Introduction
Rheumatoid arthritis (RA) is a type of chronic and
systemic autoimmunity disease that is characterized by erosive
joint synovitis (1). The morbidity
of women is 2 fold higher than men (2). The morbidity of RA is 0.01–0.05% and
the nosometry is 0.18–1.07% in different populations worldwide
(2). As a result, RA is a disease
which has incapacitated numerous laborers in China (3). According to the epidemiological
investigation in the United States, the difference between the
mortality rate of patients with RA and healthy individuals is
increasing (4). Nosogenesises of RA
is currently unclear, but heredity, infection, immunity, endocrine
and other factors are possible relevant pathogenic factors
(4,5).
Dysfunction of the immune system is the primary
cause of RA, but the underlying mechanisms are unclear (6). Animal models must be established to
determine the mechanism. There are two types of existing animal
models of arthritis; one is adjuvant-induced arthritis and the
other is arthritis induced by arthrogenic autoantigen, which is the
arthritis induced by collagen (CIA) (7). CIA has been studied frequently, and its
clinical symptoms and pathological characteristics are the same as
RA (8). As a result, CIA is the most
commonly used animal model for RA (8).
Ranunculaceae Aconitum is a traditional
Chinese medicine that is used as a long-term cardiotonic drug,
whose active constituent is a type of alkaloid named higenamine,
which is extracted from Ranunculaceae Aconitum's rhizome
(9,10). However, the mechanism underlying the
protective effect of higenamine on CIA has, to the best of our
knowledge, never been investigated. In the present study, the
protective effect and associated underlying mechanism of higenamine
against CIA is investigated.
Materials and methods
Materials
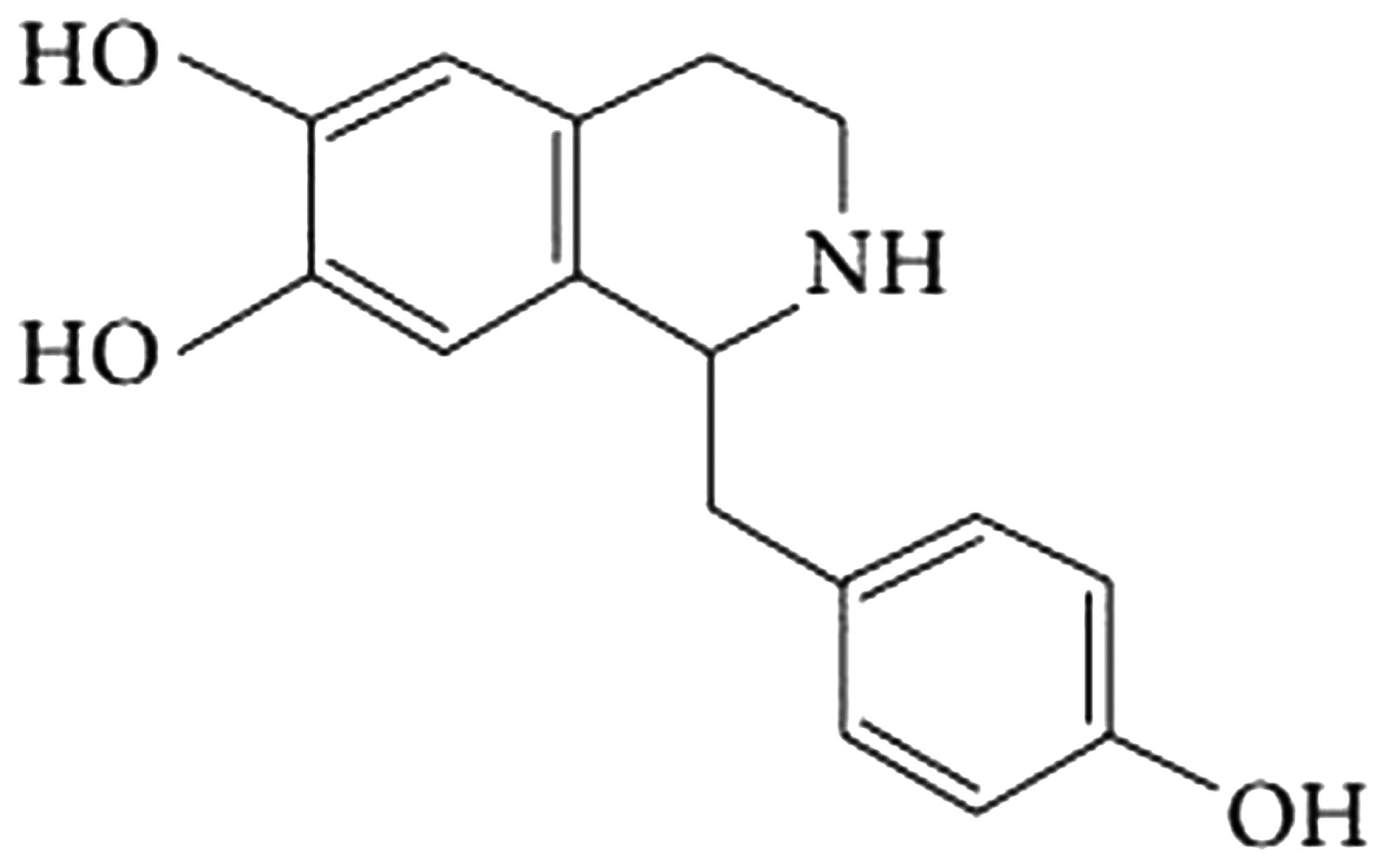
Higenamine was purchased from Sigma-Aldrich (Merck
Millipore, Darmstadt, Germany); its chemical structure is presented
Fig. 1. Freund's Incomplete
Adjuvant, bovine type II collagen, tumor necrosis factor-α (TNF-α),
interleukin-1β (IL-1β), malondialdehyde (MDA) and glutathione (GSH)
ELISA kits were purchased from Tauto Biotech Co., Ltd (Shanghai,
China). Caspase-3/9 florometric assay kits were purchased from
Sigma-Aldrich (Merck Millipore). Bicinchoninic Acid (BCA) assay was
purchased from Beyotime Institute of Biotechnology (Nanjing,
China).
Animals
Nine week-old male DBA/1J mice (5 weeks-old; weight,
18–20 g) were purchased from Shanghai Cell Bank of Chinese Academy
of Sciences (Shanghai, China). The animals were housed in a
controlled environment, 23±1°C, 12 h light/dark cycle, relative
humidity between 40 and 70%, and provided with standard rodent chow
and tap water. All of the animal experiments conducted were
performed in compliance with the guidelines and regulations for the
use and care of animals established by Jingdu Hospital (Nanjing,
China).
Induction of CIA and study design
DBA/1J mice were divided into four groups: Sham;
higenamine; CIA model; and CIA + higenamine (each group, n=6). Sham
and higenamine mice were sham-injected with saline or higenamine
(10 mg/kg) for 2 weeks. CIA model and CIA + higenamine mice
received injections of Freund's Incomplete Adjuvant with 2 mg/ml
bovine type II collagen at the base of the tail and two sites on
the back at 0 and 6 days. Clinically apparent disease onset
typically occurs at 10 days. Then, the CIA model and CIA +
higenamine mice were sham-injected with saline or higenamine (10
mg/kg) for 2 weeks.
CIA progression clinical arthritis
scores
Arthritis severity was evaluated with a clinical
scoring system of 0–4 for each paw: No signs of arthritis, 0;
swelling and/or redness in one joint, 1; swelling and/or redness in
more than one joint, 2; swelling and/or redness in the entire paw,
3; and severe swelling of the entire paw with deformity and/or
ankylosis, 4. The total arthritis score produced a maximum score of
16, as the sum of each score of the four limbs.
TNF-α, IL-1β, MDA, SOD, CAT and GSH-PX activities.
The blood samples from all mice were permitted to clot for 2 h were
collected via intracardiac puncture after treatment for 2 weeks,
and centrifuged at 2,000 × g at 4°C for 10 min. The activities of
TNF-α, IL-1β, MDA and GSH were measured using specific ELISA kits
in accordance with the manufacturer's instructions.
Caspase-3/9 activities
Under anesthetization by injection of rumpun (15
mg/kg) and ketamine (75 mg/kg), the mice were sacrificed using
cervical dislocation, and then the arthritis tissue samples from
all mice were permitted to clot for 2 h after treatment for 2
weeks. Then they were homogenized using 100 µl tissue lysis buffer
(Beyotime Institute of Biotechnology) and a protease inhibitor
cocktail (Sigma-Aldrich; Merck Millipore) for 30 min on ice and
centrifuged at 2,000 × g at 4°C for 10 min. The concentration of
protein was determined using a BCA assay. An equal quantity of
protein (10 µg) from every sample was separated using the
caspase-3/9 florometric assay kits, according to the manufacturer's
instructions.
Western blot analysis
The arthritis tissue samples from all mice were
permitted to clot for 2 h. Then they were homogenized using 100 µl
tissue lysis buffer (Beyotime Institute of Biotechnology) and a
protease inhibitor cocktail (Sigma-Aldrich; Merck Millipore) for 30
min on ice and centrifuged at 2,000 × g at 4°C for 10 min. The
concentration of protein was determined using a BCA assay. An equal
quantity of protein (50 µg) from every sample was separated by
10–12% SDS-PAGE and transferred to polyvinylidene floride
membranes. The membranes were blocked with 5% fat-free dry milk in
tris-buffered saline with Tween-20 for 2 h at room temperature and
incubated with anti-HO-1 (1:500; cat. no. sc-136960; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA), anti-Akt (1:500; cat. no.
sc-271149; Santa Cruz Biotechnology, Inc.), anti-p-Akt (1:500; cat.
no. sc-293125; Santa Cruz Biotechnology, Inc.), anti-nuclear factor
(erythroid-derived 2)-like 2 (Nrf-2) (cat. no. sc-365949; 1:1,500;
Santa Cruz Biotechnology, Inc.) and β-actin (1:500; cat. no.
BB-2116-1; BeastBio, Shanghai, China) overnight at 4°C. The
membranes were then blocked with appropriate secondary antibodies
(1:2,000; cat. no. C2221; Applygen Technologies, Inc., Beijing,
China) for 1 h at room temperature and ECL (Applygen Technologies,
Inc.) was applied. The blots were quantified using Image J software
(National Institutes of Health, Bethesda, MD, USA).
Statistical analysis
Data were expressed as the mean ± standard error and
analyzed by Student's t-test or analysis of variance with post-hoc
(Bonferroni) correction for multiple comparisons, or one-way
analysis of variance for multiple comparisons. SPSS 17.0 (SPSS,
Inc., Chicago, IL. USA) was used to analyze the data. P<0.05 was
considered to indicate a statistically significant difference.
Results
Protective effect of higenamine on CIA
progression clinical arthritis scores
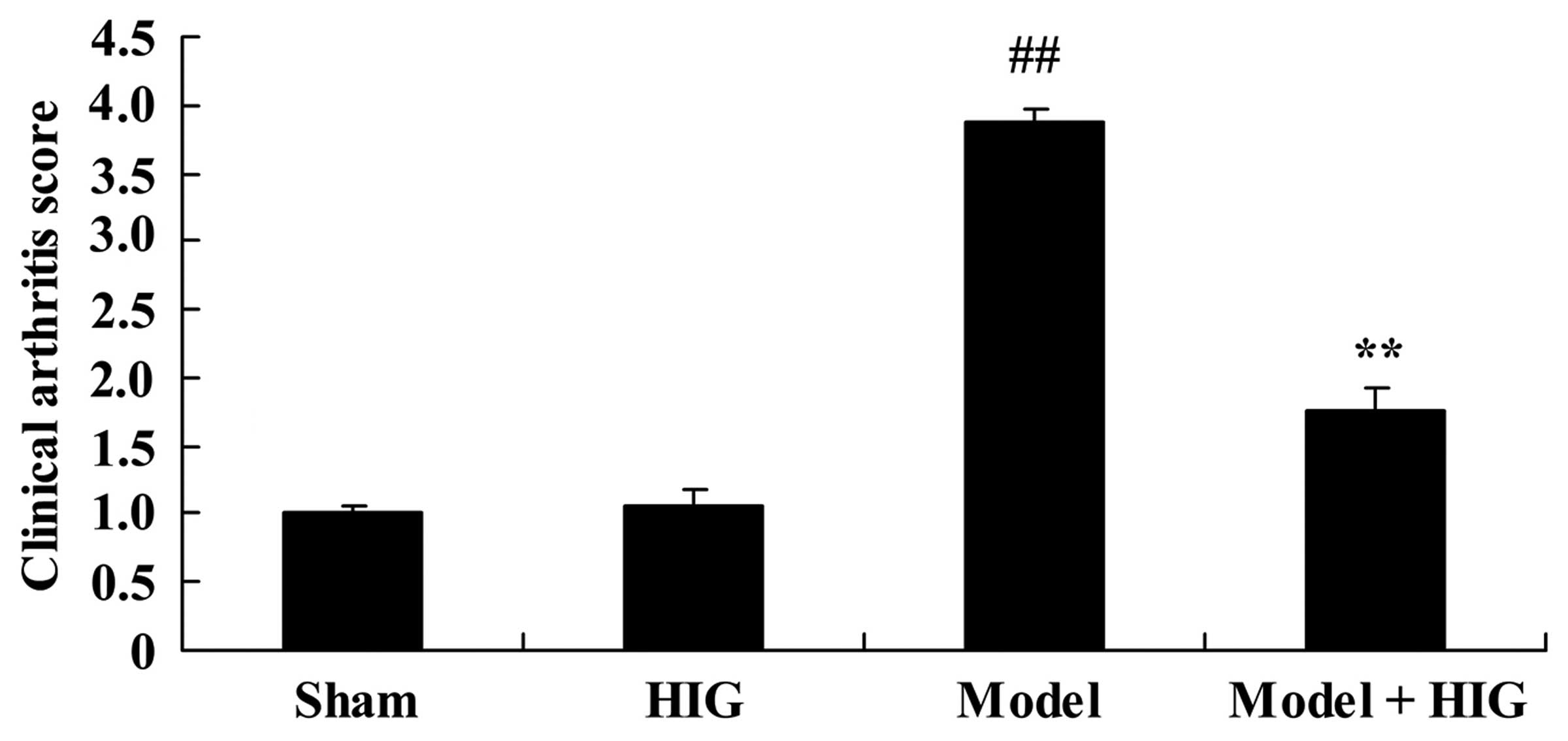
In order to evaluate the protective effect of
higenamine on CIA mice, the progression clinical arthritis scores
were evaluated. The results demonstrate that clinical arthritis
scores of the sham group were similar to those of the higenamine
group (Fig. 2). The clinical
arthritis scores of CIA mice were significantly higher compared
with those of the sham group (P<0.01; Fig. 2). The progression of clinical
arthritis scores of CIA mice were significantly reduced by
treatment with higenamine (P<0.01; Fig. 2).
Protective effect of higenamine on the
TNF-α and IL-1β activities
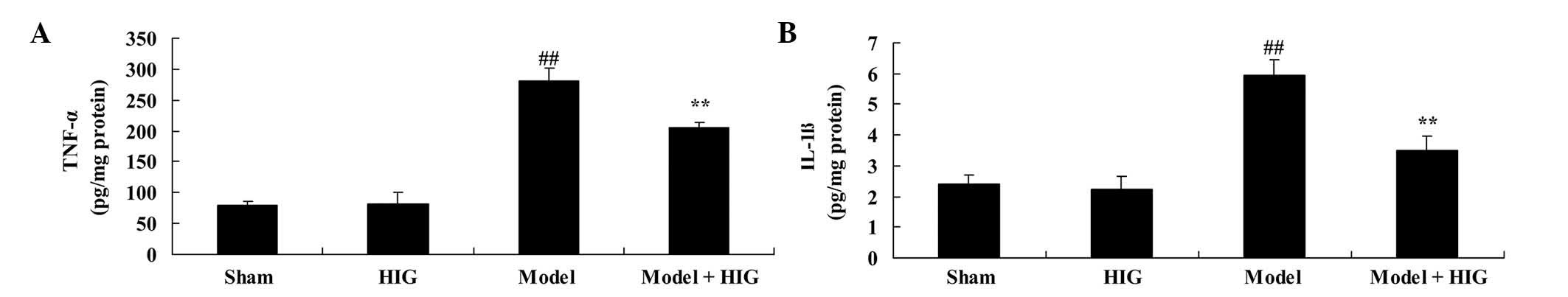
To determine the mechanism by which higenamine
treatment exerts an anti-inflammatory effect on CIA mice, the
activities of TNF-α and IL-1β were measured. TNF-α and IL-1β
activities in sham group mice were similar to those of mice in the
higenamine group (Fig. 3). CIA mice
had significantly increased inflammation factors compared with mice
in the sham group (P<0.01; Fig.
3). However, treatment with higenamine significantly reduced
these CIA-induced inflammation factors in CIA mice (P<0.01;
Fig. 3).
Protective effect of higenamine on
tMDA and GSH activities
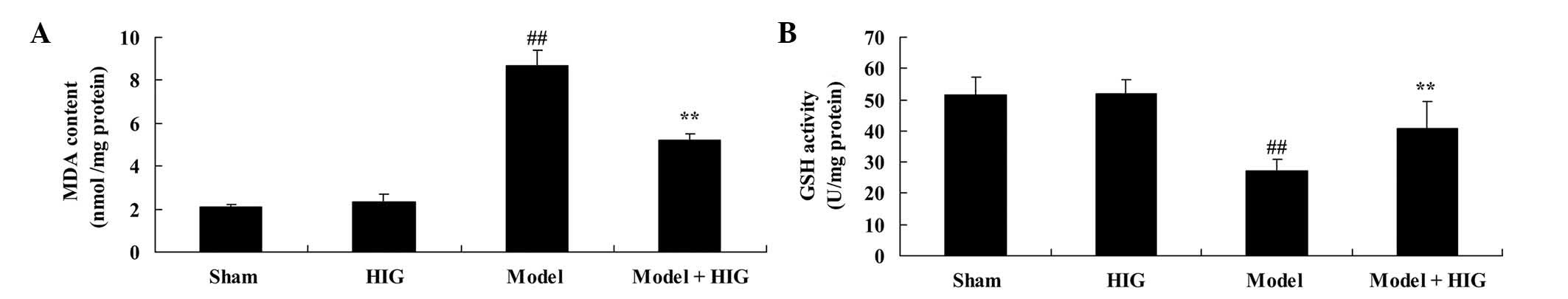
To investigate the mechanism of higenamine on
oxidative stress in CIA mice, MDA and GSH activities were measured
in each group. The MDA and GSH activities of sham mice were similar
to those in mice in the higenamine group (Fig. 4). Compared with the sham group, CIA
significantly increased the MDA activity and suppressed GSH
activity in CIA mice (P<0.01; Fig.
4). Meanwhile, higenamine treatment significantly recovered
abnormal MDA and GSH activities in CIA mice (P<0.01; Fig. 4).
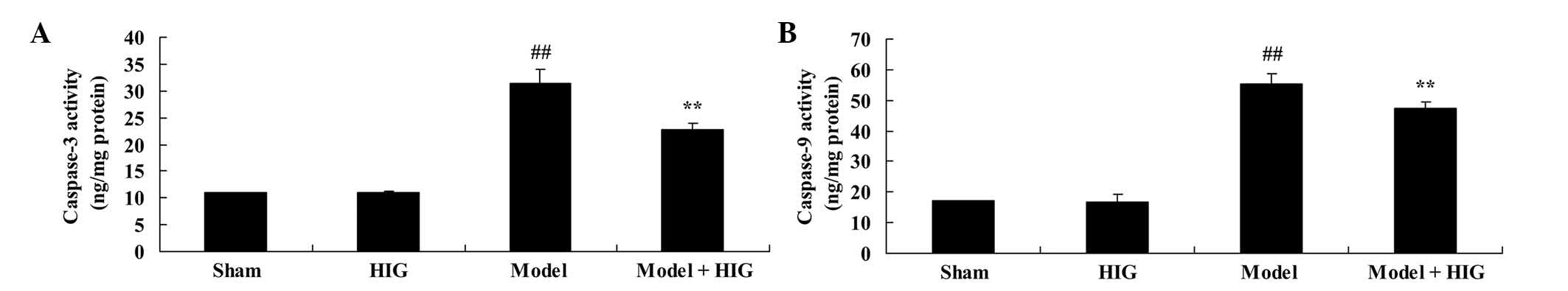
Protective effect of higenamine on
caspase-3/9 activities
In order to ascertain the anti-apoptotic effect of
higenamine on CIA mice, the caspase-3/9 activities were detected in
different groups. The caspase-3/9 activities of the sham group were
equipotent to those of mice in the higenamine group (Fig. 5). There was a significant increase in
caspase-3/9 activities of CIA mice without higenamine treatment
(Fig. 5). However, pretreatment with
higenamine significantly weakened CIA-induced caspase-3/9
activities of CIA mice, as compared with the CIA model group
(P<0.01; Fig. 5).
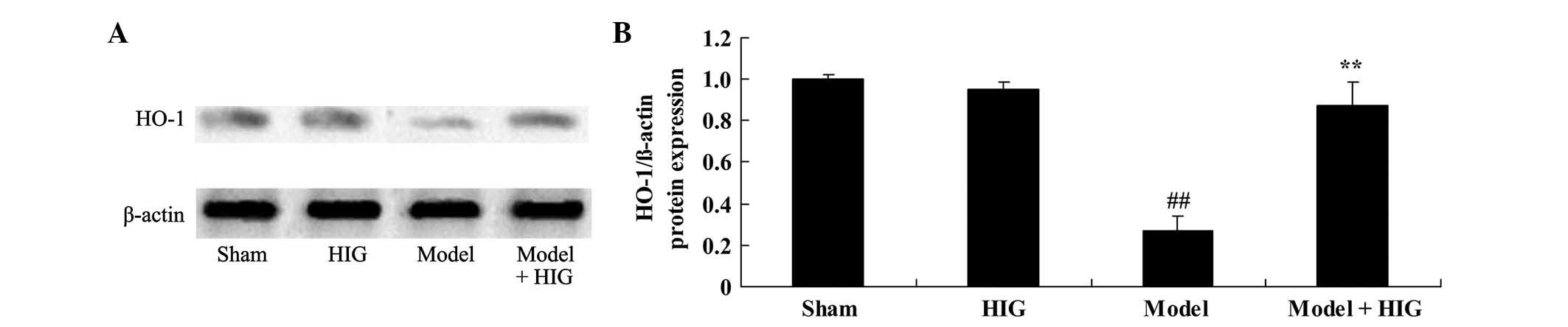
Protective effect of higenamine on
HO-1 protein expression
To investigate the mechanisms underlying the effect
of higenamine on CIA mice, HO-1 protein expression was measured
using western blot analysis. The results demonstrate that HO-1
protein expression in the sham group was similar to that of the
higenamine group (Fig. 6). Fig. 6 clearly demonstrated that HO-1
protein expression in the CIA was significantly lower compared with
that in the sham group (P<0.01). In addition, higenamine
significantly enhanced the suppression of HO-1 protein expression
in CIA mice (P<0.01; Fig. 6).
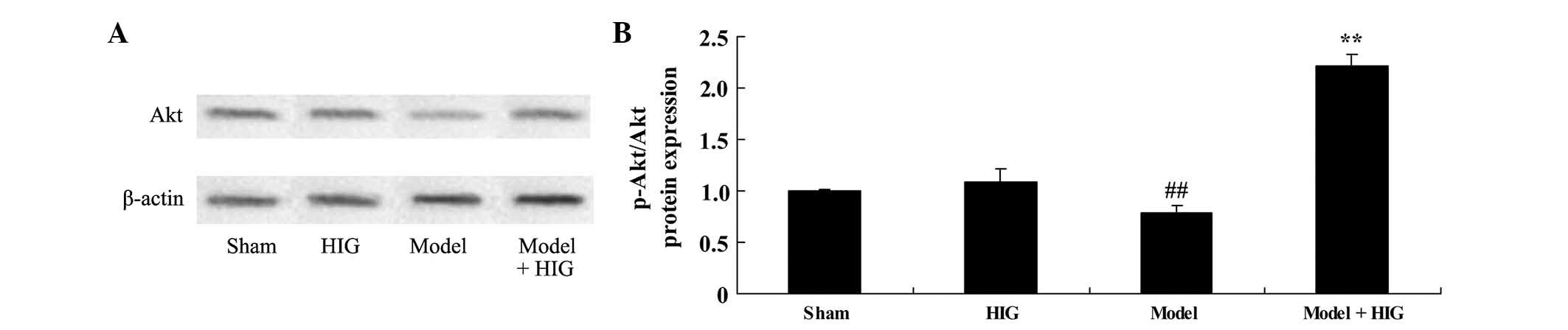
Protective effect of higenamine on Akt
protein expression
To confirm the effect of higenamine on Akt protein
expression, western blot analysis was used to analyze the Akt and
p-Akt protein expression in CIA mice. No significant changes in
p-Akt/Akt protein expression between the sham and higenamine group
were identified (Fig. 7). CIA
significantly suppressed p-Akt/Akt protein expression of CIA mice,
compared with the sham group (P<0.01; Fig. 7). In addition, higenamine
significantly activated the suppression of p-Akt/Akt protein
expression in CIA mice (P<0.01; Fig.
7).
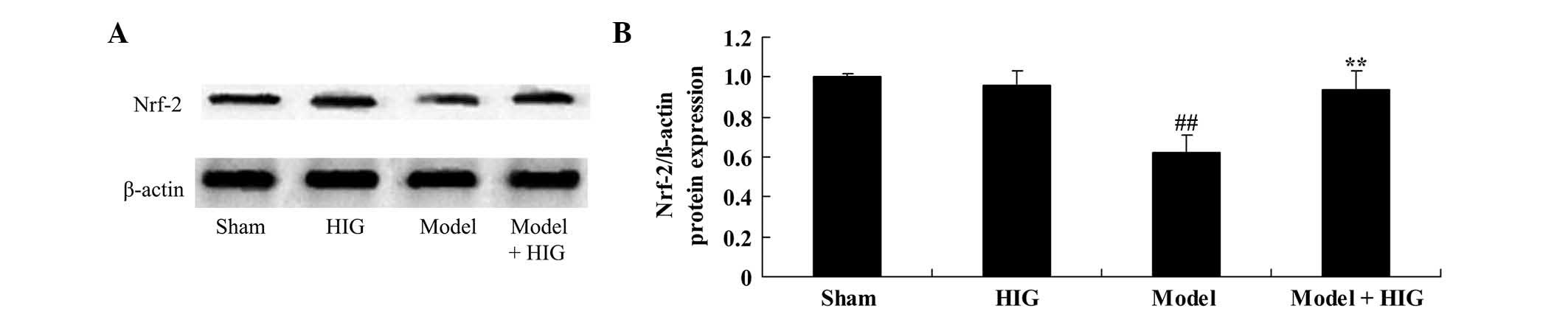
Protective effect of higenamine on
Nrf-2 protein expression
To investigate the mechanisms underlying the effect
of higenamine on CIA mice, Nrf-2 protein expression was observed
using western blot analysis. It was identified that Nrf-2 protein
expression in the sham group was similar to that in the higenamine
group (Fig. 8). CIA mice had
significantly reduced Nrf-2 protein expression compared with that
in the sham group (P<0.01; Fig.
8). As expected, Nrf-2 protein expression was significantly
increased by treatment with higenamine in CIA mice (P<0.05;
Fig. 8).
Discussion
RA is a type of chronic and systemic autoimmune
disease that is characterized by erosive joint synovitis, which
harms body systems and organs, such as joints, the heart and the
kidney (11). RA attacks facet
joints symmetrically, for instance, articulationes digitorum manus,
articulationes digitorum pedis and wrist joints (12). In the advanced stage of RA,
ankylosis, malformation and dysfunction of these facet joints will
arise. In addition, fever, anemia, scleritis and pericarditis are
likely to develop (13). In the
present study, treatment with higenamine effectively inhibited the
promotion of clinical arthritis scores of CIA mice.
According to a previous pathological study,
histopathologic features of RA are pannus formation and destruction
of cartilage and bone, as well as leukopedesis and lymphocyte
infiltration (particularly T-lymphocytes) in affected articular
tissue (14). The high expression
level of IL-1β and TNF-α in the blood of patients with RA suggests
that these two factors are associated with RA. Secreted by synovial
cells, in autocrine and paracrine forms, TNF-α can promote
inflammatory mediator secretion, such as prostaglandin E2 (PGE2),
by which inflammation will be stimulated and the cartilago
articularis will be destroyed (15).
As a result, TNF-α becomes a factor reflecting the activity and
severity of RA. IL-1β, another inflammatory mediator, is secreted
by monocyte-macrophages, and can induce synovial and cartilage
cells to produce collagenase and PGE2, which leads to inflammation
of the synovium and disintegration of the mesochondrium (16). At the same time, PGE2 can stimulate
neutrophil, macrophage and lymphocyte aggregation in the articular
cavity so that the arthritis will be exacerbated, which is a
vicious cycle. The results of the present study demonstrated that
higenamine effectively reduces CIA-induced TNF-α and IL-1β
activities in CIA mice. Ha et al (17) reported that higenamine reduces HMGB1
during hypoxia-induced brain injury by the induction of
inflammation.
MDA is an end product of lipid oxidation, the
expression level of which can reflect the severity of oxidative
damage. According to clinical research, the peroxide levels of
patients with RA is raised and their antioxidant capacity is
reduced, suggesting that there is a higher MDA expression level in
patients with RA compared with healthy individuals. GSH can protect
cells from oxidative damage, so the expression level of GSH can
reflect the state of RA. Individuals with lower activity levels of
GSH have a lower antioxidative ability. The present study
demonstrated that pretreatment with higenamine significantly
recovered abnormal CIA-induced MDA and GSH activities in CIA mice.
Lee et al (18) concluded
that higenamine reduces apoptotic cell death through suppression of
oxidation in rat myocardial ischemia-reperfusion injury.
Different from necrosis, apoptosis is an active
process which is concerned with gene activation, expression and
control. Mitochondria are stimulated by numerous signals and when
their functions are depressed they eventually release apoptotic
factors. Caspase is activated by the mitochondria's morphologic and
functional change, which results in the release of cytochrome
c. The present data clearly demonstrate that higenamine
significantly weakens CIA-induced caspase-3/9 activities of CIA
mice. In addition, Lee et al (18) concluded that higenamine reduces
apoptotic cell death through suppression of oxidation and caspase-3
activity in rat myocardial ischemia-reperfusion injury.
As one of the rate-limiting enzymes of the heme
oxygenase, HO-1 expression will be increased in stress, which is
associated with the combination of NF-κB and genomic accessible
sites of HO-1 (19). HO-1 is
involved in anti-inflammatory, antioxidant and anti-apoptosis
pathways (20). Research aimed at
evaluating the possibility of using HO-1 to diagnose RA has
demonstrated that HO-1, which is potentially associated with RA, is
found in the synovial tissues of patients with RA (20). This suggests that higenamine
significantly enhanced the suppression of HO-1 protein expression
of CIA mice. Zhang et al (21) reported that higenamine promotes M2
macrophage activation via induction of HO-1 in spinal cord
injury.
As one of the important intracellular transduction
signal pathways, the PI3K/Akt signaling pathway regulates cell
division, differentiation, apoptosis and other cell activities
(22). The loss and activation
failure of Nrf-2 can result in the increase of sensibility to
stressors, the extension of inflammation repair time and the
promotion of cancer and cell apoptosis (23). HePG2 cells are stimulated by
capsaicin to increase the expression of HO-1, and this is achieved
by increasing the nuclear translocation of Nrf-2 to activate the
PI3K/Akt signaling pathway (24).
This regulation of activation serves an important role in the
induction and maintenance of the expression of metabolizing and
antioxidant enzymes. In addition, this regulation can protect cells
from oxidative injury (25). In the
present study, it was demonstrated that higenamine activates the
suppression of p-Akt/Akt and Nrf-2 protein expression in CIA mice.
In addition, Ha et al (17)
demonstrated that higenamine reduces HMGB1 via the PI3K/Akt/Nrf-2
signaling pathway in rats with hypoxia-induced brain injury.
In conclusion, the current study demonstrates that
the protective effect of higenamine ameliorates collagen-induced
arthritis through the induction of HO-1 and PI3K/Akt/Nrf-2
signaling pathways. Thus, higenamine may be an important novel
therapeutic agent for the treatment of CIA.
Acknowledgements
This work was supported by Nanjing city science and
technology project (210303089).
References
|
1
|
Bae J and Park JW: Topical delivery of
leflunomide for rheumatoid arthritis treatment: Evaluation of local
tissue deposition of teriflunomide and its anti-inflammatory
effects in an arthritis rat model. Drug Dev Ind Pharm. 27:1–9.
2015.(Epub ahead of print).
|
|
2
|
Smolen JS, Weinblatt ME, van der Heijde D,
Rigby WF, van Vollenhoven R, Bingham CO III, Veenhuizen M, Gill A,
Zhao F, Komocsar WJ, et al: Efficacy and safety of tabalumab, an
anti-B-cell-activating factor monoclonal antibody, in patients with
rheumatoid arthritis who had an inadequate response to methotrexate
therapy: results from a phase III multicentre, randomised,
double-blind study. Ann Rheum Dis. 74:1567–1570. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Westhovens R, Robles M, Ximenes AC,
Wollenhaupt J, Durez P, Gomez-Reino J, Grassi W, Haraoui B, Shergy
W, Park SH, et al: Maintenance of remission following 2 years of
standard treatment then dose reduction with abatacept in patients
with early rheumatoid arthritis and poor prognosis. Ann Rheum Dis.
74:564–568. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chung KC, Kotsis SV, Fox DA, Regan M,
Burke FD, Wilgis EF and Kim HM: Differences between the United
States and the United Kingdom in the treatment of rheumatoid
arthritis: Analyses from a hand arthroplasty trial. Clin Rheumatol.
29:363–367. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kavanaugh A, St Clair EW, McCune WJ,
Braakman T and Lipsky P: Chimeric anti-tumor necrosis factor-alpha
monoclonal antibody treatment of patients with rheumatoid arthritis
receiving methotrexate therapy. J Rheumatol. 27:841–850.
2000.PubMed/NCBI
|
|
6
|
Richter J, Capková K, Hříbalová V,
Vannucci L, Danyi I, Malý M and Fišerová A: Collagen-induced
arthritis: Severity and immune response attenuation using
multivalent N-acetyl glucosamine. Clin Exp Immunol. 177:121–133.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kamada H, Goto M, Matsuura S, Takaoka Y
and Nagai H: Immunopharmacological studies on collagen-induced
arthritis in dark Agouti (DA) rats. Jpn J Pharmacol. 74:313–322.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Pimentel TA, Sampaio AL, D'Acquisto F,
Perretti M and Oliani SM: An essential role for mast cells as
modulators of neutrophils influx in collagen-induced arthritis in
the mouse. Lab Invest. 91:33–42. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bonamore A, Barba M, Botta B, Boffi A and
Macone A: Norcoclaurine synthase: Mechanism of an enantioselective
pictet-spengler catalyzing enzyme. Molecules. 15:2070–2078. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Feng S, Jiang J, Hu P, Zhang JY, Liu T,
Zhao Q and Li BL: A phase I study on pharmacokinetics and
pharmacodynamics of higenamine in healthy Chinese subjects. Acta
Pharmacol Sin. 33:1353–1358. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang Z, Chen Z, Yang S, Wang Y, Yu L,
Zhang B, Rao Z, Gao J and Tu S: (1)H NMR-based metabolomic analysis
for identifying serum biomarkers to evaluate methotrexate treatment
in patients with early rheumatoid arthritis. Exp Ther Med.
4:165–171. 2012.PubMed/NCBI
|
|
12
|
Luz KR, Furtado RN, Nunes CC, Rosenfeld A,
Fernandes AR and Natour J: Ultrasound-guided intra-articular
injections in the wrist in patients with rheumatoid arthritis: A
double-blind, randomised controlled study. Ann Rheum Dis.
67:1198–1200. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Nishiya K, Hisakawa N, Tahara K, Matsumori
A, Ito H, Hashimoto K, Nakatani K and Takatori K: Additive triple
DMARD combination therapy of a low dose of sulfhydryl compounds,
sulfasalazine and methotrexate in the treatment of rheumatoid
arthritis: A clinical trial. Acta Med Okayama. 53:275–279.
1999.PubMed/NCBI
|
|
14
|
Wislowska M and Jakubicz D: Preliminary
evaluation in rheumatoid arthritis activity in patients treated
with TNF-alpha blocker plus methotrexate versus methotrexate or
leflunomide alone. Rheumatol Int. 27:641–647. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Huang C, Wan L and Liu J: Effect of
Xinfeng capsule on nuclear factor Kappa B/tumor necrosis factor
alpha and transforming growth factor beta 1/Smads pathways in rats
with cardiac injuries induced by adjuvant arthritis. J Tradit Chin
Med. 36:92–100. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ma Z, Piao T, Wang Y and Liu J: Astragalin
inhibits IL-1beta-induced inflammatory mediators production in
human osteoarthritis chondrocyte by inhibiting NF-kappaB and MAPK
activation. Int Immunopharmacol. 25:83–87. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ha YM, Kim MY, Park MK, Lee YS, Kim YM,
Kim HJ, Lee JH and Chang KC: Higenamine reduces HMGB1 during
hypoxia-induced brain injury by induction of heme oxygenase-1
through PI3K/Akt/Nrf-2 signal pathways. Apoptosis. 17:463–474.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lee YS, Kang YJ, Kim HJ, Park MK, Seo HG,
Lee JH, Yun-Choi HS and Chang KC: Higenamine reduces apoptotic cell
death by induction of heme oxygenase-1 in rat myocardial
ischemia-reperfusion injury. Apoptosis. 11:1091–1100. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chi PL, Liu CJ, Lee IT, Chen YW, Hsiao LD
and Yang CM: HO-1 induction by CO-RM2 attenuates TNF-α-induced
cytosolic phospholipase A2 expression via inhibition of
PKCα-dependent NADPH oxidase/ROS and NF-κB. Mediators Inflamm.
2014:2791712014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kirino Y, Takeno M, Murakami S, Kobayashi
M, Kobayashi H, Miura K, Ideguchi H, Ohno S, Ueda A and Ishigatsubo
Y: Tumor necrosis factor alpha acceleration of inflammatory
responses by down-regulating heme oxygenase 1 in human peripheral
monocytes. Arthritis Rheum. 56:464–475. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang Z, Li M, Wang Y, Wu J and Li J:
Higenamine promotes M2 macrophage activation and reduces Hmgb1
production through HO-1 induction in a murine model of spinal cord
injury. Int Immunopharmacol. 23:681–687. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Torres-Arzayus MI, de Mora J Font, Yuan J,
Vazquez F, Bronson R, Rue M, Sellers WR and Brown M: High tumor
incidence and activation of the PI3K/AKT pathway in transgenic mice
define AIB1 as an oncogene. Cancer Cell. 6:263–274. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Pareek TK, Belkadi A, Kesavapany S,
Zaremba A, Loh SL, Bai L, Cohen ML, Meyer C, Liby KT, Miller RH, et
al: Triterpenoid modulation of IL-17 and Nrf-2 expression
ameliorates neuroinflammation and promotes remyelination in
autoimmune encephalomyelitis. Sci Rep. 1:2012011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ha do T, Oh J, Khoi NM, Dao TT, Dung V, Do
TN, Lee SM, Jang TS, Jeong GS and Na M: In vitro and in vivo
hepatoprotective effect of ganodermanontriol against t-BHP-induced
oxidative stress. J Ethnopharmacol. 150:875–885. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ugur M, Yildirim K, Kiziltunc A, Erdal A,
Karatay S and Senel K: Correlation between soluble intercellular
adhesion molecule 1 level and extracellular superoxide dismutase
activity in rheumatoid arthritis: A possible association with
disease activity. Scand J Rheumatol. 33:239–243. 2004. View Article : Google Scholar : PubMed/NCBI
|