Introduction
Contour enhancements of the face constitute an
important aspect of facial plastic surgeries for cosmetic, as well
as traumatic, congenital, and extirpative defect corrections
(1). There are various options to
consider for the reconstruction of a facial depressed deformity
depending on whether the underlying defect is a skeletal or a soft
tissue depression, including autologous tissue grafts, allogenic
tissue grafts and alloplastic materials.
Autologous tissues, such as grafted adipose tissue
(lipofilling), are thought to produce a more natural reconstruction
of the contour of the face, but they are highly invasive with
significant donor site morbidity and have drawbacks including
limited availability, limited moldability, and unpredictable
resorption (2). These disadvantages
constrict their clinical use. Various alloplastic materials have
been used in facial cosmetic and reconstructive surgery. The
material should have good biocompatibility, be easy to remodel at
the operating table, maintain its desired form and consistency
in situ, and be inert in body tissue (3). The most commonly used materials in
clinical practice include silicone, Gore-Tex, medpore, and expanded
polytetrafluoroethylene (ePTFE) (1,2).
Silicone implants lack the ability for vascularization, promote
thick capsule formation, cause resorption of the underlying bone,
and display a tendency for the implant to shift or extrude over a
long period of time. With Gore-tex the risk of infection, seroma
formation, and shifting from optimal place increases. Medpor has
good biocompatiblility, but due to its characteristic of stiffness,
it is mainly used on the bone reconstruction and cannot be used in
the soft tissue.
W.L. Gore and Associates, Inc. first produced ePTFE
in 1969 (4,5). The material was first used in 1982 to
reconstruct soft-tissue deficiencies. Since then, ePTFE has been
used safely and effectively in the human body for various
applications in vascular and cosmetic surgery. In cosmetic surgery,
ePTFE was often used for facial wrinkles, and for chin and nasal
augmentation.
In the present study, the ePTFE was used for
large-area facial depressed deformities. The results showed that,
ePTFE constituted a viable alternative treatment for facial
depressed deformity.
Patients and methods
Patient selection
In theory, any healthy patient with a facial
depressed deformity is suitable for ePTFE implantation. However, to
obtain a more satisfactory outcome for the surgeon and the patient,
patient selection should be deliberate. An ePTFE implant is more
suitable for patients with thick skin and subdermal tissue, and
especially for patients who do not want to undergo a more invasive
surgery of free-flap transfer. ePTFE is a long-lasting solid
implant that is palpable and visible on animation when it is
implanted in thin subdermal tissue.
In the present study, 31 ePTFE implants were used
for facial augmentation in 26 patients (12 women, 14 men) between
September 2008 and January 2014. The patient age range was 17–45
years (mean age 23.2 years). Indications for the augmentation
procedure were congenital malformations, post-traumatic defects,
and reconstruction after tumor surgery. Of the 26 patients,
diagnoses included stable hemifacial atrophy (3 cases),
craniofacial microsomia (13 cases), bony depression after trauma (8
cases), and other unclear reasons for soft tissue atrophy (2
cases). The implants were used for augmentation in the
nasal/paranasal area, zygomatico-orbital area, and chin and
mandibular area. The postoperative follow-up periods ranged from 6
to 18 months (average 9 months).
Surgical technique
The procedures were performed under local anesthesia
(a solution of 0.5% lidocaine with 1:200,000 epinephrine). The
depressed deformity area was marked with methylene blue, and an
ePTFE implant with the proper thickness, was trimmed on the edges
for a polished smooth transition effect.
For the frontal area, the surgeon first marked the
region to be treated with an indelible marker. If the patient had
displayed a strong frontalis movement during the pre-operatory
examination, the botulinum toxin was injected 1 week before the
implanting procedure. After marking, a solution of 0.5% lidocaine
and 1:200,000 epinephrine was infiltrated along the planned
dissection area and the two supraorbital ridges to block the
supratrochlear and supraorbital nerves. A no. 15 Bard-Parker blade
(Aspen Surgical Products, Inc., Chicago, IL, USA) was used to make
an incision along the 5-cm in length marked line behind the
anterior hairline down through the level of the periosteum. A
subperiosteal dissection was carefully made, inferiorly to the
level of the superior orbital rim and laterally to the temporal
ridge, taking care not to tear the periosteum or cross over the
temporal fusion line, and carefully avoiding the supraorbital
neurovascular bundles. The range of dissection extended into normal
tissue 0.5–1 cm beyond the junctional transition zone of the
defect. The trimmed ePTFE implant was inserted into the
subperiosteal pocket with utmost precision and the edges were
inspected carefully to confirm that there was no buckling or
folding. Then, the incision was sutured.
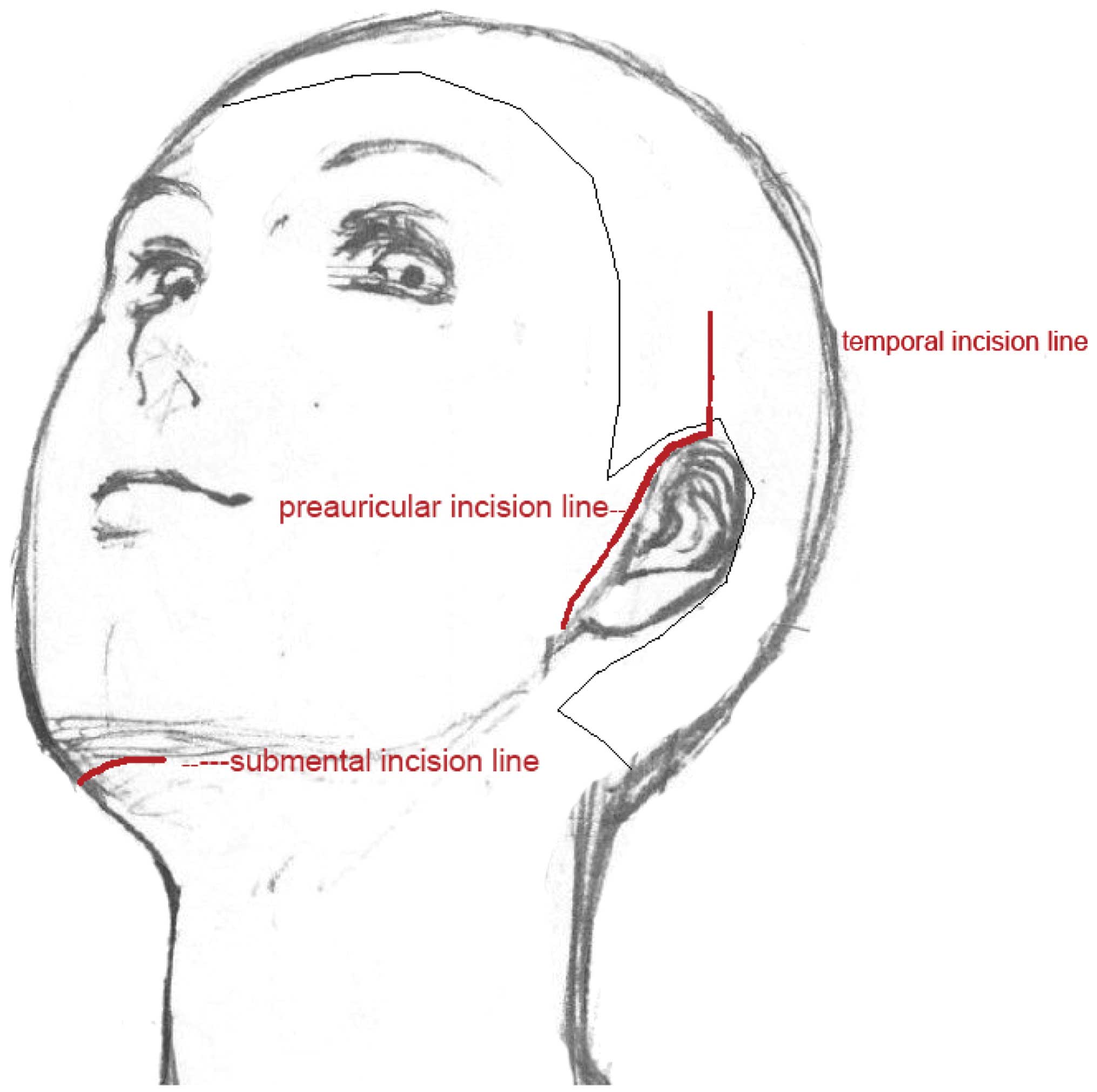
For the cheek, temporal, and mandibular areas, the
procedure was almost the same except for the obvious differences
regarding the tissue plane for implanting and the access sites. For
patients with a recipient site on the cheek and zygomatic areas, a
preauricular incision was made to create a pocket between the
subcutaneous tissue and the superficial musculoaponeurotic system
(SMAS). For the temporal and frontal depressed deformities, an
incision behind the hairline was selected, the dissected space lay
between the deep and superficial temporal fascias, and the utmost
care was taken to preserve the facial nerve intact. For
augmentation of the mandibular area, a submental incision was made
to access the implantation site (Fig.
1).
Results
In order to treat facial depression, 31 ePTFE were
implanted into the temporal areas (3 implants), cheek areas (5
implants), zygomatic areas (10 implants), mandibular areas (8
implants) and frontal areas (5 implants) in 26 patients.
No major complications occurred during the follow-up
of the 26 patients who had ePTFE implants inserted for varied
depressed deformities in the frontal, temporal, zygomatic, and
mandibular areas. There were no cases of infection, implant
exposure, delayed hematoma, or seroma. Minor complications, such as
immediate postoperative hematoma, visible or palpable lateral
border, asymmetries of shape, visible scars, or hair loss were also
rare. There was 1 patient (3.8%) with an under-corrected depressed
contour, while the remaining 25 patients were satisfied with the
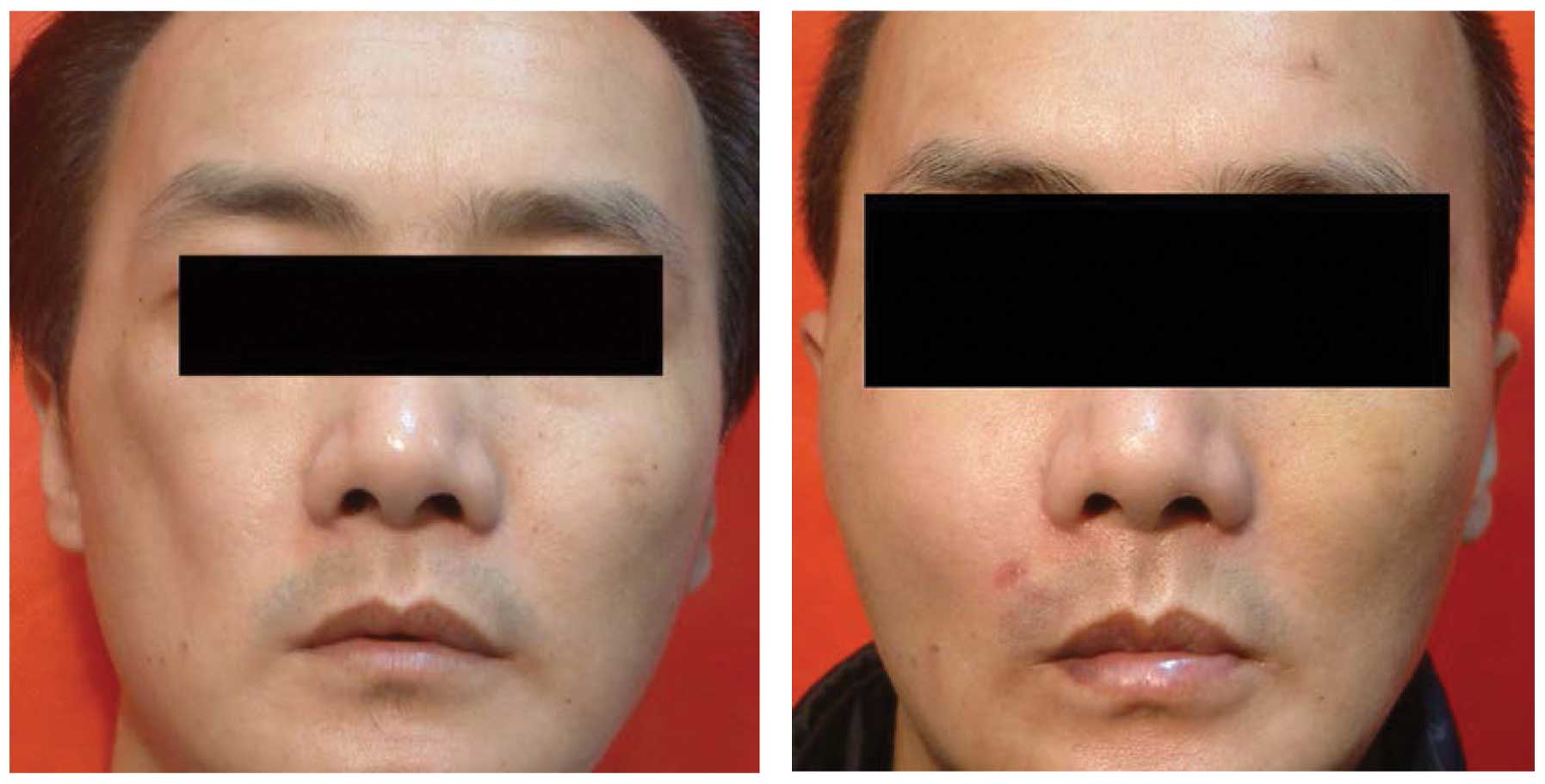
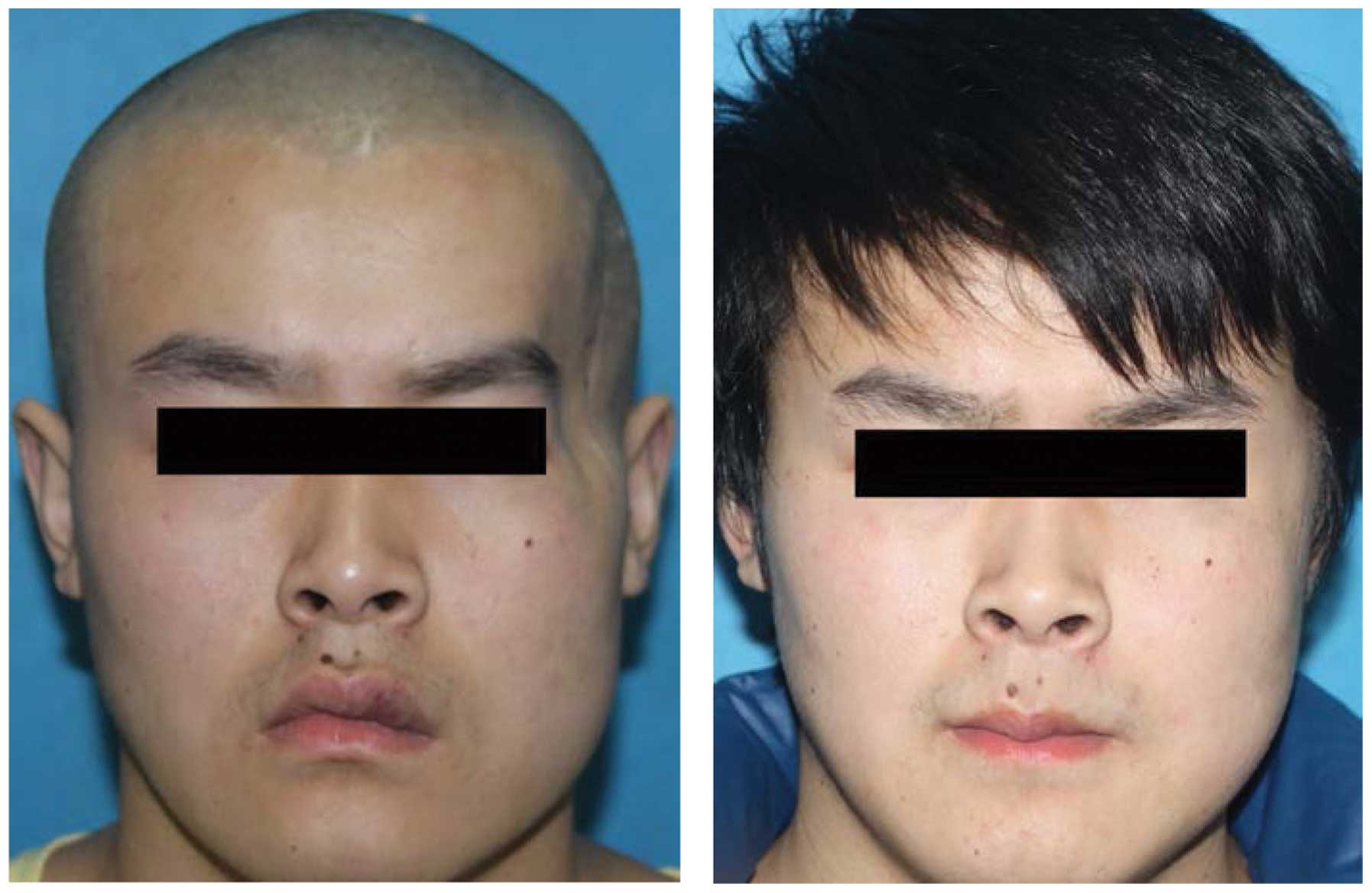
outcomes (satisfaction rate, 96.2%) (Figs. 2 and 3).
Discussion
ePTFE is a woven form of PTFE that creates a
mesh-like structure. It is flexible, soft and strong, non-toxic,
biocompatible, and not water-soluble. It has been reported that
although the ePTFE soft-tissue patch is a porous material (average
pore size is 22 µm), it does not appear to allow extensive fibrous
tissue ingrowth, as do other porous alloplastic materials (4,5).
Previous findings have shown that the pores of ePTFE provide a
lattice for incorporating connective tissue (6). The phenomenon of little to no tissue
adhesion allows for easy removal in case of complication or if the
patient is not satisfied with the augmentation result. The level of
tissue reaction to ePTFE is little, and previous reports confirm
fibrotic capsules are minimal (1–3,5–8). Scant
and focal chronic inflammatory cell reactions to ePTFE material may
be explained by micro-motion at the tissue-implant interface,
contamination, or reaction to the material configuration. However,
the implant material itself does not appear to be a direct stimulus
for inflammation, unlike Proplast, another form of
polytetrafluoroethylene, which elicits an intense and ongoing
inflammatory cell reaction that does not subside but rather
markedly increases over time (4).
Various methods for correcting facial depressed
deformities have been described in the literature (6,9).
Silicone and Gore-Tex are the most widely used implant materials
for skeletal augmentation. However, certain intrinsic properties
such as stiffness and molding difficulty, hinder their use in soft
tissue augmentation. If the patient has a thin skin texture or the
implant is placed in a dynamic expression rich area, the borders of
the hard implant can be identified or palpated. Fat injections or
autologous fat grafting for facial contouring are one of the most
frequently employed methods as they are easy to perform and involve
a relatively minor invasive procedure. However, autologous fat
grafting has the critical pitfall of unpredictable resorption over
time and the risk of uneven distribution of fat throughout the area
that the surgeon wants to modify.
During the procedure, attention should be paid to
the implanting tissue plane and implanting region. As mentioned
earlier, aside from frontal and temporal regions, ePTFEs can be
inserted into the tissue plane between the SMAS and subcutaneous
tissue. Particularly important for such a procedure is to ensure
the skin is sufficiently thick to conceal the traces of the edges
of the ePTFE. It is not recommended to implant ePTFE materials in
the regions of lips and nasolabial folds, however, as there is an
increased risk of a discomforting firm or stiff feeling (10).
Biotolerability is defined as the ability of a
material to reside in the body for long periods of time with only
low degrees of inflammatory reaction (11). Various factors affect
biotolerability, including the biomaterial itself and the
implanting procedure. Bacterial infections and repeated frictions
in the implantation regions are important contributing factors for
inflammatory reactions. Thus, to increase biotolerability, we
refined our operational procedures to decrease the possibility for
inflammatory reactions. We stringently adhered to the principles of
aseptic technique, avoided frequent placing in and removal from the
dissected pocket, suturing the incision tightly away from
potentially invading bacteria.
In conclusion, ePTFE is biocompatible and can be
well tolerated by the host. Through mature preoperative planning
and skillful manipulation, ePTFE can yield a good outcome. Aside
from lipofilling and a free-flap transfer, ePTFE constitutes a
viable alternative to consider for the treatment of depressed
deformities of the face.
Acknowledgements
The present study was supported by the Chongqing
Science and Technology Commission (Cstc2013kjrc-qnrc10003).
References
|
1
|
Cuzalina LA and Hlavacek MR: Complications
of facial implants. Oral Maxillofac Surg Clin North Am. 21:91–104,
vi-vii. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sclafani AP and Romo T III: Biology and
chemistry of facial implants. Facial Plast Surg. 16:3–6. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rubin JP and Yaremchuk MJ: Complications
and toxicities of implantable biomaterials used in facial
reconstructive and aesthetic surgery: A comprehensive review of the
literature. Plast Reconstr Surg. 100:1336–1353. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Maas CS, Merwin GE, Wilson J, Frey MD and
Maves MD: Comparison of biomaterials for facial bone augmentation.
Arch Otolaryngol Head Neck Surg. 116:551–556. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Maas CS, Gnepp DR and Bumpous J: Expanded
polytetrafluoroethylene (Gore-Tex soft-tissue patch) in facial
augmentation. Arch Otolaryngol Head Neck Surg. 119:1008–1014. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Artz JS and Dinner MI: The use of expanded
polytetrafluoroethylene as a permanent filler and enhancer: An
early report of experience. Ann Plast Surg. 32:457–462. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Schranz D, Jux C, Vogel M, Bauer J,
Akintürk H and Valeske K: Large-diameter graft-stent (Advanta V12)
implantation in various locations: Early results. Cardiol Young.
21:66–73. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Niamtu J III: Advanta ePTFE facial
implants in cosmetic facial surgery. J Oral Maxillofac Surg.
64:543–549. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Baek R, Heo C and Kim BK: Use of various
free flaps in progressive hemifacial atrophy. J Craniofac Surg.
22:2268–2271. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cox SE: Who is still using expanded
polytetrafluoroethylene? Dermatol Surg. 31:1613–1615. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ratner BD: The biocompatibility manifesto:
Biocompatibility for the twenty-first century. J Cardiovasc Transl
Res. 4:523–527. 2011. View Article : Google Scholar : PubMed/NCBI
|