Introduction
The genus Rhodiola consists of numerous
species. The best known is R. rosea (1–3). Less
reported in Europe are other members of this genus, R.
quadrifida and R. kirilowii. Plants of Rhodiola
genus, a member of the Crassulaceae family, are
traditionally used in Asiatic medicine for their adaptogenic and
anti-inflammatory properties, and have been recognized as
immunomodulators in the past few decades (4,5). In
addition, these plants exhibit inhibitory effects against a number
of pathogens without the involvement of an immune system. It has
been reported that extracts from R. rosea inhibit
Clostridium perfringens and influenza virus neuraminidases
in vitro (6). Moreover, R.
rosea extract inhibits the replication of dengue virus,
vesicular stomatitis virus and coxsackievirus B3 (7–9). Plants
from Rhodiola genus are useful in bacterial infections,
particularly those displaying antibiotic resistance. Cybulska et
al (10) demonstrated that
addition of R. rosea extract to Neisseria ghonorrhea
culture inhibits its growth in vitro. Based upon these
findings, it is possible that Rhodiola-based therapeutic
agents may be useful adjuvants or alternatives to antibiotics in
the treatment of viral/bacterial infections during pregnancy.
Previous studies have investigated the immunotropic
activity of alcoholic and aqueous extracts of roots and rhizomes of
Rhodiola plants in mice, rats and pigs. During the initial
investigation (11), not a large
quantity of information was available about the immunotropic
activity of Rhodiolas. Further studies showed that the
majority of Rhodiola extracts stimulated immunity (12,13). The
present study investigates the effects of aqueous Rhodiola
kirilowii (RKW) or 50% hydro-alcoholic (RKW-A) extracts,
administered to pregnant and lactating mice, on the immune system
of the resulting six-week old progeny. This experimental model is
used by the present study to evaluate whether the use of R.
kirilowii extract as an immunostimulant in pregnancy is safe
for the developing immune system of the progeny (14).
Materials and methods
Plant cultivation
R. kirilowii roots and rhizomes were
cultivated, identified and collected in the Research Institute of
Medicinal Plants, now the Institute of Natural Fibers and Medicinal
Plants (Poznań, Poland). The voucher specimen was kept in the
herbarium of Department of Botany, Breeding and Agriculture
(Plewiska, Poland).
Preparation and chemical analysis of
extracts
Extracts were prepared as previously described
(12). Briefly, to produce RKW
extract, finely powdered R. kirilowii roots were extracted
twice with water (first for 2 h and then for 1 h) in a raw
material: Solvent ratio of 1:5, at between 40 and 45°C by the water
extraction method. Resulting supernatants were mixed together,
centrifuged (15 min, room temperature, 2000 × g) and
lyophilized. To produce RKW-A extract: Finely powdered R.
kirilowii roots were extracted with a 1:1 ethanol: Water
solution, in a raw material: Solvent ratio of 1:10 using the
percolation method. Then, percolates were lyophilized following
distillation at between 40 and 45°C. Dry extract ratio were 5.09:1
for RKW and 3.27:1 for RKW-A. Extracts were stored at −70°C until
required.
Chemical analysis of extracts
Chemical analysis of extracts was performed as
previously described (15),
according to the methods proposed by Hertog et al (16). Briefly, the polyphenol concentration
of the extracts was assayed using high-performance liquid
chromatography (HPLC; Dionex system, pump P680, autosampler ASI100;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) equipped with a
CoulArray Coloumetric Array Electrochemical Detector (ECD; Thermo
Fisher Scientific, Inc.).
Experimental animals
Experiments were performed on 202 6-week old progeny
(80 from control, 59 from RKW and 63 from RKW-A mothers) of 70
adult inbred female BALB/c mice (8–9 weeks old; ~20 g; Mossakowski
Medical Research Centre Polish Academy of Sciences, Warsaw,
Poland), which were mated with adult males of the same strain.
Between the appearance of a copulatory plug and 28 days following
delivery, females were fed daily with lyophilized RKW (n=20) or
RKW-A (n=19) extract. The extracts were given at a dosage of 20
mg/kg (7 mg/m2), to mice weighing ~20 g with a body
surface area of 0.007 m2, which corresponds to a dose of
100 mg (1.6 mg/kg) given to a person weighing 60 kg with a body
surface area of 1.6 m2 (1.6 mg/kg) (17). The control group (n=31) received
distilled water instead of extract. The substances given were
applied on a corn crisp and served to the mice in a Petri dish.
Experimental animals were handled in accordance with
the Polish regulations concerning the wellness of laboratory
animals (Polish National Institute of Health, Warsaw, Poland). All
experiments were accepted by and conducted in accordance with the
ethical guidelines of the Local Ethics Committee (IV) on Animal
Testing, National Medicines Institute, Warsaw, Poland (permission
no. 73/2011). Mice were maintained under typical conditions
(22.5–23.0°C, relative humidity 50–70%, 12 h day/night cycle) with
ad libitum access to breeding rodent feed (Labofeed H;
Factory of Fodder, Kcynia, Poland) and water. Female and male
progeny were housed separately. Pups were withdrawn from mothers 24
days following delivery.
Sera and spleen isolation
Mice were retro-orbitally bled under anesthesia
[intraperitoneal injection of ketamine (120 mg/kg) and xylazine (12
mg/kg); Polypharm S.A., Warsaw, Poland]. Serum was separated by
clotting for 1 h at room temperature, followed by centrifugation at
2000 × g for 20 min at 4°C and then stored at −70°C until
required. Following bleeding, mice were sacrificed by anesthetic
overdose (pentobarbital, 400 mg/kg; Polypharm S.A, Warsaw, Poland)
and spleens were isolated under aseptic conditions (laminar flow
cabinet), for immediate use.
Morphometric evaluation of
spleens
Histological evaluation and quantitative microscopic
analysis of lymphatic nodules was performed on hematoxylin and
eosin stained paraffin sections of the spleens obtained from the
progeny (n=10 from each group). The histotechnical criteria applied
for quantitative analysis were as follows: i) A thickness of
between 3 and 5 µm; ii) complete transversal section of the spleen
including white and red pulp structures; and iii) no evidence of
traumatic artifacts within the sample, such as fragmentation or
hemorrhage. The diameter of splenic nodules and the number of
lymphatic nodules per microscopic field were measured. The surface
of the section of spleen was then analyzed in regards to the
following: i) The total area and number of white pulp lymphatic
nodules, with the results expressed as the number per microscopic
field (5.5 mm2); and ii) lymphatic nodule diameters
measured in consecutive nodules. Images were acquired and processed
using ToupView software (version 3.7; ToupTek Photonics Co., Ltd.,
Hangzhou, China).
Light microscopy examination, using an Evolution 100
Trino optical microscope (Delta Optical; Mińsk Mazowiecki, Poland)
connected to a photometric color CCD camera (UCMOS05100KPA;
Hangzhou ToupTek Photonics Co., Ltd.), of the red and white pulp
(sites of hematopoiesis) was performed. In the red pulp attention
was paid to splenic cords and venous sinuses. In the white pulp,
the follicles, germinal centers, periarteriolar lymphoid sheath
(PALS) and marginal zone were assessed. Fixed spleen preparations
were examined using a panchromatic lens (numerical aperture, 0.25)
at ×100 magnification.
Preparation of splenocyte
suspension
Spleens were gently pressed through a sterile nylon
strainer (40 µm) into a 50 ml Flacon tube with 20 ml of culture
medium [Roswell Park Memorial Institute (RPMI)-1640 medium with
GlutaMAX; Thermo Fisher Scientific, Inc., Warsaw, Poland],
supplemented with 10% fetal bovine serum (FBS; Thermo Fisher
Scientific, Inc., Warsaw, Poland) and antibiotics (50 IU/ml
penicillin; 50 µg/ml streptomycin; Thermo Fisher Scientific, Inc.).
Strainers were rinsed twice with media to remove remaining cells.
Then, cells were centrifuged (500 × g, 5 min, room
temperature), the resulting pellet resuspended in media and cells
counted in a hematological analyzer (Exigo veterinary hematological
system; Boule Medical AB, Stockholm, Sweden). A cell suspension of
1×106 cells/ml was used to evaluate response to
mitogens. Splenocyte viability was determined using the trypan blue
exclusion test and amounted to >95% cell viability. Following
preparation, cells were immediately used.
Phenotypic determination of
splenocytes
Spleen cell suspensions (1×106 cell/ml)
were washed twice with phosphate buffered saline (PBS) and
centrifuged (500 × g, 5 min, room temperature). Cell pellets
were resuspended in 1 ml of PBS and 100 µl of the suspension was
labeled by cell-surface marker staining with the following
fluorochrome-coniugated anti-mouse monoclonal antibodies: Mouse T
lymphocyte Subset Antibody Cocktail with Isotype Control [hamster
anti-mouse phycoerythrin (PE)-Cyanine 7 cluster of differentiation
(CD) 3e, rat anti-mouse PE CD4 and rat anti-mouse allophycocyanin
(APC) CD8a; cat. no 558431; ready-to-use], Mouse B Lymphocyte
Activation Antibody Cocktail with Isotype Control (rat anti-mouse
PE-Cy7 CD25, hamster anti-mouse PE CD69 and rat anti-mouse APC
CD19; BD Biosciences, Warsaw, Poland; cat. no. 558063;
ready-to-use) and PE rat anti-mouse CD335 (natural killer cell
p46-related protein; cat. no. 560757) (all BD Biosciences, Warsaw,
Poland), according to the manufacturer's instructions (20 min
incubation at room temperature). Red blood cells from splenocyte
suspensions were lysed (10 min, Lysing Solution 10X Concentrate; BD
Biosciences). Phenotypic analysis was then performed using flow
cytometry (FACSCalibur; BD Biosciences). Results of this analysis
are presented as the mean % of splenocytes of a particular
phenotype ± the standard error of the mean.
Response of splenocytes to
mitogens
Response to mitogens was measured using two tests;
the alamarBlue assay and [3H] thymidine incorporation
assay. For the alamarBlue assay, splenocytes were seeded into
96-well plates (1×105 cell/well), incubated for 1 h
under standard condition (5% CO2, 95% humidity, 37°C)
and then a mitogen was added to each well: Lipopolysaccharide (LPS;
20 µg/ml), Concanavalin A (ConA; 5 µg/ml) or phytohaemagglutinin
(PHA; 2 µg/ml) (all purchased from Sigma-Aldrich, Poznań, Poland).
Following 24 h of incubation, alamarBlue (1:10, v/v; Thermo Fisher
Scientific, Inc., Warsaw, Poland) was added to the wells. Cells
were incubated for 24 h at standard conditions (37°C, 5%
CO2, 95% relative humidity). Then, alamarBlue
fluorescence (excitation 544 nm, emission 590 nm) of the wells was
measured using a FLUOstar Omega Microplate Reader (BMG Labtech
GmbH, Ortenberg, Germany) as previously described (18). For the [3H] thymidine
incorporation assay (19), cells
were cultured and treated with mitogens as described above. Then,
the cultures were incubated for 48 h prior to being pulsed with
thymidine (3HTdR, 2 Ci/mM, 0,4 µC/20 µl/culture) and
cultured for a further 24 h. Following culture, cells were
transferred onto Whatman filter paper discs (Labo Plus, Warsaw
Poland), extracted with 30% trichloroacetic acid, dehydrated using
alcohol and ether, and transferred to glass scintillation vessels
filled with liquid scintillation mixture (cat no: 327123;
Sigma-Aldrich; Thermo Fisher Scientific, Inc.). The measurements
were taken using a Packard Tri-Carb 2100TR scintillation counter
(PerkinElmer, Inc., Waltham, MA, USA). Tests were performed in
triplicate and an unstimulated control was included.
Cytokine determination
Flow cytometry determination of the concentration of
selected cytokines [interleukin (IL)-2, −4, −6, −10 and −17A, tumor
necrosis factor-α (TNF-α) and interferon-γ (IFN-γ)] in the sera was
evaluated using the Mouse Th1/Th2/Th17 Cytokine Kit (cat. no.
560485, BD Biosciences), according to the manufacturer's
protocol.
Anti-sheep red blood cell (SRBC) CD2
antibody production
Progeny mice (6-weeks old) were immunized with 5%
SRBC (0.2 ml intraperitoneal injection; Graso Biotech, Gdański,
Poland) 7 days prior to being bled under anesthesia from the
retro-orbital plexus. Anti-SRBC antibody levels were evaluated by
performing a hemagglutination assay, as previously described
(20), on a series sera dilutions.
Briefly, following heat inactivation of the sera (56°C, 30 min), 1%
SRBC was added and the mixture was incubated for 60 min at 37°C,
incubated for 18 h at 4°C, and centrifuged (10 min, 150 × g,
4°C) and shaken. The hemagglutination titer was defined, using
light microscopy, as the highest dilution in which ≥3 cell
conglomerates were present in ≥3 consecutive fields at an objective
magnification of ×20. For the purposes of statistical analysis,
results were transformed into logarithm inversions of the
titers.
Statistical analysis
Statistical evaluation of the results obtained, from
the control and experimental groups, was performed using unpaired
t-tests and one- or two-way analysis of the variance, followed by
the Tukey test or Bonferroni correction (in the case of a normal
distribution) or non-parametric Kruskal-Wallis and Mann-Whitney U
tests (in the case of abnormal distribution). Assessment of the
distribution of the data was evaluated using the Shapiro-Wilk test.
GraphPad Prism software was used to carry out these tests (version
5; GraphPad Software, Inc., La Jolla, CA, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
Chemical analysis of extracts
RKW and RKW-A extracts were found to contain
phenyloethanoid salidroside and thyrozol, four phenolic acids
(chlorogenic, ferulic, ellagic and p-coumaric), and flavonoids
[fisetin, naringenin, kaempferol, epicatechin, luteolin, quercetin,
epigallocatechin and (+)-catechin]. HPLC-ECD analysis revealed a
significant difference in the content of biologically active
compounds between RKW and RKW-A extracts (P<0.0001). Typically,
RKW-A extract presented a higher concentration of the identified
compounds than RKW. The total concentration of polyphenols amounted
to 16.16 µg/mg in RKW and 23.75 µg/mg in RKW-A.
Spleen morphology
No macroscopic abnormalities were identified in the
anatomy of spleens from the experimental and control groups.
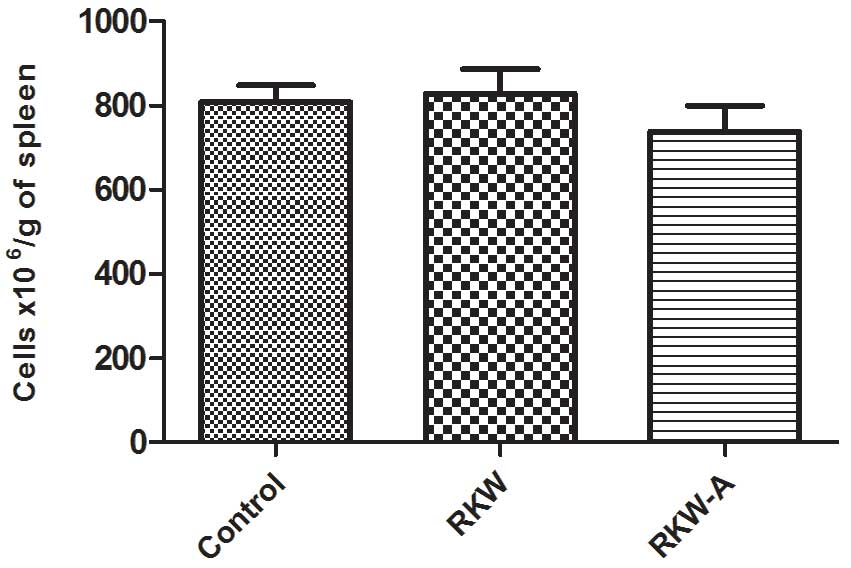
Similarly, no differences in the relative weight, cellularity
(Fig. 1) and morphological picture
of spleens were observed between the groups. Splenic lymphatic
nodules were large, with well-developed germinal centers. In
addition, the PALS and marginal zone were found to be normal in all
groups. The red pulp of the spleen was moderately plethoric.
Morphometric evaluation did not reveal differences between the
experimental and control groups in regards to the number of
lymphatic nodules per microscopic field and their diameter
(Table I).
 | Table I.Morphometric analysis of the spleen
in control and experimental animals. |
Table I.
Morphometric analysis of the spleen
in control and experimental animals.
| Group | Number of images
analyzed | Number of lymphatic
nodules/field ± SEM | Number of lymphatic
nodules analyzed | Mean diameter of
nodule (mm) ± SEM |
|---|
| Control | 34 | 9.23±0.49 | 316 | 0.323±0.0074 |
| RKW | 41 | 10.34±0.52 | 414 | 0.318±0.0059 |
| RKW-A | 32 | 9.62±0.59 | 308 | 0.334±0.0062 |
Phenotype of splenocytes
The percentage of CD4+ cells was highest
in spleens collected from the progeny of mice fed RKW extract
during pregnancy, and amounted to 63% of all CD3+
splenocytes from the group. In comparison, in splenocytes from the
progeny of RKW-A extract-fed mothers and control mothers,
corresponding values were 52 and 55%, respectively. The
CD4+:CD8+ ratio was 2.35±0.2 in the RKW
group, in comparison to 1.88±0.1 in the RKW-A group and 2.41±0.2 in
the control group. The results described are presented in Fig. 2.
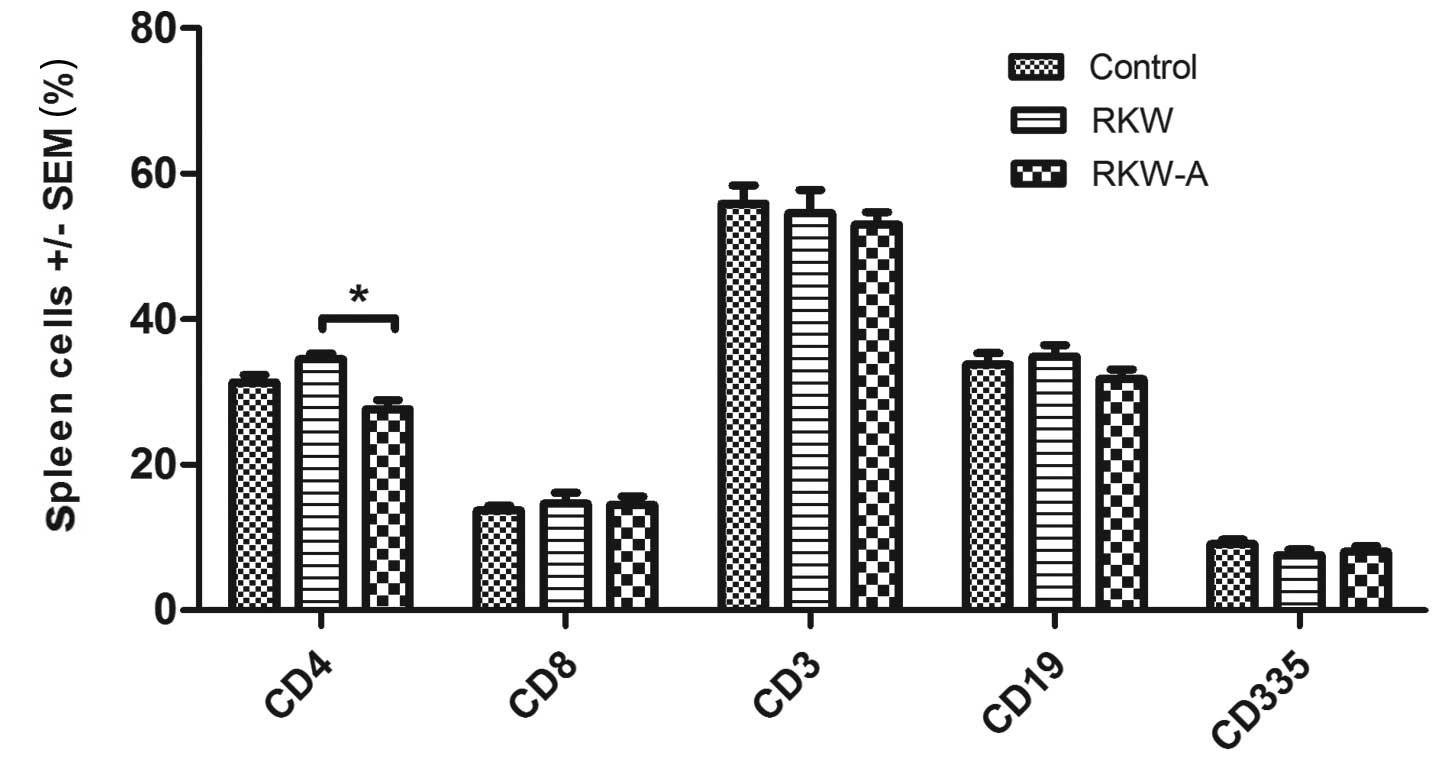 | Figure 2.Phenotypes of spleen lymphocytes.
Phenotypic analysis was performed by flow cytometry [number of
spleens tested, 46 (control, 15; RKW, 13; RKW-A, 18)]. Results are
presented as the mean percentage of spleen cells ± SEM. Statistical
analysis performed: Unpaired t-test, Shapiro-Wilk normality
test, two-way analysis of the variance and Bonferroni correction.
SEM, standard error of the mean; CD, cluster of differentiation;
RKW, Rhodiola kirilowii water extract; RKW-A, Rhodiola
kirilowii hydro-alcoholic extract. *P=0.0004. |
Splenocytic response to mitogens
The alamarBlue assay identified that offspring of
mice fed with RKW extract during pregnancy and lactation showed a
significantly higher level of metabolic activity following addition
of the mitogen PHA compared with the offspring of mice fed with
RKW-A (P=0.0496), which did not affect metabolic activity and
slightly decreased proliferation following PHA stimulation
(Fig. 3A). However, no significant
differences between groups in response to PHA were found by the
[3H] thymidine incorporation assay (Fig. 3B). In cells from ConA-stimulated
spleens the alamarBlue assay found no differences in proliferation
between the study and control groups in ConA stimulated cells
(control, 156±3.9, n=31; RKW, 153±5.2, n=16; RKW-A, 151±4.5, n=12)
(Fig. 4A), while the [3H]
thymidine incorporation assay identified significantly lower
[3H] thymidine incorporation in cells collected from
experimental groups in comparison to the control (RKW, P=0.0306;
RKW-A, P=0.0356; Fig. 4B). In
LPS-stimulated cells the alamarBlue assays found no significant
difference between groups (Fig. 5A),
while the [3H] thymidine incorporation assays found
significantly lower cell proliferation in the RKW-A group compared
with the control (P=0.0175; Fig.
5B).
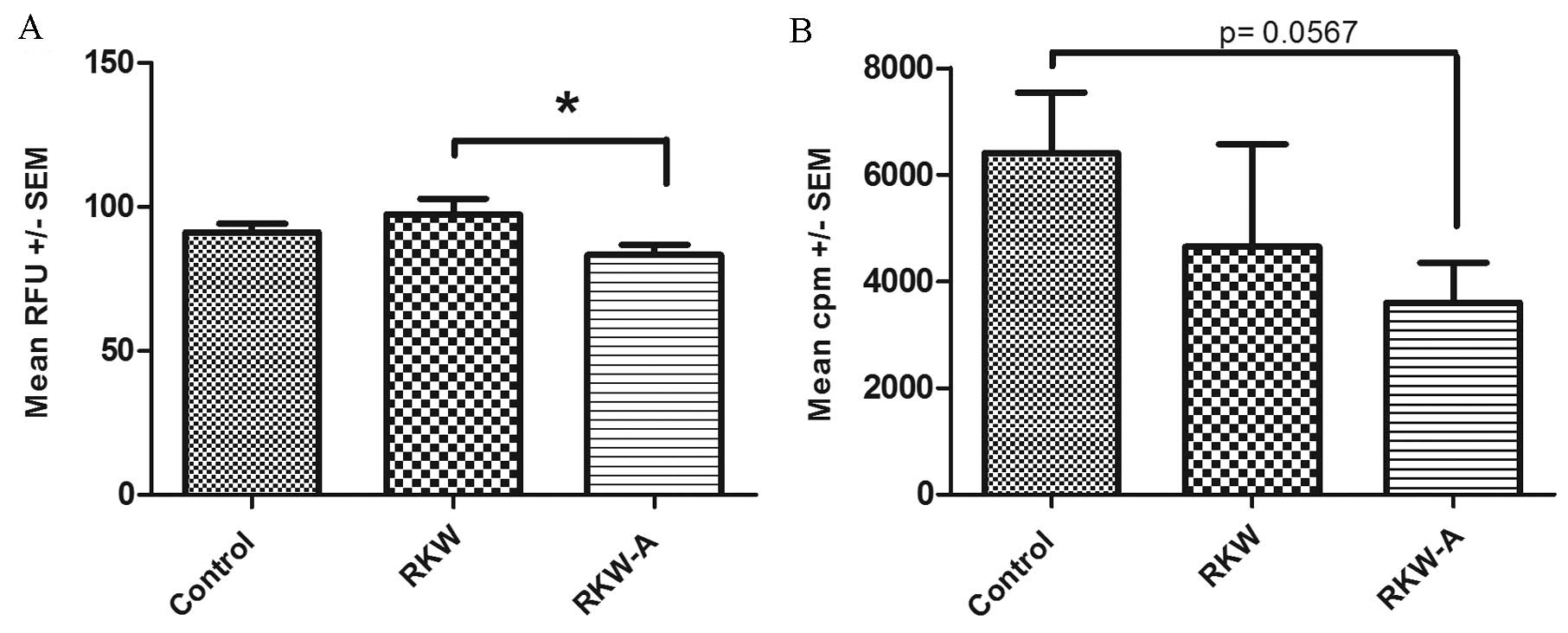 | Figure 3.Proliferation index of splenocytes
following phytohemagglutinin (PHA) stimulation. Results are
presented as the mean RFU or cpm ± SEM, measured by the (A)
alamarBlue assay [number of spleens tested, 59 (control, 31; RKW,
16; RKW-A, 12)] or (B) [3H] thymidine incorporation
assay following PHA (2 µg/ml) stimulation [number of spleens
tested, 27 (control, 9; RKW, 9; RKW-A, 9)]. Statistical analysis
performed: Unpaired t-test, Shapiro-Wilk normality test,
one-way analysis of the variance and the Tukey comparison test.
RFU, relative fluorescence units; SEM, standard error of the mean;
cpm, counts per minute; RKW, Rhodiola kirilowii water
extract; RKW-A, Rhodiola kirilowii hydro-alcoholic extract.
*P=0.0496. |
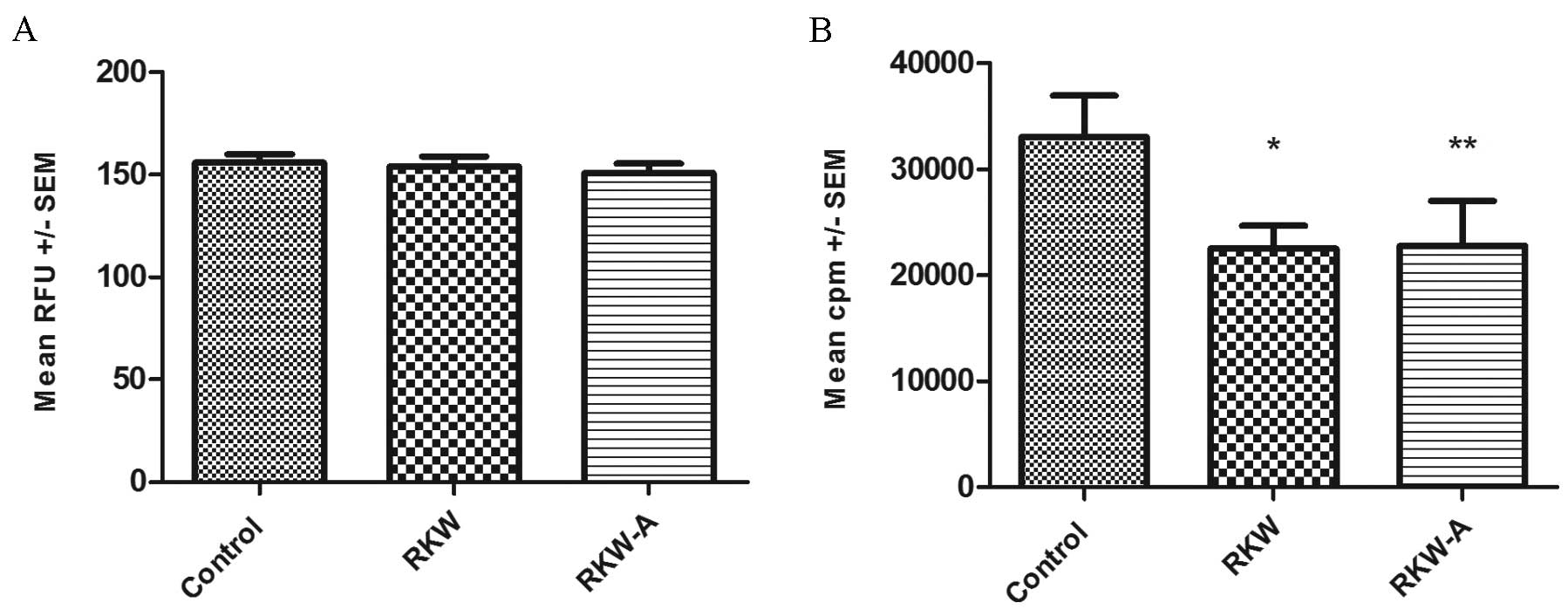 | Figure 4.Proliferation indexes of splenocytes
following Concanavalin A (ConA) stimulation. Results are presented
as the mean RFU or cpm ± SEM, measured by the (A) alamarBlue assay
[number of spleens tested, 59 (control, 31; RKW, 16; RKW-A, 12)] or
(B) [3H] thymidine incorporation assay following ConA (5
µg/ml) stimulation [number of spleens tested, 27 (control, 9; RKW,
9; RKW-A, 9)]. Statistical analysis performed: Unpaired
t-test, Shapiro-Wilk normality test, one-way analysis of the
variance and the Tukey comparison test. RFU, relative fluorescence
units; SEM, standard error of the mean; cpm, counts per minute;
RKW, Rhodiola kirilowii water extract; RKW-A, Rhodiola
kirilowii hydro-alcoholic extract. *P=0.0306 vs. control;
**P=0.0356 vs. control. |
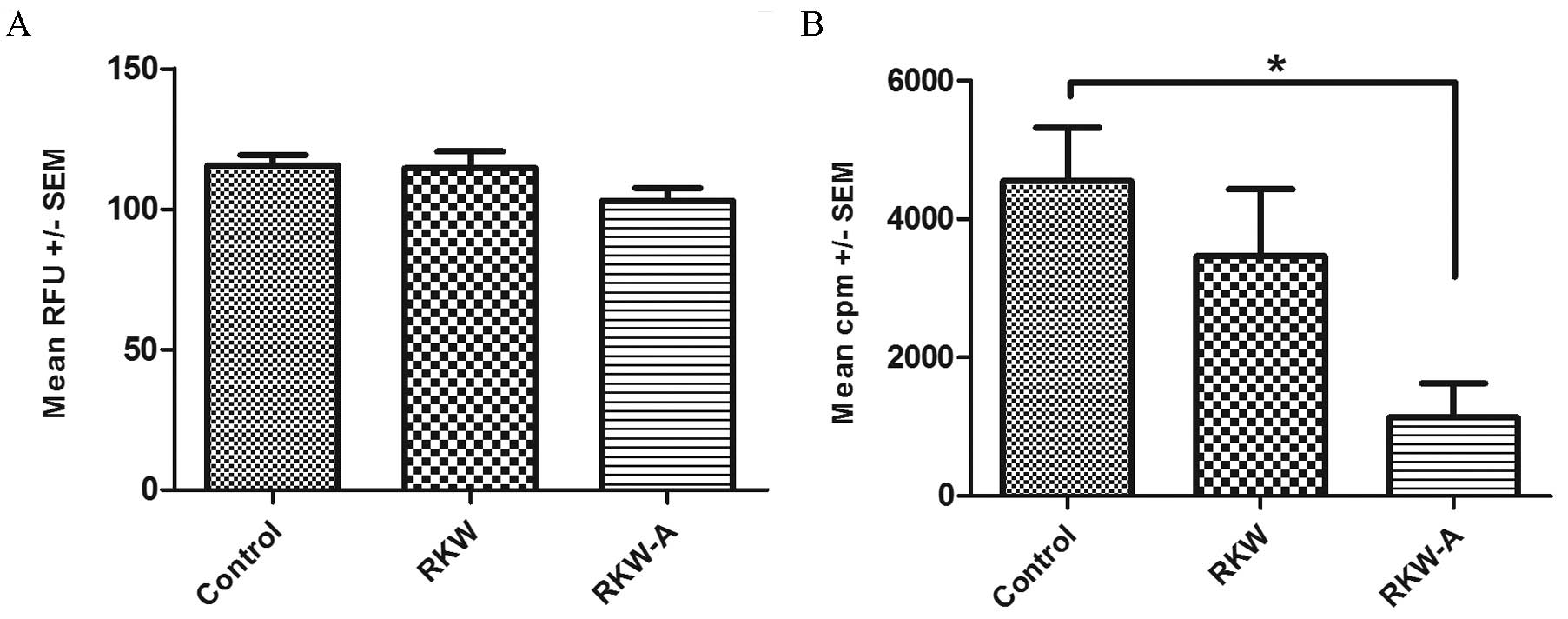 | Figure 5.Proliferation index of splenocytes
following lipopolysaccharide (LPS) stimulation. Results are
presented as the mean RFU or cpm ± SEM, measured by the (A)
alamarBlue assay [number of spleens tested, 59 (control, 31; RKW,
16; RKW-A, 12)] or (B) [3H] thymidine incorporation
assay following LPS (20 µg/ml) stimulation [number of spleens, 27
(control, 9; RKW, 9; RKW-A, 9)]. Statistical analysis performed:
Unpaired t-test, Shapiro-Wilk normality test, one-way
analysis of the variance and the Tukey comparison test. RFU,
relative fluorescence units; SEM, standard error of the mean; cpm,
counts per minute; RKW, Rhodiola kirilowii water extract;
RKW-A, Rhodiola kirilowii hydro-alcoholic
extract.*P=0.0175. |
Cytokine concentrations in mice
sera
The serum concentrations of selected cytokines
(IL-2, −4, −6, −10 and −17a, TNF-α, and IFN-γ) were evaluated by
flow cytometry. No statistically significant differences were found
in the serum concentrations of IL −2, −4 and −6, and IFN-γ between
groups (Fig. 6A-D). The serum
concentration of TNF-α was significantly higher in the RKW-A group
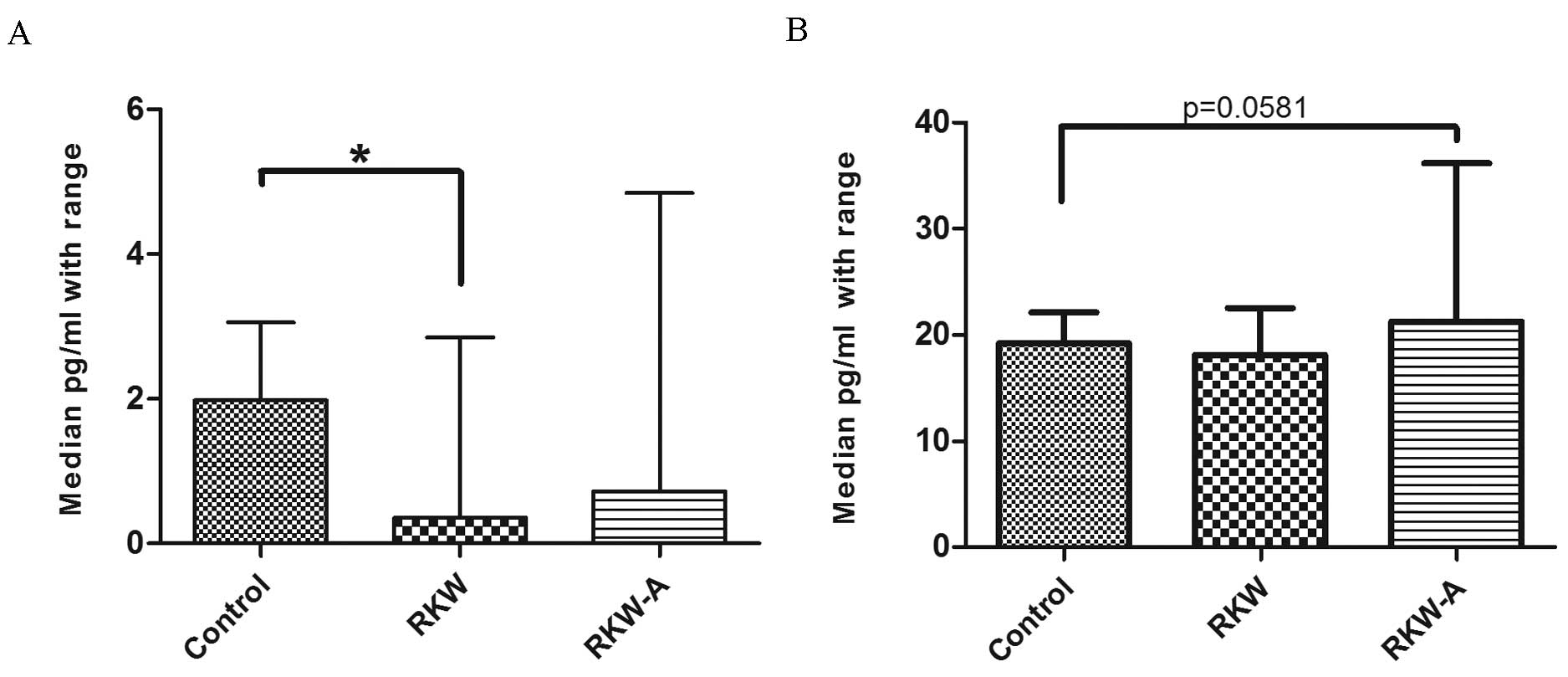
compared with the control (P=0.0123; Fig. 6E). Serum IL-17a concentration was
significantly lower in mice whose mothers were fed during pregnancy
and lactation with RKW extract compared with the control (P=0.0347;
Fig. 7A). However, there were no
differences between mice of RKW-A and control mothers (Fig. 7A). In addition, IL-10 serum
concentration was higher (P=0.0581) in the RKW-A group compared
with the control (Fig. 7B).
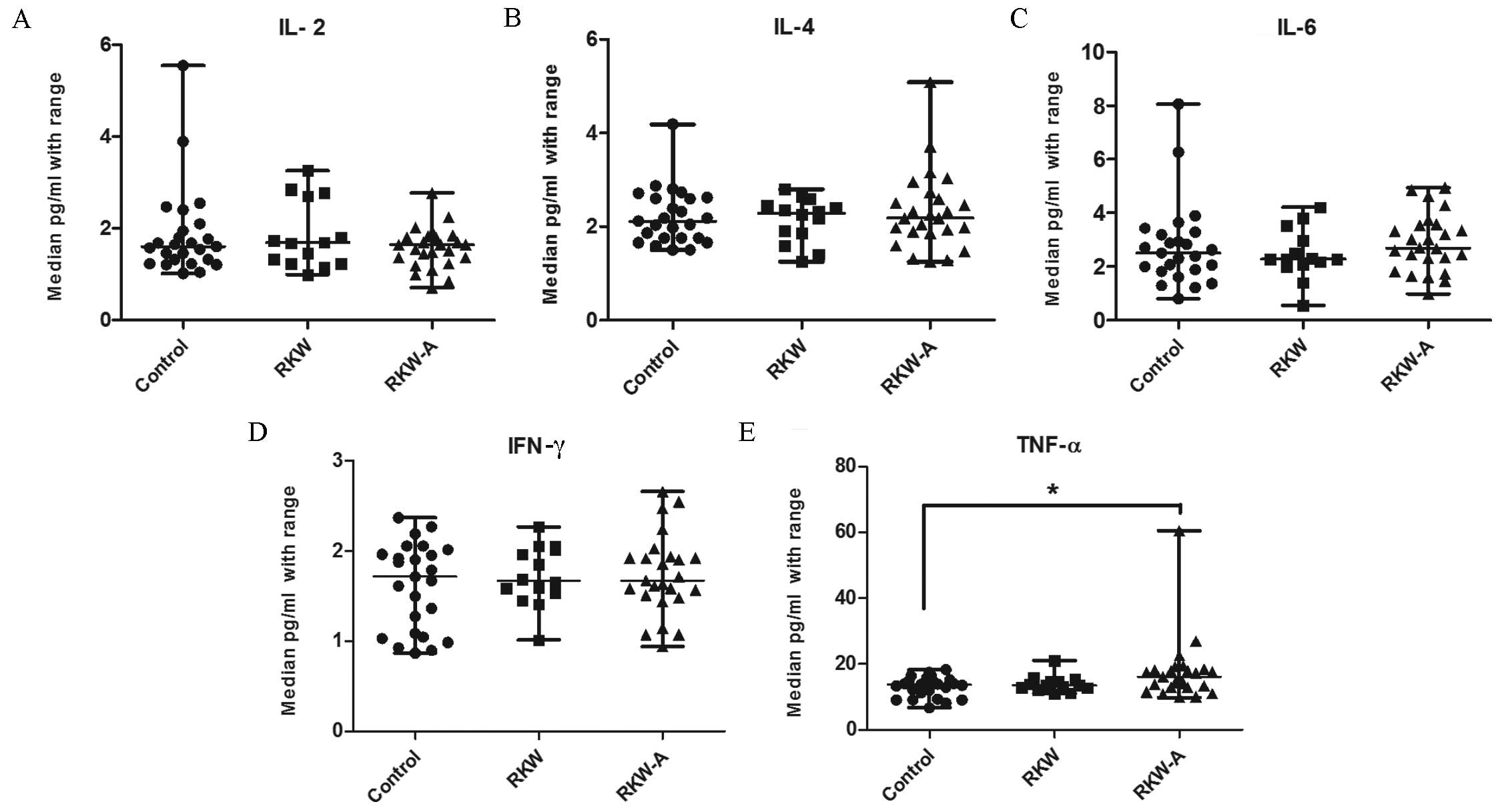 | Figure 6.Concentration of selected cytokines
(A) IL-2, (B) IL-4, (C) IL-6, (D) IFN-γ and (E) TNF-α in the sera.
n=54 mice (control, 20; RKW, 18; RKW-A, 16). Results presented are
the median ± the range of the cytokine in pg/ml. Statistical
analysis performed: Unpaired t-test, Shapiro-Wilk normality
test, Kruskal-Wallis test and Dunn's test. RKW, Rhodiola
kirilowii water extract; RKW-A, Rhodiola kirilowii
hydro-alcoholic extract. *P=0.0123 from Mann Whitney test. |
Anti-SRBC antibody production
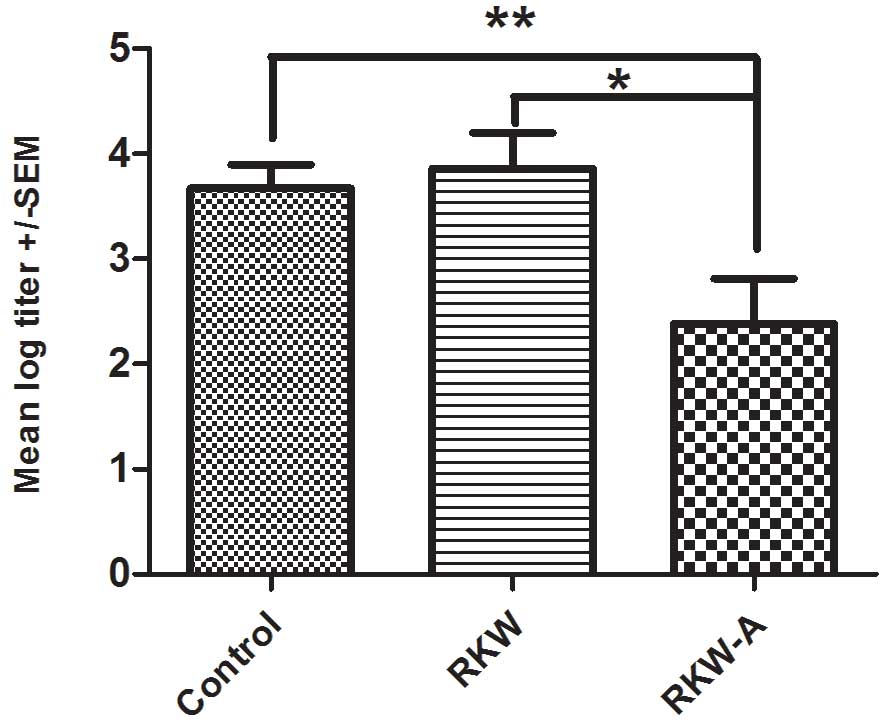
The mean of log titer of anti-SRBC antibody
production was significantly lower in progeny of mice fed RKW-A
extract (P=0.0305) compared with those of mice fed water (Fig. 8). There was no difference between
progeny of mice fed with RKW and those of mice fed water control
(Fig. 8).
Discussion
To best of our knowledge, the present study is the
first to investigate the influence of R. kirilowii extract,
administered to pregnant and lactating mice, on the immune system
of their progeny. The immune system is crucial for the survival of
complex organisms, such as mice and humans, because of its function
in defense against diseases and in regulation of homeostasis. The
primary cells of the immune system are lymphocytes, therefore, the
present study investigated their proliferative activity and
expression of cytokines.
The results of the present study determined that the
spleen lymphocytes of mice whose mothers were fed during pregnancy
and lactation with RKW extract were more able to metabolize
resazurin when stimulated with PHA, compared with splenocytes from
mice whose mothers were fed RKW-A extract. Differences in the
results obtained from the alamarBlue and [3H] thymidine
incorporation assays are a result of the test specificity. Tge
alamarBlue assay measures the metabolic activity of the cells,
whereas [3H] thymidine incorporation is based on de
novo DNA synthesis during proliferation.
Lymphocyte activation is usually typically
associated with the expression of IL-2, −4, −6, −10 and −17, and
TNF-α, (21,22). IL-2 promotes proliferation of and
cytokine production by T-cells, and serves an important role in the
maintenance of the functional properties of B cells (23). IL-4 is a key regulator of the immune
response and promotes the differentiation of naive T cells into T
helper (Th) 2 cells (24). IL-6 has
been shown to influence inflammatory action and the
antigen-specific immune response (25). In addition, IL-6 serves an important
role in cellular defense mechanisms trough regulating hematopoiesis
and the immune response (26).
Primarily produced by Th2 lymphocytes, IL-10 generates and promotes
T cell tolerance, via downregulation of IFN-γ, IL-2 and −5
production, proinflammatory cytokines, and eosinophil function and
activity (27). TNF-α functions in
the initiation of cellular and humoral immune responses (28).
In the present study, a minor increase was observed
in the production of immunoregulatory IL-10 in mice whose mothers
were fed with RKW-A extract, with a simultaneous increase in sera
TNF-α concentration. This may be the reason for the altered spleen
cell metabolism observed earlier, confirming the results of the
alamarBlue and [3H] thymidine incorporation assays.
TNF-α directly promotes the growth and differentiation of
neutrophils, macrophages and B cells.
IFN-γ, synthesized by natural killer cells and T
lymphocytes, serves a role in the immune response against
pathogens. IFN-γ activates macrophages and promotes differentiation
of CD4+ T lymphocytes into Th1 cells. However, in the
present study this was not the case, because there were no
differences in sera IFN-γ concentrations between the progeny of
mice fed Rhodiola extracts and the progeny of mice fed water
(control).
Th17 cells primarily produce IL-17a, which is
important in inducing and mediating the proinflammatory responses.
In addition, IL-17a induces and promotes the production of the
cytokines IL-6, TNF-α, granulocyte-colony stimulating factor,
granulocyte-macrophage colony-stimulating factor, IL-1β, TGF-β
cytokines and the chemokines IL-8, growth related oncogene-a and
monocyte chemoattractant protein-1 (29,30). A
previous study found that IL-17a can down-regulate Th1
differentiation (31).
The present study identified that RKW and RKW-A
extracts did not change progeny sera expression of IFN-γ, IL-2, −4
or −6 compared with the control. However, TNF-α and IL-10
expression were increased in the progeny of mice fed with RKW-A
extract. In addition, mice whose mothers were fed with RKW-A had
lower antibody titers following immunization with SRBC, which may
be connected with the increased serum concentration of IL-10 and
decreased splenocyte response to LPS (a B cell mitogen) identified
in the current study.
Interestingly, a significantly lower concentration
of IL-17a in the sera and a significantly higher metabolic response
to PHA was observed in the splenocytes of mice born to RKW-fed
mothers, compared with mice born to mothers fed with RKW-A. This is
consistent with the results of a previous study in a mouse model of
acute graft-vs.-host disease, in which the authors determined that
the absence of Th17 cells lead to augmented Th1 differentiation
(31). These results suggest that
PHA-responsive spleen cells are T helper lymphocytes, members of
the Th1 population.
A previous study (14), in addition to the present study,
observed a higher number of pups in the litters delivered by
mothers that were fed RKW-A extracts. This is in agreement with a
number of pre-clinical and clinical observations on the beneficial
reproductive effects of other Rhodiola species, such as
R. rosea (32,33).
Antibody production by B-cells is the primary
component of the adaptive immunity response. Disorders in antibody
production impair the ability of an organism to defend against
microbial infections. In the present study, administration of RKW-A
extract to mothers significantly decreased SRBC antibody production
in comparison with RKW extract and the control (water). A previous
study found water and hydro-alcoholic extracts of R.
quadrifida had no effect on anti- SRBC antibody production
(34). In contrast, Mishra et
al reported that R. imbricata aqueous extract
significantly enhanced tetanus toxoid-specific immunoglobulin
levels and ovalbumin-induced antibody responses in a rat model
(35). Differences in antibody
production following supplementation of water or hydro-alcoholic
extracts may be the result of the various Rhodiola species
used in studies.
A recent study, performed in the same model, found
that thymuses obtained from the progeny of mice treated with both
types of R. kirilowii extract showed significantly lower
total apoptotic cell counts compared with the progeny of control
mice, and that such treatment of mothers had no significant
influence upon IL-7 expression of progeny thymocytes (36). The study concluded that R.
kirilowii extracts may help to preserve thymus function in the
progeny of treated animals.
The question of what causes the differences in the
observed effects of RKW and RKW-A extracts arose from the results
of the present study. In the present study, analysis of the content
of selected polyphenolic compounds in RKW and RKW-A showed
quantitative differences only, for which there are two possible
explanations. Firstly, RKW-A extract may contain substances other
than the analyzed polyphenolic compounds, which in RKW are absent
or present in very low concentrations. Secondly, the different
influences of RKW and RKW-A extracts on developing fetuses may be a
result of having different concentrations of the analyzed
polyphenols. Thirdly, there may differences in the bioavailability
and biodistribution of selected polyphenols in serum and milk of
mice-mothers.
In conclusion, progeny of RKW-A mothers differ from
the control and RKW offspring in lower antibody production, lower
response of splenocytes to LPS and ConA, and higher serum
concentrations of TNF-α and IL-10. Spleens of RKW progeny were
found to contain more CD4+ cells compared with the
spleens of control and RKW-A progeny. In addition, spleen cells
collected from the progeny of RKW mice responded more to PHA
(significantly in the almarBlue assay, on the borderline of
significance in the [3H] thymidine incorporation assay)
compared with corresponding cells from RKW-A progeny. However, RKW
and RKW-A offspring splenocytes presented significantly lower
responses to ConA in the [3H] thymidine incorporation
assay compared with control group. Therefore, caution is
recommended in the use of RKW and RKW- extracts, particularly
long-term, as immunostimulants in pregnancy prior to further
research.
Acknowledgments
The present study was supported by the National
Centre of Science (Kraków, Poland; grant no. 2012/05/B/NZ
7/03219).
References
|
1
|
Zhang S, Gao W, Xu K, Guo Y, Lin S, Xue X,
Lu G, Li N, Liu H and Liu W: Early use of Chinese drug rhodiola
compound for patients with post-trauma and inflammation in
prevention of ALI/ARDS. Zhonghua Wai Ke Za Zhi. 37:238–240.
1999.(In Chinese). PubMed/NCBI
|
|
2
|
Panossian A, Wikman G and Sarris J:
Rosenroot (Rhodiola rosea): Traditional use, chemical composition,
pharmacology and clinical efficacy. Phytomedicine. 17:481–493.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hung SK, Perry R and Ernst E: The
effectiveness and efficacy of Rhodiola rosea L.: A systematic
review of randomized clinical trials. Phytomedicine. 18:235–244.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mishra KP, Ganju L and Singh SB:
Anti-cellular and immunomodulatory potential of aqueous extract of
Rhodiola imbricata rhizome. Immunopharmacol Immunotoxicol.
34:513–518. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chen SP, Liu R Huang, Lu TM, Wei JC, Wu
TC, Tsai WY, Tsai CH and Yang CC: Complementary usage of Rhodiola
crenulata (L.) in chronic obstructive pulmonary disease patients:
The effects on cytokines and T cells. Phytother Res. 29:518–525.
2015. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jeong HJ, Ryu YB, Park SJ, Kim JH, Kwon
HJ, Kim JH, Park KH, Rho MC and Lee WS: Neuraminidase inhibitory
activities of flavonols isolated from Rhodiola rosea roots and
their in vitro anti-influenza viral activities. Bioorg Med Chem.
17:6816–6823. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Diwaker D, Mishra KP, Ganju L and Singh
SB: Rhodiola inhibits dengue virus multiplication by inducing
innate immune response genes RIG-I, MDA5 and ISG in human
monocytes. Arch Virol. 159:1975–1986. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ahmed M, Henson DA, Sanderson MC, Nieman
DC, Zubeldia JM and Shanely RA: Rhodiola rosea exerts antiviral
activity in athletes following a competitive marathon race. Front
Nutr. 2:242015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang H, Ding Y, Zhou J, Sun X and Wang S:
The in vitro and in vivo antiviral effects of salidroside from
Rhodiola rosea L. against coxsackievirus B3. Phytomedicine.
16:146–155. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cybulska P, Thakur SD, Foster BC, Scott
IM, Leduc RI, Arnason JT and Dillon JA: Extracts of Canadian first
nations medicinal plants, used as natural products, inhibit
neisseria gonorrhoeae isolates with different antibiotic resistance
profiles. Sex Transm Dis. 38:667–671. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Furmanowa M, Skopińska-Różewska E, Rogala
E and Hartwich M: Rhodiola rosea in vitro culture-phytochemical
analysis and antioxidant action. Acta Societ Botanic Pol. 67:69–73.
1998. View Article : Google Scholar
|
|
12
|
Siwicki AK, Skopińska-Różewska E, Hartwich
M, Wójcik R, Bakuła T, Furmanowa M, Bałan BJ, Sommer E, Mielcarek
S, Buchwald W, et al: The influence of Rhodiola rosea extracts on
non-specific and specific cellular immunity in pigs, rats and mice.
Centr Eur J Immunol. 32:84–91. 2007.
|
|
13
|
Skopińska-Różewska E, Wójcik R, Siwicki
AK, Sommer E, Wasiutyński A, Furmanowa M, Malinowski M and
Mazurkiewicz M: The effect of Rhodiola quadrifida extracts on
cellular immunity in mice and rats. Pol J Vet Sci. 11:105–111.
2008.PubMed/NCBI
|
|
14
|
Zdanowski R, Lewicki S, Sikorska K,
Żmigrodzka M, Buchwald W, Wilczak J and Skopińska-Różewska E: The
influence of aqueous and hydro-alcoholic extracts of roots and
rhizomes of Rhodiola kirilowii on the course of pregnancy in mice.
Centr Eur J Immunol. 39:471–475. 2014. View Article : Google Scholar
|
|
15
|
Lewicki S, Stankiewicz W,
Skopińska-Różewska E, Wilczak J, Leśniak M, Suska M, Siwicki AK,
Skopiński P and Zdanowski R: Spleen content of selected
polyphenols, splenocytes morphology and function in mice fed
Rhodiola kirilowii extracts during pregnancy and lactation. Pol J
Vet Sci. 18:847–855. 2015.PubMed/NCBI
|
|
16
|
Hertog MGL, Hollman PCH and Venema DP:
Optimization of a quantitative HPLC determination of potentially
anticarcinogenic flavonoids in vegetables and fruits. J Agric Food
Chem. 40:1591–1598. 1992. View Article : Google Scholar
|
|
17
|
Shin JW, Seol IC and Son CG:
Interpretation of animal dose and human equivalent dose for drug
development. J Korean Med. 31:1–7. 2010.
|
|
18
|
Zdanowski R, Stankiewicz W, Kamiński A,
Grzela T, Skopiński P, Lewicki S and Skopińska-Rózewska E: The
effect of sterilization by irradiation of human pericardium and
skin frozen tissues on their ability to influence the proliferation
of endothelial cells in vitro. Centr Eur J Immunol. 37:119–122.
2012.
|
|
19
|
Ahmed SA, Gogal RM Jr and Walsh JE: A new
rapid and simple non-radioactive assay to monitor and determine the
proliferation of lymphocytes: An alternative to [3H]thymidine
incorporation assay. J Immunol Methods. 170:211–224. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Skopińska-Różewska E, Siwicki AK and
Sommer E: Stimulation of humoral immunity in mice by some
commercial fragrances. Centr Eur J Immunol. 34:232–234. 2009.
|
|
21
|
Alberts B, Johnson A, Lewis J, Raff M,
Roberts K and Walter P: Helper T cells and lymphocyte
activationMolecular biology of the Cell. 4th. New York: Garland
Science; 2002
|
|
22
|
Ferrari L, Martelli P, Saleri R, De
Angelis E, Cavalli V, Bresaola M, Benetti M and Borghetti P:
Lymphocyte activation as cytokine gene expression and secretion is
related to the porcine reproductive and respiratory syndrome virus
(PRRSV) isolate after in vitro homologous and heterologous recall
of peripheral blood mononuclear cells (PBMC) from pigs vaccinated
and exposed to natural infection. Vet Immunol Immunopathol.
151:193–206. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bachmann MF and Oxenius A: Interleukin 2:
From immunostimulation to immunoregulation and back again. EMBO
Rep. 8:1142–1148. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhu J and Paul WE: Peripheral CD4+ T-cell
differentiation regulated by networks of cytokines and
transcription factors. Immunol Rev. 238:247–262. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Nishimoto N and Kishimoto T: Interleukin
6: From bench to bedside. Nat Clin Pract Rheumatol. 2:619–626.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Riether C, Schürch CM and Ochsenbein AF:
Regulation of hematopoietic and leukemic stem cells by the immune
system. Cell Death Differ. 22:187–198. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhou X, Schmidtke P, Zepp F and Meyer CU:
Boosting interleukin-10 production: Therapeutic effects and
mechanisms. Curr Drug Targets Immune Endocr Metabol Disord.
5:465–475. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Shevach EM, DiPaolo RA, Andersson J, Zhao
DM, Stephens GL and Thornton AM: The lifestyle of naturally
occurring CD4+ CD25+ Foxp3+ regulatory T cells. Immunol Rev.
212:60–73. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ouyang W, Kolls JK and Zheng Y: The
biological functions of T helper 17 cell effector cytokines in
inflammation. Immunity. 28:454–467. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Onishi RM and Gaffen SL: Interleukin-17
and its target genes: Mechanisms of interleukin-17 function in
disease. Immunology. 129:311–321. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yi T, Zhao D, Lin CL, Zhang C, Chen Y,
Todorov I, LeBon T, Kandeel F, Forman S and Zeng D: Absence of
donor Th17 leads to augmented Th1 differentiation and exacerbated
acute graft-versus-host disease. Blood. 112:2101–2110. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Khanum F, Bawa AS and Singh B: Rhodiola
rosea: A Versatile Adaptogen. Compr Rev Food Sci F. 4:55–62. 2005.
View Article : Google Scholar
|
|
33
|
Brown RP and Gerbarg PL: Rhodiola rosea: A
Phytomedicinal Overview. HerbalGram. 56:2002.
|
|
34
|
Skopińska-Rózewska E, Stankiewicz W,
Zdanowski R, Siwicki AK, Furmanowa M, Buchwald W and Wasiutyński A:
The in vivo effect of Rhodiola quadrifida extracts on the antibody
production, on the blood leukocytes subpopulations and on the
bacterial infection in mice. Centr Eur J Immunol. 37:140–144.
2012.
|
|
35
|
Mishra KP, Chanda S, Shukla K and Ganju L:
Adjuvant effect of aqueous extract of Rhodiola imbricata rhizome on
the immune responses to tetanus toxoid and ovalbumin in rats.
Immunopharmacol Immunotoxicol. 32:141–146. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Bień K, Lewicki S, Zdanowski R,
Skopińska-Różewska E and Krzyżowska M: Feeding pregnant and
lactating mice Rhodiola kirilowii extracts helps to preserve thymus
function of their adult progeny. Pol J Vet Sci. 19:581–587.
2016.
|