Introduction
Pegylated IFN (PEG-IFN) and ribavirin (RBV)
treatment for chronic hepatitis C (CHC) infection in 2005 has
improved treatment outcomes (1).
Furthermore, the approval of a new PEG-IFN/RBV/telaprevir (TVR)
triple treatment for CHC in 2011 improved the rate of achieving a
sustained virologic response (SVR) and shortened the length of
treatment (1–3).
Qualitative and quantitative methods have identified
that IFN-based CHC treatment has a negative impact on the
health-related quality of life (HRQOL) of patients (4–6). To the
best of our knowledge, there are no previous reports on the impact
of TVR-based treatment on the QOL of patients with CHC infection.
In TVR-based triple treatment, the possibility of side effects,
such as high-grade anemia, skin disorders and renal insufficiency,
has made understanding its impact on patient QOL important.
Therefore, the present study aimed to investigate the HRQOL of
patients with CHC treated with TVR-based triple therapy using the
36-item short form health survey (SF-36) (7).
Materials and methods
Study participants and treatment
The present study examined 34 patients with CHC (18
men and 16 women; mean age, 62.8±10.1 for men and 63.6±10.4 years
for women), who could be followed up long term, following triple
treatment with PEG-IFN/RBV/TVR at Saiseikai Niigata Daini Hospital
(Niigata, Japan) between November 2011 and March 2014. Written
informed consent was obtained from all patients, and the Ethical
Committee of Saiseikai Niigata Daini Hospital (Niigata, Japan)
approved this study, which was conducted in accordance with the
Declaration of Helsinki.
All patients received TVR (Mitsubishi Tanabe Pharma
Corporation, Osaka, Japan) in combination with PEG-IFN α2b and RBV
(both MSD, Tokyo, Japan) for 12 weeks. This was followed by an
additional 12 weeks of PEG-IFN α2b and RBV alone. TVR (750 mg) was
orally administered every 8 h, PEG-IFN α2b (1.5 µg/kg) was injected
subcutaneously weekly and oral RBV was given daily at a dose of
between 600 and 800 mg based on the body weight of the patient.
Assessment of HRQOL
The association between the HRQOL of patients with
CHC treated with TVR-based treatment and gender, age or treatment
history was examined. The HRQOL of study participants was measured
using a 36-item self-administered questionnaire, the SF-36. The
SF-36 evaluates the following eight physical and mental health
areas: Physical functioning (PF), physical role functioning (RP),
bodily pain (BP), general health (GH), vitality (VT), social role
functioning (SF), emotional role functioning (RE) and mental health
(MH) (7). Each of the 8 areas was
scored on a scale of 0–100, where a higher score indicated better
health subjectively. These scores were calculated from the
questionnaires as described previously (8–10).
Briefly, a Japanese-validated version of SF-36 was used and the
results were compared to a Japanese normative sample score of the
SF-36 in 2,966 individuals (10,11).
Scores were calculated based on the standard value (100 points) for
the Japanese population.
Evaluation of HRQOL using the SF-36 was performed
prior to treatment (0W), 2 weeks following the start of treatment
(2W), 3 months following the start of treatment (12W), 6 months
following the start of treatment (24W) and 1 year following the
start of treatment (48W). In examination on the basis of treatment
history, patients were classified as naïve (no prior CHC treatment;
n=14), relapsed (recurrence of virus following treatment for CHC;
n=19) or null responders (no change in viral load following CHC
treatment; n=1, excluded from analysis due to low number).
Statistical analysis
Intergroup HRQOL was compared using the Student's
t-test, the chi-squared test and the Mann-Whitney U test, while
scores prior to and following the start of treatment were compared
using a paired t-test. P<0.05 was considered to indicate a
statistically significant difference. Statistical analysis was
performed using StatView software (version 5.0; SAS Institute,
Inc., Cary, NC, USA).
Results
Examination on the basis of
gender
The clinical characteristics of patients were
compared on the basis of gender (Table
I). The men showed significantly higher values for height
(P<0.01), weight (P<0.01), and body mass index (BMI;
P<0.05), whereas the women exhibited a significantly higher
serum concentration of RBV (12.6±2.1 mg/kg vs. 10.6±2.1 mg/kg;
P<0.05) and TVR (39.8±7.7 mg/kg vs. 30.6±5.6 mg/kg; P<0.01).
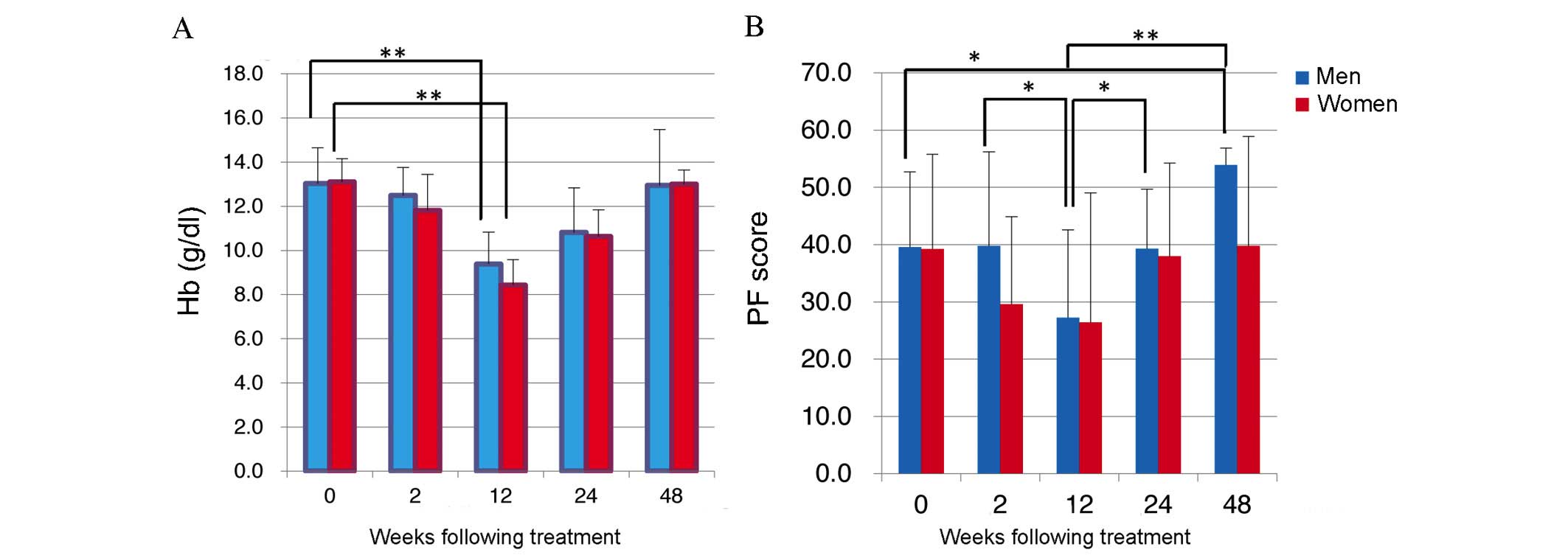
Hemoglobin (Hb) levels dropped in men and women during treatment as
SF-36 scores fell (Fig. 1A). Mean Hb
levels were 13.0±1.6 g/dl in men and 13.1±1.0 g/dl in women prior
to starting treatment, but dropped significantly to 9.4±1.4 g/dl
and 8.4±1.2 g/dl, respectively, by 12W (P<0.05; Fig. 1A). However, at 48W Hb values were
13.0±2.5 g/dl in men and 13.0±0.6 g/dl in women (Fig. 1A), indicating that baseline Hb levels
had been recovered.
 | Table I.Clinical characteristics of patients
with chronic hepatitis C during TVR-based triple treatment
according to gender. |
Table I.
Clinical characteristics of patients
with chronic hepatitis C during TVR-based triple treatment
according to gender.
|
| Gender |
|
|---|
|
|
|
|
|---|
| Characteristic | Male (n=18) | Female (n=16) | P-value |
|---|
| Age (years) | 62.8±10.1 | 63.6±10.4 | nsa |
| Height (cm) | 165.2±5.8 | 155±5.1 | P<0.0a |
| Weight (kg) | 66.5±9.3 | 52.7±5.8 | P<0.0a |
| BMI
(kg/m2) | 24.3±2.6 | 22.0±2.6 |
P<0.05a |
| Treatment history
(no. naïve/relapse/null) | 8/9/1 | 6/10/0 | nsb |
| Serum PEG-IFN
concentration (µg/kg) | 1.55±0.2 | 1.76±0.3 | nsa |
| Serum RBV
concentration (mg/kg) | 10.6±2.1 | 12.6±2.1 |
P<0.05a |
| Serum TVR
concentration (mg/kg) | 30.6±5.6 | 39.8±7.7 |
P<0.01a |
HRQOL improved markedly following completion of
TVR-based triple treatment in complete-responders, with higher
scores compared with those prior to treatment (data not shown).
Interestingly, anemia (Hb <8 g/dl) and skin symptoms appeared
frequently during treatment, corresponding to scores for physical
functioning dropped (data not shown).
Comparison of PF scores revealed a downward trend
for men (n=18) and women (n=16) following TVR-based triple
treatment until 12W, with PF scores in men dropping significantly
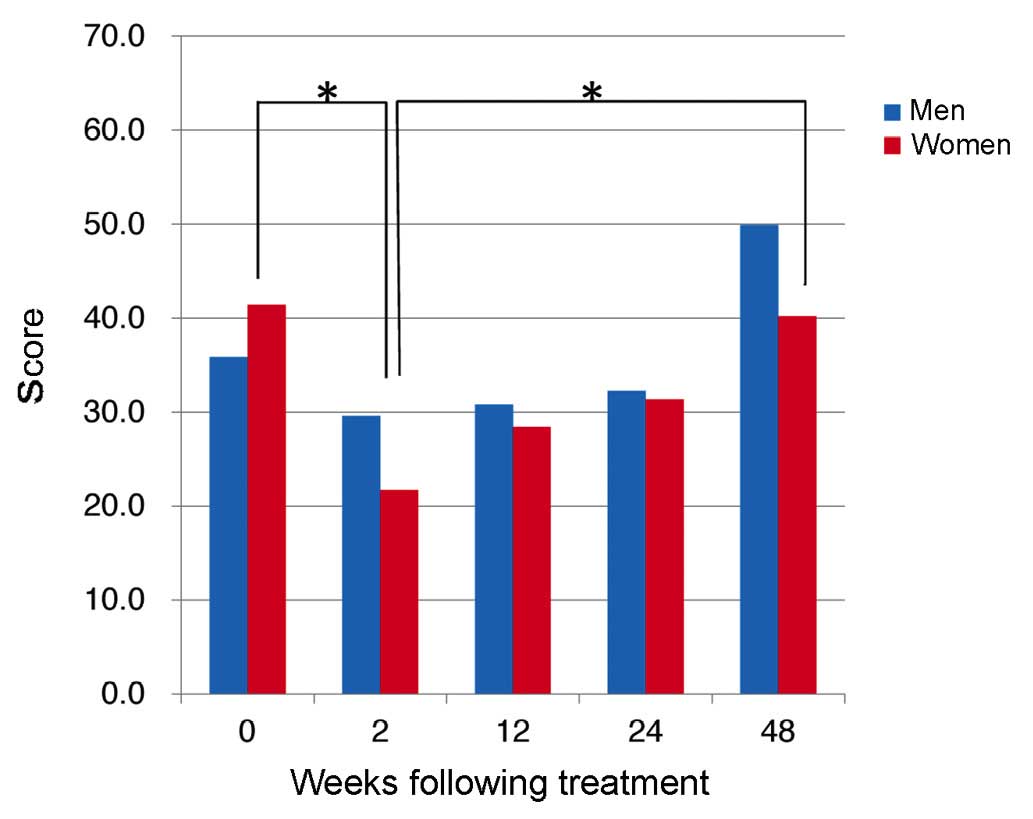
from 39.8±16.4 at 2W to 27.2±15.4 at 12W (P=0.037; Fig. 1B). In addition, PF improved from 12W,
with a mean PF score in men of 53.9±3.0 at 48W, significantly
higher than the pre-treatment score of 39.6±13.1 (P=0.0009;
Fig. 2). In addition, scores for RP
dropped significantly in women between 0W and 2W (P=0.0046;
Fig. 2).
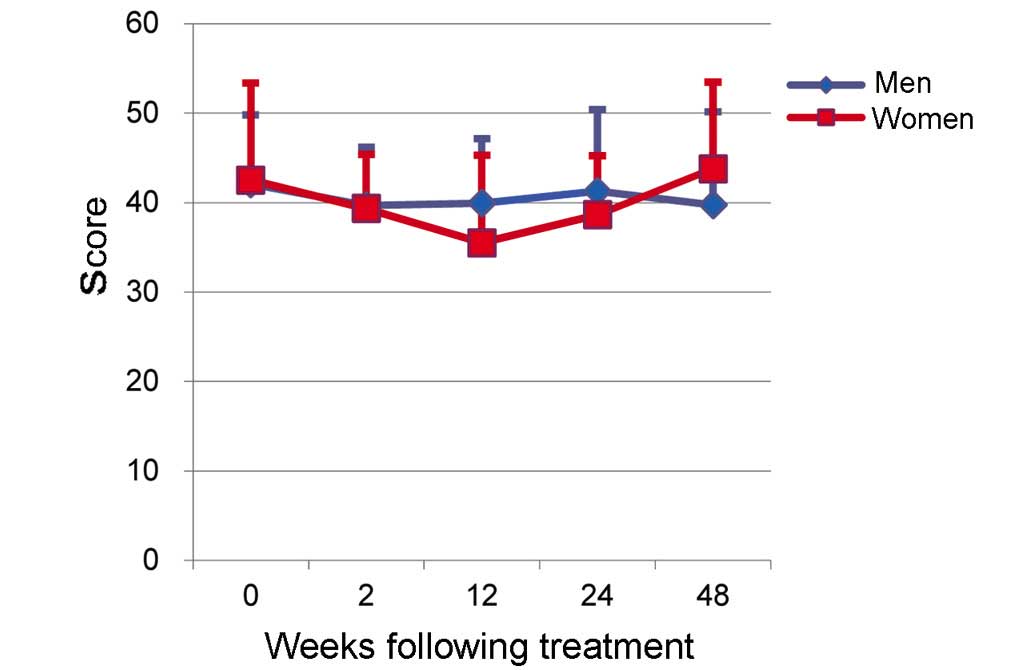
The score for MH in women was 42.5±10.8 prior to
treatment (0W) and 35.5±9.8 at 12W (Fig.
3). Although a downward trend was seen, this difference was not
significant. No significant differences in MH scores were seen
between men and women, and no effects of TVR-based treatment on MH
scores were observed (Fig. 3).
Examination on the basis of age
Patient characteristics were compared between
patients ≥65 years old (elderly group, n=19) and those <65 years
old (non-elderly group, n=15). The results of this comparison are
presented in Table II. While
significant differences in height (P<0.05) and treatment history
(P<0.01) were seen between the elderly and non-elderly groups,
no significant differences were seen in PEG-IFN, RBV or TVR
dosage.
 | Table II.Clinical characteristics of patients
with chronic hepatitis C during TVR-based triple treatment
according to age. |
Table II.
Clinical characteristics of patients
with chronic hepatitis C during TVR-based triple treatment
according to age.
|
| Age |
|
|---|
|
|
|
|
|---|
| Characteristic | Non-elderly group
(<65 years old; n=15) | Elderly group (≥65
years old; n=19) | P-value |
|---|
| Age (years) |
52.8±4.8 | 70.4±4.9 |
P<0.01a |
| Gender
(male/female) | 8/7 | 10/9 | nsb |
|
BMI(kg/m2) | 22.9±2.6 | 23.5±3.1 | nsa |
| Height (cm) | 163.8±7.3 | 158.4±6.9 |
P<0.05a |
| Weight (kg) | 62.0±11.1 | 59.1±10.0 | nsa |
| Treatment history
(Naïve/Relapse/Null) | 10/5/0 | 4/14/1 |
P<0.01b |
| Serum PEG-IFN
concentration (µg/kg) | 1.65±0.2 | 1.64±0.3 | nsa |
| Serum RBV
concentration (mg/kg) | 11.68±1.4 | 11.3±2.8 | nsa |
| Serum TVR
concentration (mg/kg) | 36.3±7.2 | 33.6±8.6 | nsa |
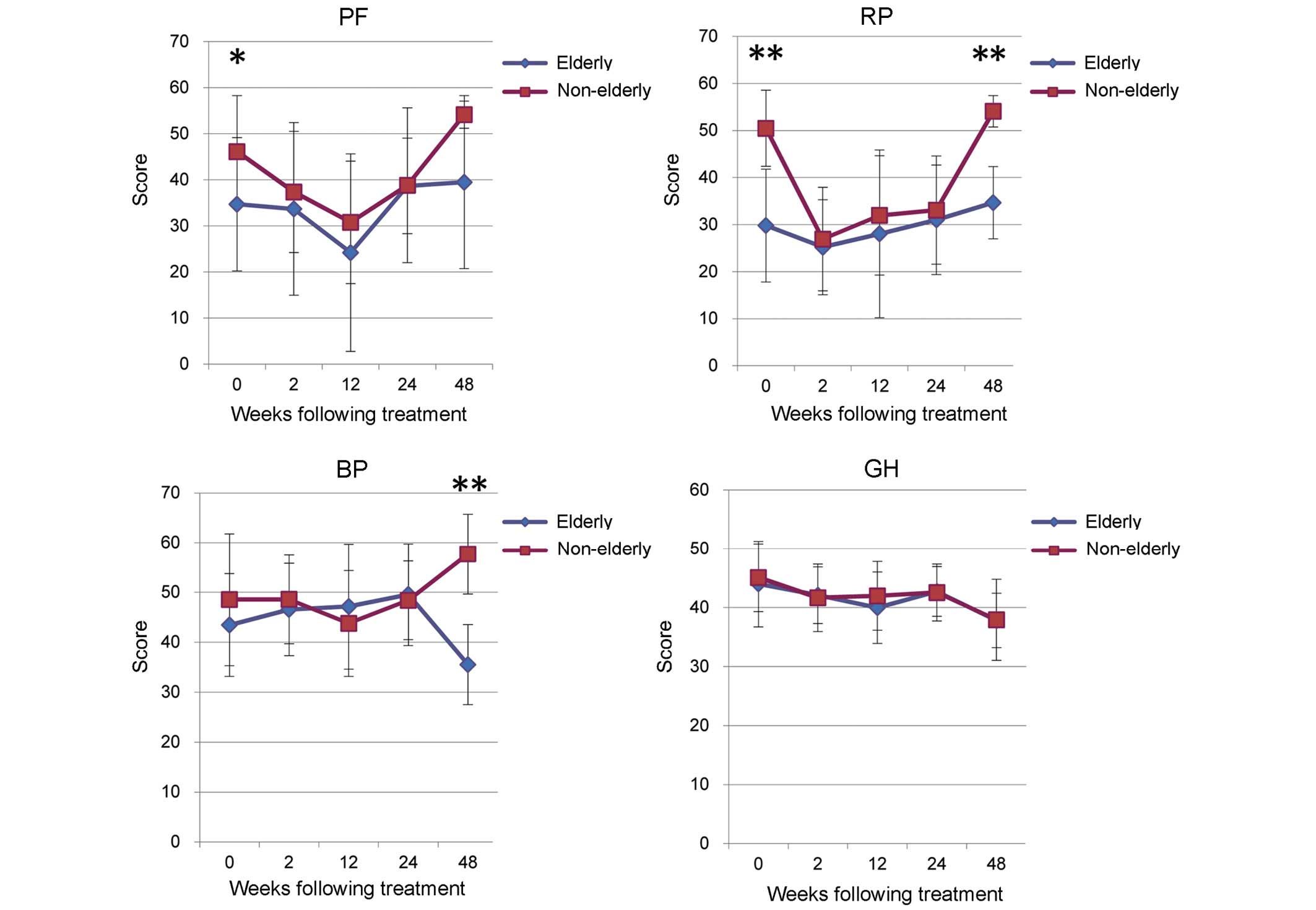
The non-elderly group exhibited significantly higher
scores in PF (P<0.05) and RP (P<0.01) prior to treatment,
which are correlated with physical health (Fig. 4). However, a drop in scores was seen
the non-elderly and elderly groups during TVR treatment (Fig. 4). A significant rise in RP score was
seen in the non-elderly group at 48W, following completion of
treatment (P<0.01; Fig. 4).
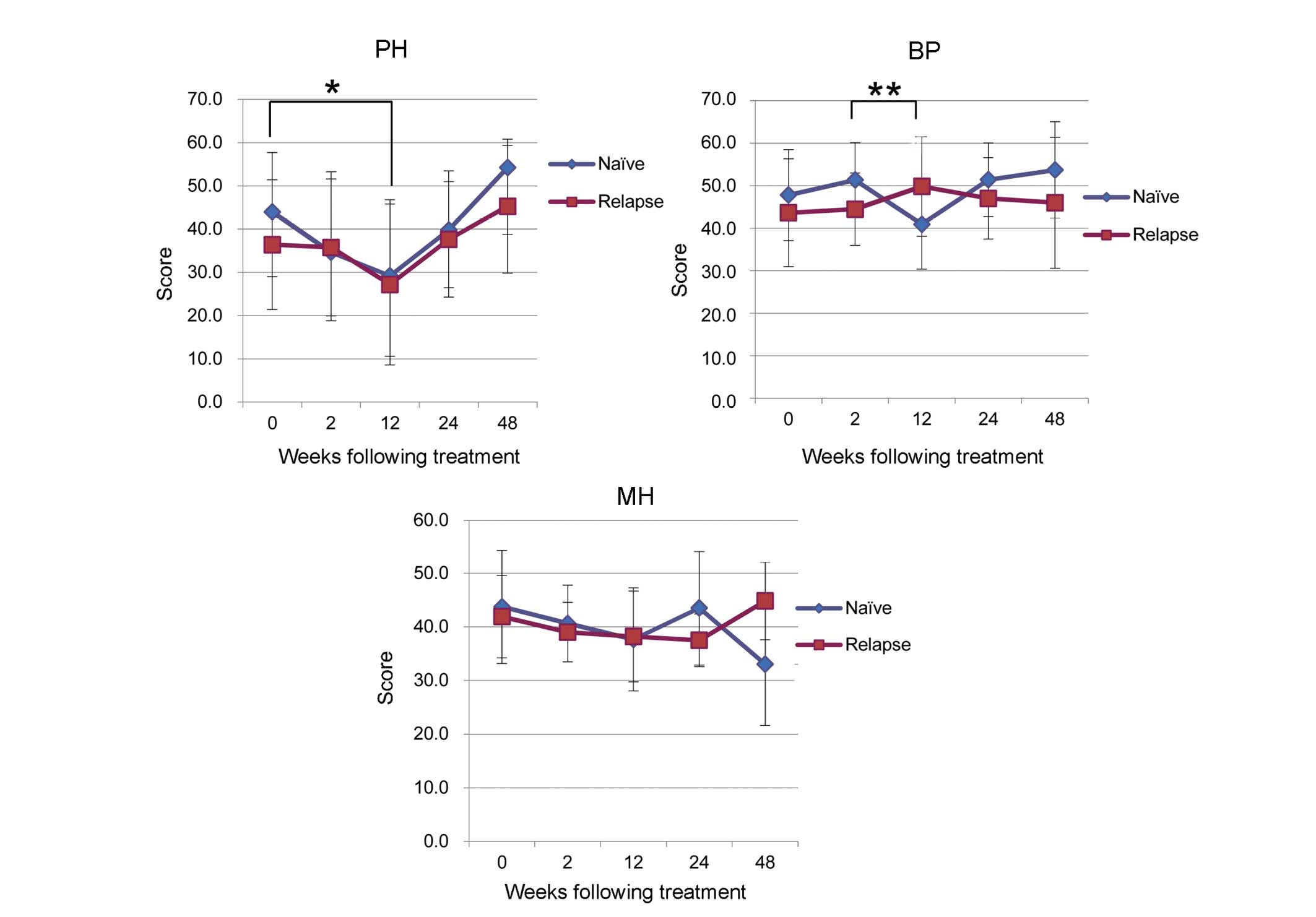
Examination on the basis of prior
treatment history
A comparison of patient characteristics according to
prior treatment history is presented in Table III. Patients were classified as
naïve (n=14), relapsed (n=19) or null responders (n=1). However,
the null responders group was excluded from this analysis. A
tendency towards low PF scores, correlated with poor physical
health, was seen in the naïve and relapse groups during TVR-based
treatment. In the naïve group, the mean PF score was 44.0±13.7
prior to treatment and decreased significantly to 29.2±17.6 between
0W and 12W (P=0.02; Fig. 5). In the
relapse group the decrease was not significant (Fig. 5). MH tended to decrease during
TVR-based treatment, however, no significant difference was seen
between groups (Fig. 5).
 | Table III.Clinical characteristics of patients
with chronic hepatitis C during TVR-based triple treatment
according to prior treatment history. |
Table III.
Clinical characteristics of patients
with chronic hepatitis C during TVR-based triple treatment
according to prior treatment history.
|
| Treatment
history |
|
|---|
|
|
|
|
|---|
| Characteristic | Naïve (n=14) | Relapse (n=19) | P-value |
|---|
| Age (years) | 58.8±10.9 | 66.3±8.7 | nsa |
| Gender
(male/female) | 8/6 | 9/10 | nsb |
| BMI
(kg/m2) | 22.7±2.9 | 23.6±2.9 | nsa |
| Height (cm) | 163.4±7.25 | 158.5±7.4 | nsa |
| Weight (kg) | 60.7±9.8 | 59.7±11.4 | nsa |
| Serum PEG-IFN
concentration (µg/kg) | 1.7±0.2 | 1.6±0.4 | nsa |
| Serum RBV
concentration (mg/kg) | 11.0±1.7 | 11.8±2.7 | nsa |
| Serum TVR
concentration (mg/kg) | 35.7±6.8 | 34.1±9.2 | nsa |
Discussion
CHC infection may be associated with a considerable
reduction in HRQOL, regardless of the disease stage (12–15).
However, the precise mechanism of decreased HRQOL in patients with
CHC has not been elucidated, although there are a number of reports
indicating an association between the degree of fibrosis, gender
and age (16,17).
IFN treatment requires long-term outpatient
treatment, making QOL evaluations essential. IFN-free hepatitis C
treatment has now been approved (18) and is being actively implemented.
However, QOL evaluations prior to and following IFN treatment
remain important, even in this era of IFN-free treatment.
HRQOL evaluations include comprehensive
health-related and disease-specific assessments. Comprehensive
HRQOL evaluations include the SF-36, developed in the United States
and used worldwide. A Japanese version, developed by Fukuhara et
al (10), is used in various
fields in Japan and reports of its usefulness have emerged
(10,11). The SF-36 allows patients to
quantitatively self-evaluate their physical and emotional QOL with
relative ease. The SF-36 is a multidimensional, comprehensive QOL
measure comprising of 36 items covering 8 areas, which are related
to physical or emotional health. Each of the 8 areas contains 2–5
levels of questions that are scored on an ordinal scale, allowing
the patient to understand their health from multiple aspects and
evaluate their QOL. Each of the 8 areas was scored on a scale of
0–100, where a higher score indicated better health
subjectively.
A systematic review by Spiegel et al
(19) found 15 studies that compared
the HRQOL of patients with hepatitis C virus (seropositive) with
healthy controls, which identified a decrease in weighted mean
SF-36 scores including PF and RP. Previous studies have reported a
decreased HRQOL in 6 SF-36 areas in patients with CHC compared with
patients without CHC, particularly in VT, GH and RP (20,21). In
the current study, patients with CHC undergoing TVR-based treatment
exhibited significantly lower SP-36 scores in 5 areas (PF, RP, BP,
GH and MH), indicating a decrease in their HRQOL. The results of
the current study are in agreement with previous studies comparing
the HRQOL of patients with CHC patients prior to and following
antiviral treatment (IFN or IFN/RBV) (22,23).
In the current study, SF-36 scores dropped in
conjunction with the progression of anemia and were lowest at 12W.
It is important to assess subjective symptoms with the SF-36 early
and to adjust dosages accordingly, in order to minimize the number
of patients who drop out of treatment, improve therapeutic efficacy
and maintain patient QOL. Furthermore, the findings of the current
study suggest that anemia is an important factor in maintaining
QOL, highlighting the importance of controlling drug dosage during
treatment.
A number of improvements have been made to IFN
treatment in recent years, enhancing its therapeutic effects and
increasing the patient response rate. In the present study, the
negative effects of TVR-based triple treatment on HRQOL were
stronger in regards to physical health compared with emotional
health. This is because PF scores decreased until 12W, but then
followed an upward trend, which may be the result of the side
effects of TVR decreasing. Conversely, PF scores, which correlate
strongly with physical health, tended to exceed scores from prior
to treatment (0W) by 48W, regardless of gender, age or treatment
history. This suggests that TVR-based triple treatment improves
physical HRQOL, which is likely associated with a SVR. In further
studies, the HRQOL in patients with IFN vs. IFN-free treatment will
be investigated.
In conclusion, the present study evaluated HRQOL,
using the SF-36, in patients with CHC during TVR-based triple
treatment. Surveys using the SF-36 offer a useful indicator of
patient QOL, which may be used to reduce the number of patients who
discontinue treatment and to improve IFN treatment in the
future.
References
|
1
|
McHutchison JG, Everson GT, Gordon SC,
Jacobson IM, Sulkowski M, Kauffman R, McNair L, Alam J and Muir AJ:
PROVE1 Study Team: Telaprevir with peginterferon and ribavirin for
chronic HCV genotype 1 infection. N Engl J Med. 360:1827–1838.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hézode C, Forestier N, Dusheiko G, Ferenci
P, Pol S, Goeser T, Bronowicki JP, Bourlière M, Gharakhanian S,
Bengtsson L, et al: Telaprevir and peginterferon with or without
ribavirin for chronic HCV infection. N Engl J Med. 360:1839–1850.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
McHutchison JG, Manns MP, Muir AJ,
Terrault NA, Jacobson IM, Afdhal NH, Heathcote EJ, Zeuzem S,
Reesink HW, Garg J, et al: Telaprevir for previously treated
chronic HCV infection. N Engl J Med. 362:1292–1303. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kinder M: The lived experience of
treatment for hepatitis C. Gastroenterol Nurs. 32:401–408. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Liu J, Lin CS, Hu SH, Liang ML, Zhao ZX
and Gao ZL: Quality of life in patients with chronic hepatitis C
after PEG-Interferon a-2a treatment. Zhonghua Gan Zang Bing Za Zhi.
19:890–893. 2011.(In Chinese). PubMed/NCBI
|
|
6
|
Marcellin P, Chousterman M, Fontanges T,
Ouzan D, Rotily M, Varastet M, Lang JP, Melin P and Cacoub P:
CheObs Study Group: Adherence to treatment and quality of life
during hepatitis C therapy: A prospective, real-life, observational
study. Liver Int. 31:516–524. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ware JE, Snow KK, Kosinski M and Gandek B:
SF-36 Health Survey Manual and Interpretation Guide. The Health
Institute, New England Medical Center; Boston, MA: 1993
|
|
8
|
Aaronson NK, Acquadro C, Alonso J, Apolone
G, Bucquet D, Bullinger M, Bungay K, Fukuhara S, Gandek B and
Keller S: International quality of life assessment (IQOLA) project.
Qual Life Res. 1:349–351. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ware JE Jr and Gandek B: Overview of the
SF-36 health survey and the international quality of life
assessment (IQOLA) project. J Clin Epidemiol. 51:903–912. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fukuhara S, Bito S, Green J, Hsiao A and
Kurokawa K: Translation, adaptation, and validation of the SF-36
health survey for use in Japan. J Clin Epidemiol. 51:1037–1044.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fukuhara S and Suzukamo Y: Manual of
SF-36v2 Japanese version. Institute for Health Outcomes and Process
Evaluation Research; Kyoto: 2004
|
|
12
|
Foster GR, Goldin RD and Thomas HC:
Chronic hepatitis C virus infection causes a significant reduction
in quality of life in the absence of cirrhosis. Hepatology.
27:209–212. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Rodger AJ, Jolley D, Thompson SC, Lanigan
A and Crofts N: The impact of diagnosis of hepatitis C virus on
quality of life. Hepatology. 30:1299–1301. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Strauss E and Teixeira MC Dias: Quality of
life in hepatitis C. Liver Int. 26:755–765. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Younossi Z, Kallman J and Kincaid J: The
effects of HCV infection and management on health-related quality
of life. Hepatology. 45:806–816. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bonkovsky HL, Snow KK, Malet PF,
Back-Madruga C, Fontana RJ, Sterling RK, Kulig CC, Di Bisceglie AM,
Morgan TR, Dienstag JL, et al: Health-related quality of life in
patients with chronic hepatitis C and advanced fibrosis. J Hepatol.
46:420–431. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Teuber G, Schäfer A, Rimpel J, Paul K,
Keicher C, Scheurlen M, Zeuzem S and Kraus MR: Deterioration of
health-related quality of life and fatigue in patients with chronic
hepatitis C: Association with demographic factors, inflammatory
activity, and degree of fibrosis. J Hepatol. 49:923–929. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kumada H, Suzuki Y, Ikeda K, Toyota J,
Karino Y, Chayama K, Kawakami Y, Ido A, Yamamoto K, Takaguchi K, et
al: Daclatasvir plus asunaprevir for chronic HCV genotype 1b
infection. Hepatology. 59:2083–2091. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Spiegel BM, Younossi ZM, Hays RD, Revicki
D, Robbins S and Kanwal F: Impact of hepatitis C on health related
quality of life: A systematic review and quantitative assessment.
Hepatology. 41:790–800. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Björnsson E, Verbaan H, Oksanen A, Frydén
A, Johansson J, Friberg S, Dalgård O and Kalaitzakis E:
Health-related quality of life in patients with different stages of
liver disease induced by hepatitis C. Scand J Gastroenterol.
44:878–887. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kwan JW, Cronkite RC, Yiu A, Goldstein MK,
Kazis L and Cheung RC: The impact of chronic hepatitis C and
co-morbid illnesses on health-related quality of life. Qual Life
Res. 17:715–724. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kang SC, Hwang SJ, Lee SH, Chang FY and
Lee SD: Health-related quality of life and impact of antiviral
treatment in Chinese patients with chronic hepatitis C in Taiwan.
World J Gastroenterol. 11:7494–7498. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hollander A, Foster GR and Weiland O:
Health-related quality of life before, during and after combination
therapy with interferon and ribavirin in unselected Swedish
patients with chronic hepatitis C. Scand J Gastroenterol.
41:577–585. 2006. View Article : Google Scholar : PubMed/NCBI
|