilepsyEpilepsy
Surgery. Lüders HO and Comair YG: 2nd. Lippincott Williams and
Wilkins; Philadelphia, PA: pp. 111–134. 2001
|
|
17
|
Yin SY: Nerve fiber disconnection
treatment for refractory epilepsy. Yi Xue Zong Shu. 20:828–832.
2014.(In Chinese).
|
|
18
|
Peters M, Oeltze S, Seminowicz D,
Steinmetz H, Koeneke S and Jäncke L: Division of the corpus
callosum into subregions. Brain Cogn. 50:62–72. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Unterberger I, Bauer R, Walser G and Bauer
G: Corpus callosum and epilepsies. Seizure. 37:55–60. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lew SM, Matthews AE, Hartman AL and
Haranhalli N: Post-Hemispherectomy Hydrocephalus Workgroup:
Posthemispherectomy hydrocephalus: Results of a comprehensive,
multiinstitutional review. Epilepsia. 54:383–389. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Massager N, Tugendhaft P, Depondt C,
Coppens T, Drogba L, Benmebarek N, De Witte O, Van BP and Legros B:
Long-term outcome of surgical disconnection of the epileptic zone
as an alternative to resection for nonlesional mesial temporal
epilepsy. J Neurol Neurosurg Psychiatry. 84:1378–1383. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Nooraine JR, Shiva K, Iyer RB, Rao RM and
Raghavendra S: Posterior quadrant disconnection for refractory
epilepsy: A case series. Ann Indian Acad Neurol l17. 392–397.
2014.
|
|
23
|
Guo XD, Wang BH, Wu YZ, Liu MH, Lu WF, Hao
WM, Wang ZH, Zhao G and Yang JG: Frontal lobe disconnection for
intractable frontal lobe epilepsy of normal imageology. Zhong Hua
Shen Jing Wai Ke Za Zhi. 29:512–515. 2013.(In Chinese).
|
|
24
|
Kim DL, Osburn LL and Cohen-Gadol AA: A
novel method for confirmation of hemispheric disconnection during
hemispherotomy surgery. Pediatr Neurosurg. 46:71–75. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhu HT, Zhu JL, Zhao TZ, Wu Y, Liu HY, Wu
T, Yang L, Zou YJ, Zhang R and Zheng G: Alteration of interictal
brain activity in patients with temporal lobe epilepsy in the left
dominant hemisphere: a resting-state MEG study. BioMed Res Int.
2014:1714872014. View Article : Google Scholar : PubMed/NCBI
|