Introduction
Obstructive sleep apnea (OSA) is a chronic sleep
disorder with high morbidity and mortality, affecting 9% of
middle-aged women and 24% of middle-aged (30–55 years old) men. OSA
increased the risk of the overall mortality by 26.2% (1). Epidemiologically, OSA is an independent
risk factor for hypertension, diabetes mellitus, coronary heart
disease and even mortality (2–5).
Intermittent hypoxemia-induced oxidative stress and inflammation,
combined with increased sympathetic activation, are potential
mechanisms linking OSA and its cardiovascular or metabolic sequelae
(6). In addition to the
OSA-associated health consequences, OSA results in increasing
social and economic burdens, including car accidents, inefficiency
at work and reduced social ability (7–9).
Although general practitioners are aware of OSA, there is a limited
availability of efficient diagnostic methods, and a diagnosis of
OSA is important, since it may be associated with other serious
complications (10). The majority of
undiagnosed OSA patients are not aware of the dangers caused by OSA
and do not seek therapy (11).
As recommended by the American Academy of Sleep
Medicine (AASM), overnight polysomnography (PSG) is the gold
standard for diagnosing OSA (12).
However, several limitations of PSG monitoring should be
considered; for instance, PSG is expensive, laborious and
inconvenient. Therefore, the development of a simple and convenient
device for diagnosing OSA is required. A micromovement sensitive
mattress (MSM) is a novel sleep-monitoring system that was first
developed by the Institute of Aviation Medicine of the Chinese Air
Force (13). The MSM monitoring
system features sensors in a sheet, and can detect the pressure of
respiratory movements, heart rate and the pressure of body
movements. The MSM converts the sensory output into digital
signals, and ultimately these may be used to reconstruct sleep
breathing patterns. The greatest advantage of this device is that
suspected OSA patients do not need to be restrained by attached
sensors and consequently experience a more comfortable sleep
(13). In Japan, a similar
sheet-type portable monitor was produced and demonstrated that the
sensitivity and specificity were relatively high (14–16).
However, the device requires validation for use in the Chinese Han
population.
The aim of the present study was to compare MSM with
nocturnal PSG monitoring in order to determine the accuracy and
validity of MSM in a clinical environment.
Subjects and methods
Study subjects and clinical
measurements
The present study enrolled 180 consecutive suspected
OSA patients between June 2013 and June 2014 from the Department of
Otolaryngology, Shanghai Jiao Tong University Affiliated Sixth
People's Hospital (Shanghai, China). All participants had
self-reported habitual snoring and excessive daytime sleepiness,
with or without nocturnal apnea. The exclusion criteria included:
i) Patients with an implanted electronic device; ii) patients who
had received or were receiving therapy for OSA; iii) patients with
severe systemic diseases; and iv) pregnant women. In addition,
patients with severe back pain and spinal deformities that would
affect the MSM monitoring application were excluded. Ultimately,
135 Han-Chinese participants (112 males, 23 females; mean age,
44.39±12.07 years; mean BMI, 26.92±3.31 kg/m2) met the
criteria and were analyzed.
Height and weight were measured, and the body mass
index (BMI) was calculated as BMI = weight / height2
(kg/m2). All subjects underwent both PSG and MSM
monitoring. It is worth to note that the PSG device did not
interfere with the results of MSM. Written informed consent was
obtained from each participant, and the study was approved by the
Ethics Committee of Shanghai Jiao Tong University Affiliated Sixth
People's Hospital.
MSM structure and measurement
The MSM monitoring system (Rising Sun Co., Ltd,
Beijing, China) consisted of a specially-designed sheet, a finger
pulse oxygen saturation (SpO2) detector (Rising Sun Co.,
Ltd.) and a personal computer. The specially-designed sheet had a
length of 195 cm, width of 90 cm and thickness of 10 cm.
Micromovement-sensitive pressure sensors were performed in the
hospital and were arranged within the sheet. They were able to
detect slight pressure caused by heartbeat, respiration and other
body movements. Following collection of aforementioned data during
patient sleep for one night, the data were recorded on a memory
card, transferred to the personal computer and then analyzed using
customized analytical software.
Respiratory efforts and subcortical arousals were
used to define apnea and hypopnea. Apnea was defined as a decrease
of ≥50% in the amplitude of respiratory movements for ≥10 sec,.
Hypopnea was defined as a ≥30% decrease in the amplitude of
respiratory movement for ≥10 sec and an oxygen desaturation
decrease of ≥5%. Both apnea and hypopnea were combined with
respiratory effort and were required to end with subcortical
arousal. The apnea-hypopnea index (AHI) detected with the MSM
(AHIMSM) was calculated using the number of apnea and
hypopnea events per hour of sleep (13). The MSM system will continuously
record the events of apnea or hypopnea during the night The mean
AHI value for the duration of the sleep was calculated after
collecting the number of events per hour.
Overnight PSG measurement
Overnight PSG was conducted using standard digital
polysomnographic evaluation with an Alice 4 or Alice 5 Diagnostic
Sleep system (Philips Respironics Inc., Murrysville, PA, USA),
simultaneously with MSM monitoring between 21:00 and 6:00. In
addition, an electroencephalogram (C4-A2, C3-A1, O2-A1 and O1-A2;
Philips Respironics, Inc., Murrysville, PA, USA), bilateral
electrooculogram, submental and anterior tibial electromyogram, and
electrocardiogram (Philips Respironics Inc.,) were recorded with
surface electrodes during sleep time. Nasal and oral flow, thoracic
and abdominal movement, body posture, snoring and finger
SpO2 were also recorded (12). The diagnosis of OSA was assessed
according to the AASM criteria, defining apnea events as the
cessation of airflow by ≥90% for at least 10 sec, and hypopnea
events as a reduction in airflow by ≥30% for at least 10 sec and
oxygen desaturation of ≥4%, which were the criteria for PSG
(12). According to AASM criteria,
diagnosis and severity of OSA was determined by AHI. Non-OSAHS,
mild, moderate and severe OSA were defined as an AHI of <5,
5–15, 15–30, and ≥30 events per hour, respectively. AHI monitored
by PSG (AHIPSG) was calculated as the total number of
apnea and hypopnea events divided by the total number of hours of
sleep. According to AASM, AHIPSG of ≥5, ≥15 and ≥30 represent mild,
moderate and severe OSA, respectively. These cut-off values of
AHIPSG were used to further classify OSA into different degrees.
Therefore, the sensitivity (specificity) for MSM detecting OSA in
mild, moderate and severe OSA, respectively, could not be
determined. Any mistakes that may have been recorded in the MSM and
PSG results were amended manually by a technician, who was blinded
to the participant information in order to avoid bias.
Statistical analysis
All the data were analyzed using SPSS software
(version 19.0; IBM SPSS, Armonk, NY, USA) and MedCalc (version
12.7.3; MedCalc Software bvba, Ostend, Belgium). Values are
presented as the mean ± standard deviation for continuous
variables, and as a number or percentage for categorical variables,
as appropriate. The correlation between AHIPSG and
AHIMSM was evaluated using Pearson's correlation
coefficient. One-sample t-test was used to evaluate the difference
between AHIPSG and AHIMSM. Bland-Altman
analysis was performed using MedCalc to assess the similarity
between AHIPSG and AHIMSM. OSA was diagnosed
in patients that have a higher AHI than the cut-off value. Thus,
cut-off points of AHIPSG were estimated to increase the
precision of the AHIMSM device using the area under the
receiver operating characteristic (ROC) curves. To assess the
predictive performance of the MSM, sensitivity and specificity were
also calculated. P<0.05 was considered to indicate a
statistically significant difference.
Results
Patient characteristics
Of the total cohort (n=135), 4 participants were
excluded from the study as they did not sleep overnight. The
remaining 131 subjects (110 males, 21 females; mean age,
44.33±11.90 years; mean BMI, 26.88±3.24 kg/m2; mean
apnea index, 28.97±24.25; mean hypopnea index, 7.47±7.13; mean
hypopnea events, 53.98±39.43; mean obstructive apnea events,
204.33±176.82; mean central apnea events, 7.11±15.31; mixed apnea
events, 8.56±20.74; and SpO2, 60.63±29.87%). were used
in the final statistical analysis. According to
electroencephalogram, electrooculogram and electromyogram analyses,
the different sleep stages were separated. Mean total sleep time
(TST) was 465.98±64.37 min, mean time in the non-rapid eye movement
(NREM) stage was 393.61±79.42 min, mean time in the rapid eye
movement (REM) stage was 69.29±41.94 min. Percentage of NREM in TST
was 84.68±9.03%, percentage of REM in TST was 15.24±9.11%, mean
time in light sleep was 347.08±89.75 min, mean time in deep sleep
was 41.96±36.22 min, percentage of light sleep in TST was
74.86±14.33%, percentage of deep sleep in TST was 9.29±7.96%. The
mean AHI calculated with the PSG and MSM devices were 36.14±25.50
and 36.04±23.89 events/h, respectively. In the 14 non-OSA patients,
the AHIPSG ranged from 1–4.5 events/h, and the mean
AHIPSG was 2.61±1.33 events/h. The mean
AHIMSM was 5.19±2.86 events/h. In the 18 mild OSA
patients, the AHIPSG ranged from 5.2–14.5 events/h, and
the mean AHIPSG was 9.65±2.77 events/h. The mean
AHIMSM was 12.91±5.98 events/h. In the 27 moderate OSA
patients, the AHIPSG ranged from 15.1–27.4 events/h, the
mean AHIPSG was 20.71±3.62 events/h, the mean
AHIMSM was 23.46±6.14 events/h and in the 72 severe OSA
patients, the AHIPSG ranged from 30.3–104.2 events/h.
The mean AHIPSG was 55.07±18.17 events/h and the mean
AHIMSM was 52.53±19.01 events/h. As Bland-Altman
analysis revealed there was a good agreement of 97% between the
AHIPSG and AHIMSM values, thus the diagnosis
was consistent between the two methods. According to AHIPSG, 14
participants were non-OSA, 18 were mild OSA, 27 were moderate OSA,
and 72 were severe OSA. The mean difference between AHIPSG and
AHIMSM devices was 0.11±6.20, and the difference between them was
not statistically significant (P=0.84).
Comparison of AHIPSG and
AHIMSM results
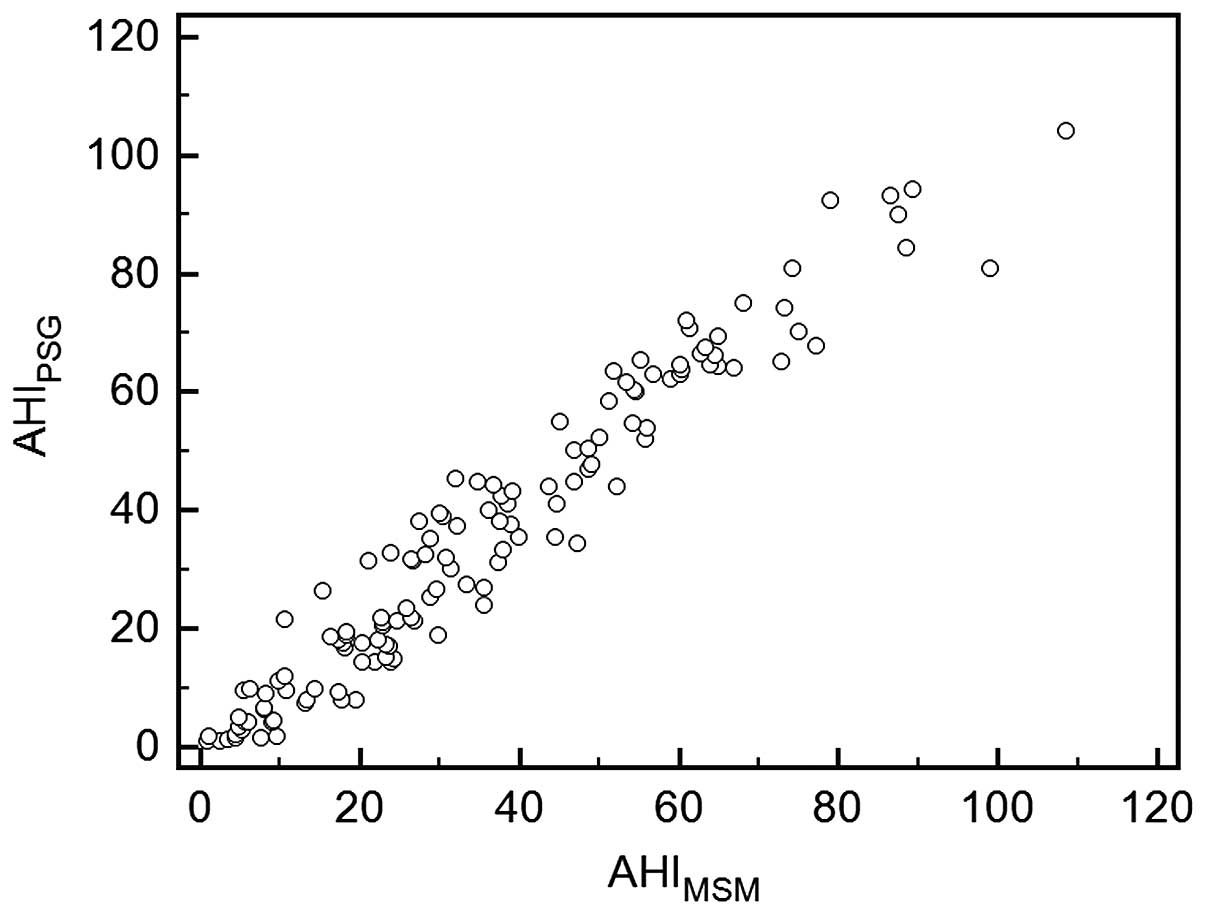
A significant correlation was identified between
AHIPSG and AHIMSM values (r=0.97,
P<0.001), which are presented as a scatterplot in Fig. 1. Thus, the AHI assessed by the MSM
significantly correlated with the AHI simultaneously assessed by
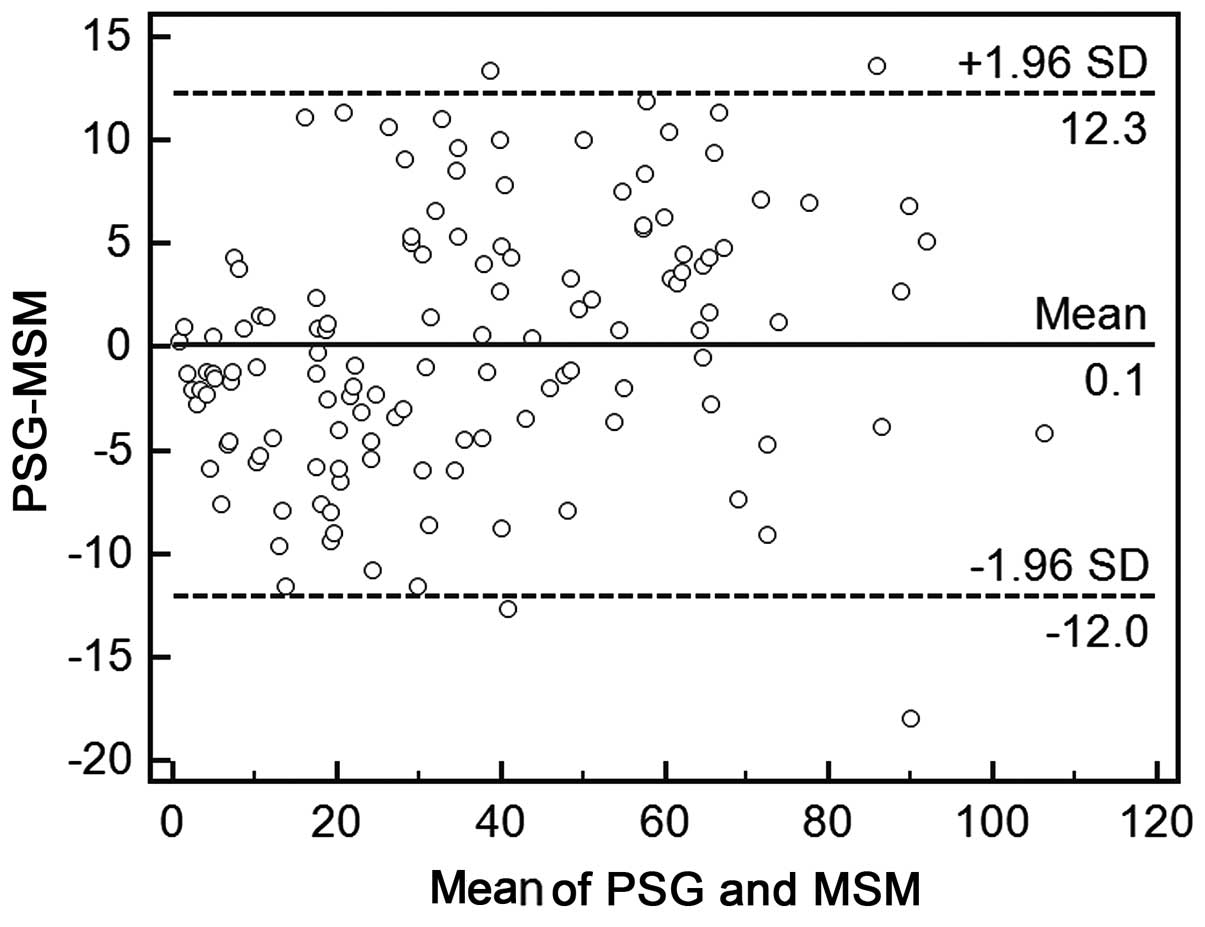
PSG. In addition, Bland-Altman analysis revealed a good agreement
(97%) between AHIPSG and AHIMSM values
(Fig. 2). A Bland-Altman plot of
AHIMSM versus PSG AHI had only 4 (3%) outliers for the MSM, and the
majority of the AHIs measured by the MSM fell within two standard
deviations of the mean PSG value, indicating that differences
between AHIPSG and AHIMSM were reasonably tightly distributed.
Sensitivity and specificity of
results
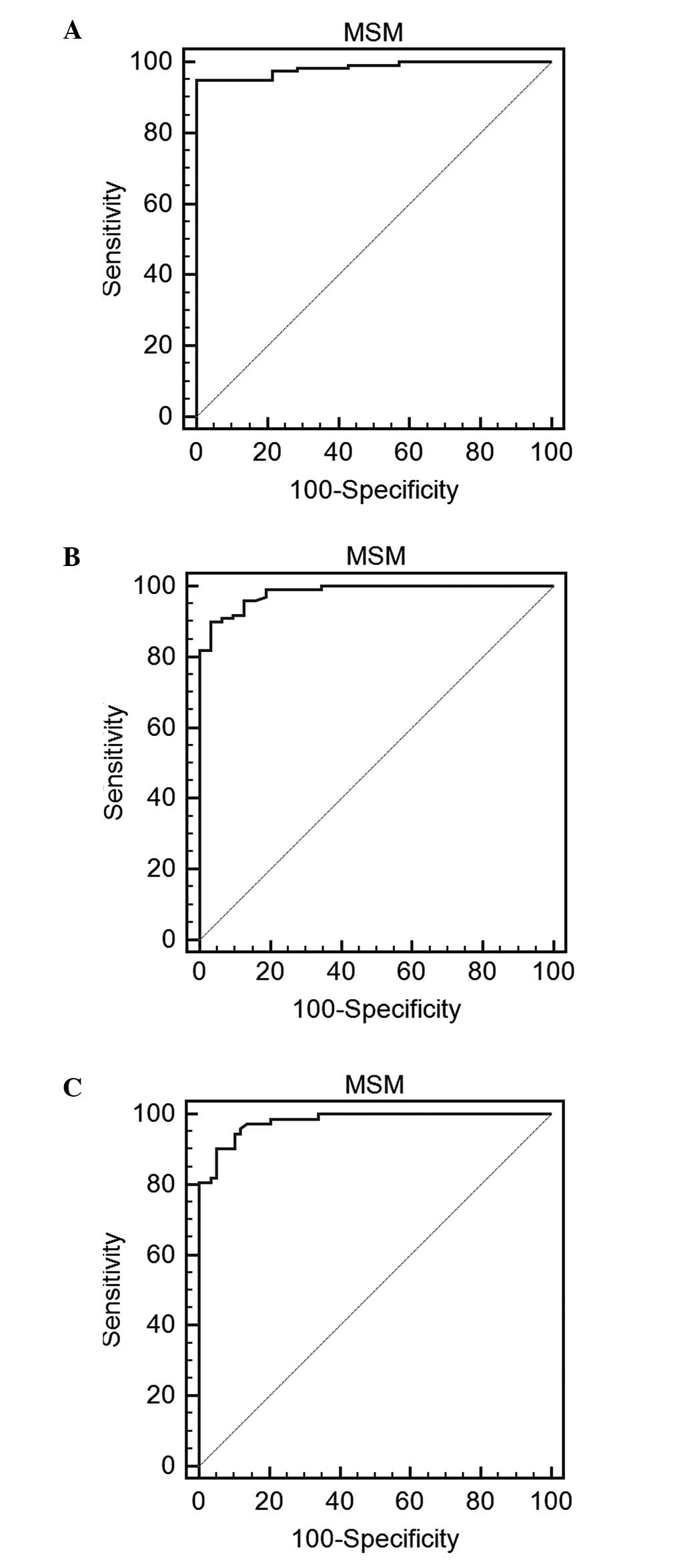
ROC curve analysis was also performed to evaluate
the clinical use of the MSM as a diagnostic tool for OSA at cut-off
values of AHIPSG of ≥5, ≥15 and ≥30 events/h. These
values were based on the results of a previous study (12). The results indicated that the
percentages of sensitivity (specificity) for detecting an
AHIMSM of ≥5, ≥15 and ≥30 events/h were 94.9 (100%),
89.9 (96.9%) and 90.3 (94.9%), respectively. The area under the
curve used to differentiate AHIPSG of ≥5, ≥15 and ≥30
events/h in the clinical diagnosis of OSA was 0.984, 0.982 and
0.980, respectively (Fig. 3A-C).
Discussion
In the present study, the results of Pearson's
correlation coefficient analysis revealed that the obtained
AHIMSM value was correlated with AHIPSG
value. In addition, Bland-Altman plots provided evidence of the
agreement between the results of AHIMSM and
AHIPSG. The promising performance of the MSM
sleep-monitoring system was also validated compared with PSG
monitoring among mild, moderate and severe OSA groups in the
Chinese Han population.
As a result of the discomfort imposed upon patients
with suspected OSA undergoing PSG monitoring, in addition to being
a laborious process for the technician, other simple screening and
diagnostic methods have been proposed (17–21).
Although mitochondrial DNA mutation appears promising in screening
OSA in male patients, the findings require further verification
(17). The Berlin questionnaire is
also used widely for diagnosis; however, the actual association
between the results of the Berlin questionnaire and OSA, is still
debated (18). Similarly, the
STOPBang questionnaire was recently revealed to have low accuracy,
with sensitivities of 81–86% and specificities of 34–57% using
cut-off values of the PSG respiratory disturbance index of 5, 15
and 30 events/h (19,22). Notably, the accuracy of the AHI
calculated using snoring analysis compared with the AHI calculated
from PSG has been reported to be 96.7, 86.7 and 96.7% in patients
with mild, moderate and severe OSA, respectively (23). Although acoustic analysis has
previously shown high agreement with the results of PSG, this
technology is sophisticated and requires considerable technical
expertise (10). Furthermore, a type
3 portable monitoring (PM) device (Stardust II Sleep Recorder;
Philips Respironics, Inc.) has been recommended for use in a
clinical environment if the recordings are reviewed manually
(21). However, the use of PM also
has limitations, since the device is sophisticated and causes
patient discomfort, as a finger probe, nasal cannula and belt must
be attached to the body (24). In
addition, PM is not recommended in suspected OSA patients with
comorbid conditions, such as insomnia, depression and mania, which
may mask symptoms of OSA (25).
Consequently, a simpler and more comfortable portable device should
be developed.
A sheet-like sleep-monitoring device, termed the
static charge sensitive bed (SCSB), was first used for detecting
sleep-associated apnea in 1986 (26). Although SCSB demonstrated a high
sensitivity, and was easy to use and relatively inexpensive, it
only had a limited capacity for identifying apnea events and was
unable to distinguish the type of respiratory event. In Japan, a
similar sheet-like sleep-monitoring system, termed SD-101, was
previously used to detect suspected OSA (14–16). In
the study by Agatsuma et al (14), ROC curve analysis of aa respiratory
disturbance index (measured by SD-101, Kentzmedico Co. Ltd,
Saitama, Japan) of 14 events/h revealed 89.5% sensitivity and 85.8%
specificity for identifying OSA. Tsukahara et al (15) calculated AHI using the time in bed,
and at a cut-off of 14 events/h, the sensitivity and specificity of
detecting an AHI of ≥20 events/h were 90.2 and 90.0%, respectively.
Kobayashi et al (16) used
the SD-101 with percutaneous oxygen saturation detecting an AHI of
>15 events/h on PSG with a sensitivity (specificity) of 96.9%
(90.5 %) compared with 87.5% (85.7 %), respectively.
The MSM sleep-monitoring system used in the present
study is a noninvasive diagnostic device that has been validated in
the Chinese Han population. The predominant merits of this MSM
sleep-monitoring system are the following: i) It is comfortable and
does not disturb sleep; ii) it is appropriate for suspected OSA
patients unwilling to undergo PSG with connected sensors; iii) the
sensitivity and specificity of the MSM sleep-monitoring device are
superior to those of the SD-101 device; and iv) it is equipped with
an oxygen detector.
Despite the aforementioned advantages, several
limitations of the present study should be addressed. First, the
relatively small number of participants may bias the results.
Therefore, future research to evaluate the clinical utility of the
MSM sleep-monitoring system needs to be performed in multiple sleep
centers and communities. Furthermore, the study focused on the
Chinese Han population, and thus it is essential to confirm the
accuracy of the MSM sleep-monitoring system in other populations.
In addition, the diagnostic accuracy of MSM in specific
subpopulations, such as in extremely obese individuals and in
children, should also be evaluated systematically.
In conclusion, the present study obtained good
accuracy for diagnosing OSA with a non-restrictive, non-invasive
MSM sleep-monitoring system with relatively high sensitivity and
specificity. Further community-based studies with larger sample
sizes are warranted to confirm whether the MSM sleep-monitoring
system is a suitable substitute for PSG monitoring in diagnosing
OSA.
References
|
1
|
Young T, Palta M, Dempsey J, Skatrud J,
Weber S and Badr S: The occurrence of sleep-disordered breathing
among middle-aged adults. N Engl J Med. 328:1230–1235. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pepin JL, Borel AL, Tamisier R, Baguet JP,
Levy P and Dauvilliers Y: Hypertension and sleep: Overview of a
tight relationship. Sleep Med Rev. 18:509–519. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Aurora RN and Punjabi NM: Obstructive
sleep apnoea and type 2 diabetes mellitus: A bidirectional
association. Lancet Respir Med. 1:329–338. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ali SS, Oni ET, Warraich HJ, Blaha MJ,
Blumenthal RS, Karim A, Shaharyar S, Jamal O, Fialkow J, Cury R, et
al: Systematic review on noninvasive assessment of subclinical
cardiovascular disease in obstructive sleep apnea: New kid on the
block! Sleep Med Rev. 18:379–391. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Marshall NS, Wong KK, Cullen SR, Knuiman
MW and Grunstein RR: Sleep apnea and 20-year follow-up for
all-cause mortality, stroke, and cancer incidence and mortality in
the Busselton Health Study cohort. J Clin Sleep Med. 10:355–362.
2014.PubMed/NCBI
|
|
6
|
Hoyos CM, Melehan KL, Liu PY, Grunstein RR
and Phillips CL: Does obstructive sleep apnea cause endothelial
dysfunction? A critical review of the literature. Sleep Med Rev.
20:15–26. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Stevenson MR, Elkington J, Sharwood L,
Meuleners L, Ivers R, Boufous S, Williamson A, Haworth N, Quinlan
M, Grunstein R, et al: The role of sleepiness, sleep disorders, and
the work environment on heavy-vehicle crashes in 2 Australian
states. Am J Epidemiol. 179:594–601. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rodenstein D: Sleep apnea: Traffic and
occupational accidents-individual risks, socioeconomic and legal
implications. Respiration. 78:241–248. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yang EH, Hla KM, McHorney CA, Havighurst
T, Badr MS and Weber S: Sleep apnea and quality of life. Sleep.
23:535–541. 2000.PubMed/NCBI
|
|
10
|
Xu H, Zheng X, Jia W and Yin S:
Chromatography/mass spectrometry-based biomarkers in the field of
obstructive sleep apnea. Medicine (Baltimore). 94:e15412015.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Strollo PJ Jr and Rogers RM: Obstructive
sleep apnea. N Engl J Med. 34:99–104. 1996. View Article : Google Scholar
|
|
12
|
Iber C, Ancoli-Israel S, Chesson AL and
Quan SF: The AASM manual for the scoring of sleep and associated
events: rules, terminology and technical specifications. 1st.
American Academy of Sleep Medicine; Westchester, IL: 2007
|
|
13
|
Zhang QF, Tong YF, She CP, Zhang XR, Song
W, Wang LM and Cheng CJ: Comparative analysis of polysomnography
and micro-sensitive mattress-sleep monitor used for obstructive
sleep apnea hypopnea syndrome. Zhonghua Er Bi Yan Hou Tou Jing Wai
Ke Za Zhi. 45:1014–1019. 2010.PubMed/NCBI
|
|
14
|
Agatsuma T, Fujimoto K, Komatsu Y,
Urushihata K, Honda T, Tsukahara T and Nomiyama T: A novel device
(SD-101) with high accuracy for screening sleep apnoea-hypopnoea
syndrome. Respirology. 14:1143–1150. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tsukahara M, Sakao S, Jujo T, Sakurai T,
Terada J, Kunii R, Tanabe N and Tatsumi K: The accuracy and
uncertainty of a sheet-type portable monitor as a screening device
to identify obstructive sleep apnea-hypopnea syndrome. Intern Med.
53:1307–1313. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kobayashi M, Namba K, Tsuiki S, Nakamura
M, Hayashi M, Mieno Y, Imizu H, Fujita S, Yoshikawa A, Sakakibara H
and Inoue Y: Validity of sheet-type portable monitoring device for
screening obstructive sleep apnea syndrome. Sleep Breath.
17:589–595. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Huang XY, Li H, Xu XM and Wang LX:
Mitochondrial DNA mutation screening of male patients with
obstructive sleep apnea-hypopnea syndrome. Exp Ther Med. 8:519–524.
2014.PubMed/NCBI
|
|
18
|
Margallo VS, Muxfeldt ES, Guimarães GM and
Salles GF: Diagnostic accuracy of the Berlin questionnaire in
detecting obstructive sleep apnea in patients with resistant
hypertension. J Hypertens. 32:2030–2037. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ha SC, Lee DL, Abdullah VJ and van Hasselt
CA: Evaluation and validation of four translated Chinese
questionnaires for obstructive sleep apnea patients in Hong Kong.
Sleep Breath. 18:715–721. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Xu H, Song W, Yi H, Hou L, Zhang C, Chen
B, Chen Y and Yin S: Nocturnal snoring sound analysis in the
diagnosis of obstructive sleep apnea in the Chinese Han population.
Sleep Breath. 19:599–605. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Santos-Silva R, Sartori DE, Truksinas V,
Truksinas E, Alonso FF, Tufik S and Bittencourt LR: Validation of a
portable monitoring system for the diagnosis of obstructive sleep
apnea syndrome. Sleep. 32:629–636. 2009.PubMed/NCBI
|
|
22
|
Nagappa M, Liao P, Wong J, Auckley D,
Ramachandran SK, Memtsoudis S, Mokhlesi B and Chung F: Validation
of the stop-bang questionnaire as a screening tool for obstructive
sleep apnea among different populations: A Systematic review and
meta-analysis. PLoS One. 10:e01436972015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Xu H, Song W, Yi H, Hou L, Zhang C, Chen
B, Chen Y and Yin S: Nocturnal snoring sound analysis in the
diagnosis of obstructive sleep apnea in the Chinese Han population.
Sleep Breath. 19:599–605. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Guerrero A, Embid C, Isetta V, Farre R,
Duran-Cantolla J, Parra O, Barbé F, Montserrat JM and Masa JF:
Management of sleep apnea without high pretest probability or with
comorbidities by three nights of portable sleep monitoring. Sleep.
37:1363–1373. 2014.PubMed/NCBI
|
|
25
|
Sunwoo B and Kuna ST: Ambulatory
management of patients with sleep apnea: Is there a place for
portable monitor testing? Clin Chest Med. 31:299–308. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Salmi T, Partinen M, Hyyppä M and Kronholm
E: Automatic analysis of static charge sensitive bed (SCSB)
recordings in the evaluation of sleep-related apneas. Acta Neurol
Scand. 74:360–364. 1986. View Article : Google Scholar : PubMed/NCBI
|