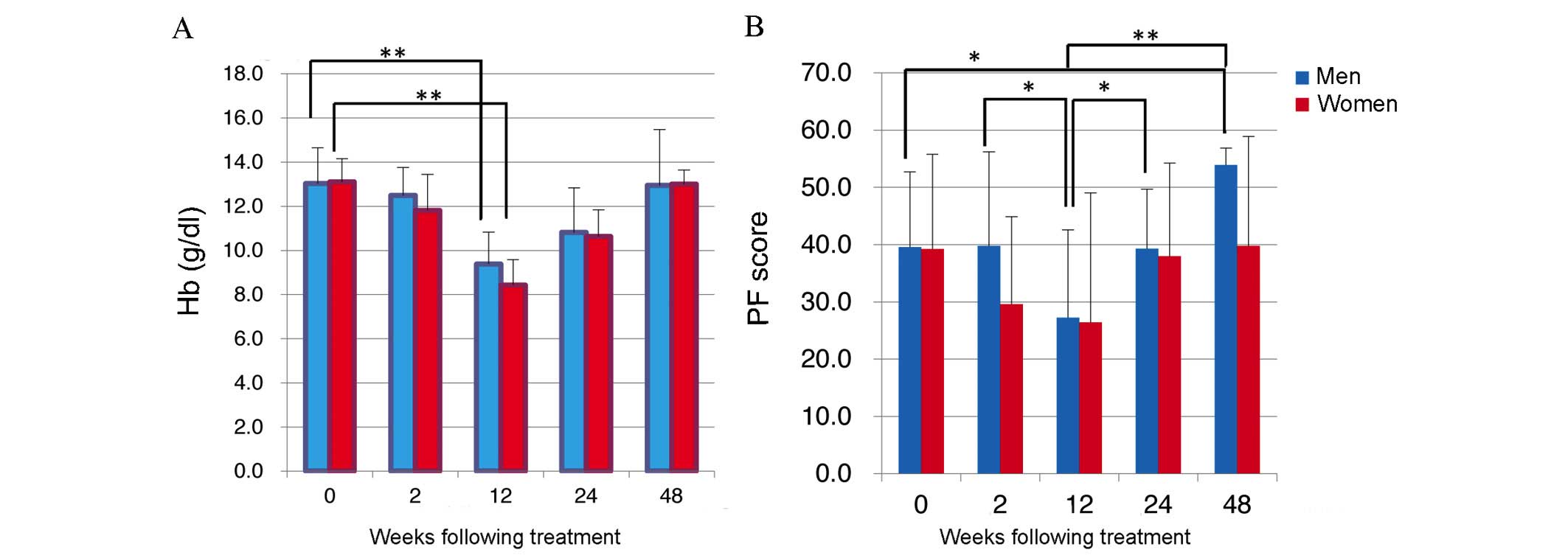
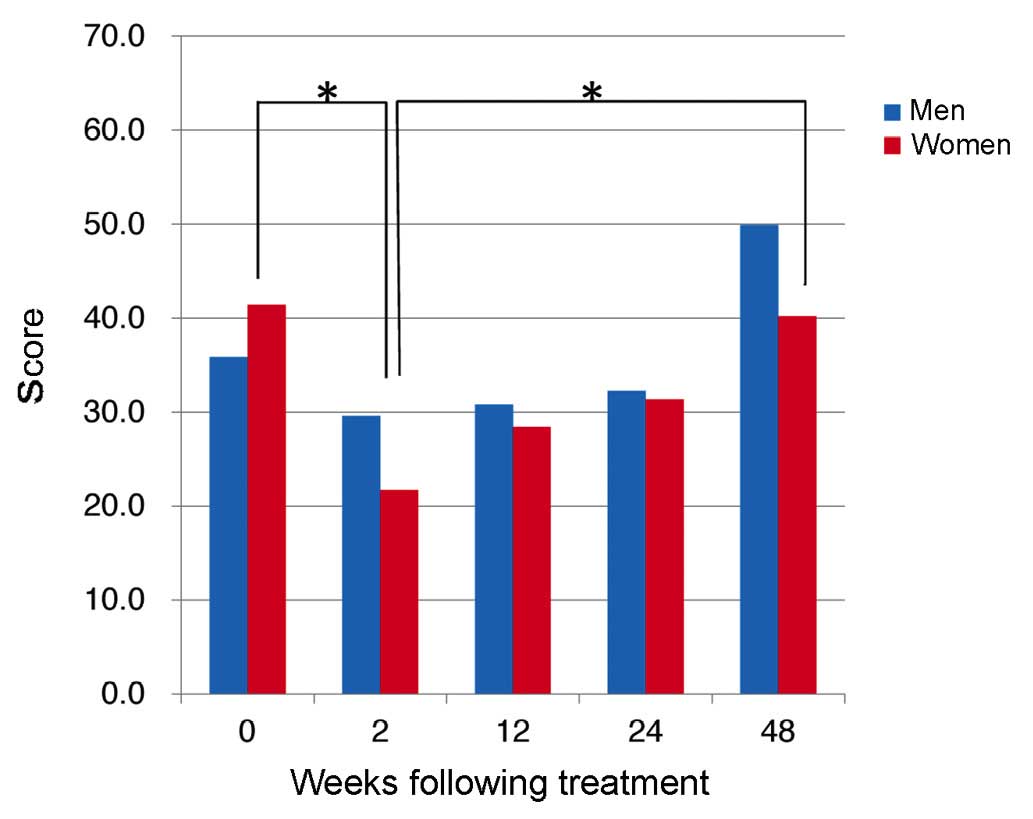
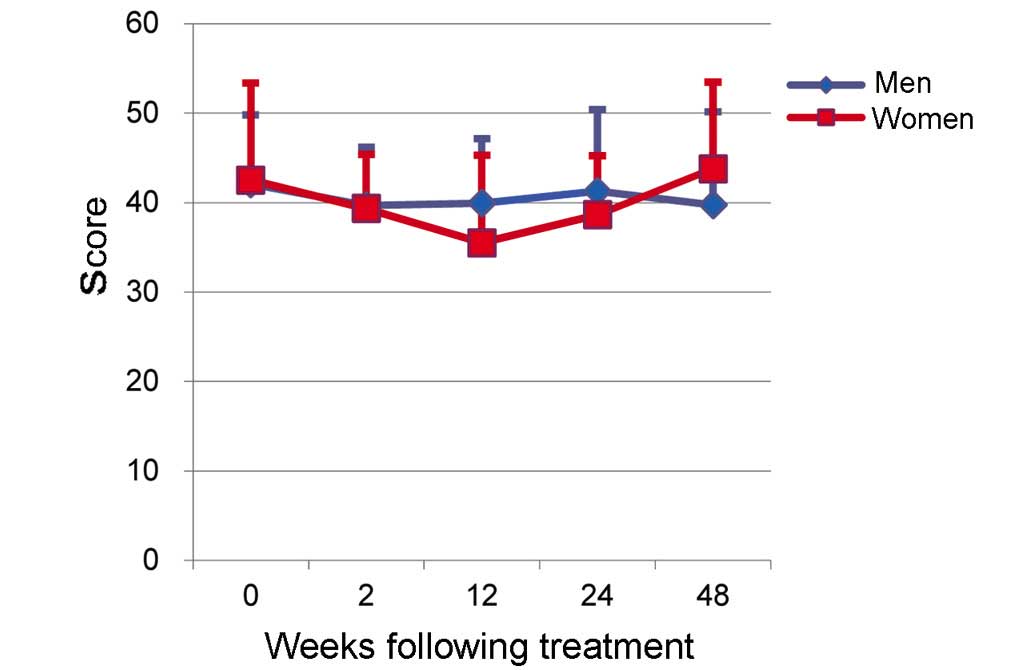
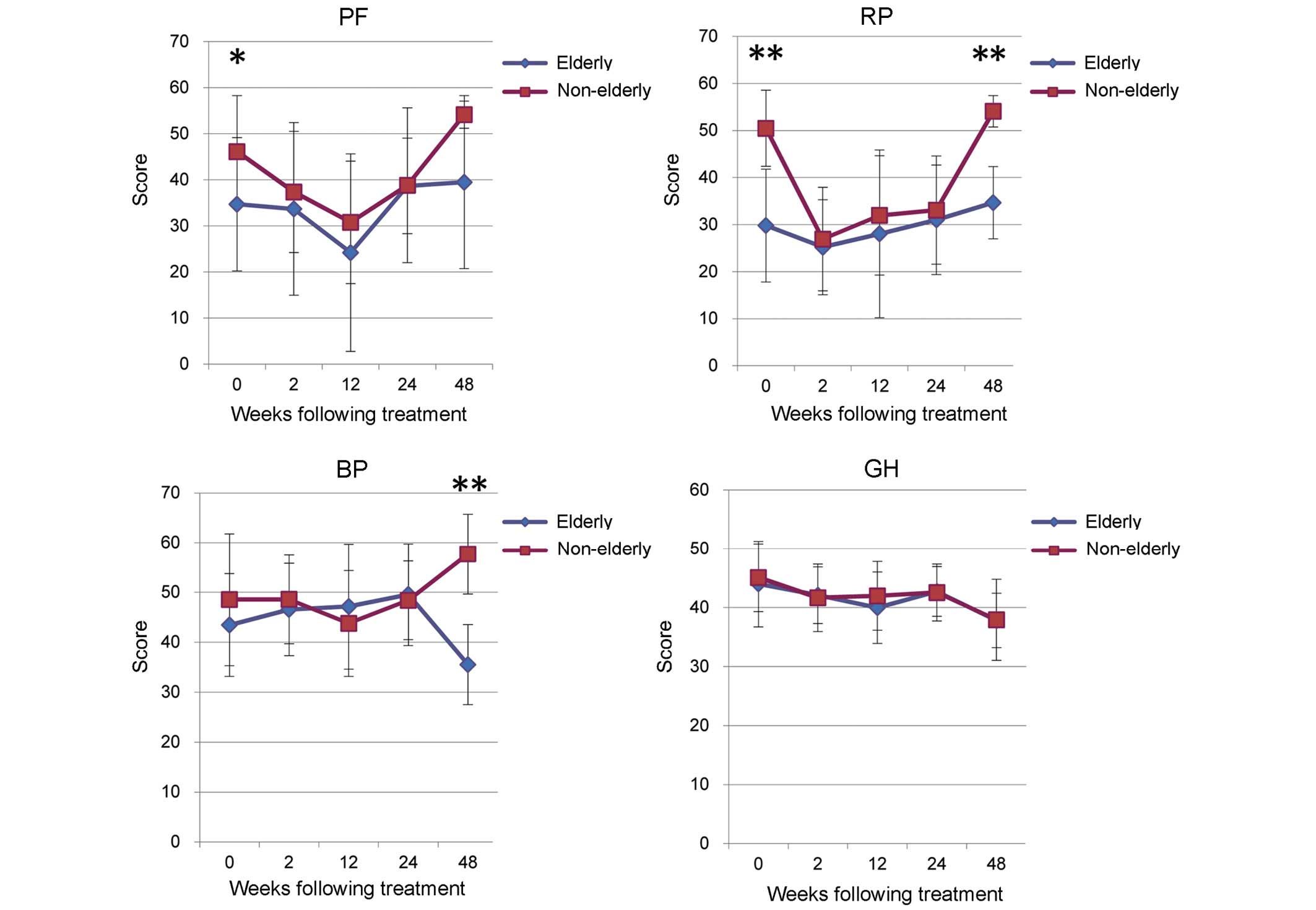
|
1
|
McHutchison JG, Everson GT, Gordon SC,
Jacobson IM, Sulkowski M, Kauffman R, McNair L, Alam J and Muir AJ:
PROVE1 Study Team: Telaprevir with peginterferon and ribavirin for
chronic HCV genotype 1 infection. N Engl J Med. 360:1827–1838.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hézode C, Forestier N, Dusheiko G, Ferenci
P, Pol S, Goeser T, Bronowicki JP, Bourlière M, Gharakhanian S,
Bengtsson L, et al: Telaprevir and peginterferon with or without
ribavirin for chronic HCV infection. N Engl J Med. 360:1839–1850.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
McHutchison JG, Manns MP, Muir AJ,
Terrault NA, Jacobson IM, Afdhal NH, Heathcote EJ, Zeuzem S,
Reesink HW, Garg J, et al: Telaprevir for previously treated
chronic HCV infection. N Engl J Med. 362:1292–1303. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kinder M: The lived experience of
treatment for hepatitis C. Gastroenterol Nurs. 32:401–408. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Liu J, Lin CS, Hu SH, Liang ML, Zhao ZX
and Gao ZL: Quality of life in patients with chronic hepatitis C
after PEG-Interferon a-2a treatment. Zhonghua Gan Zang Bing Za Zhi.
19:890–893. 2011.(In Chinese). PubMed/NCBI
|
|
6
|
Marcellin P, Chousterman M, Fontanges T,
Ouzan D, Rotily M, Varastet M, Lang JP, Melin P and Cacoub P:
CheObs Study Group: Adherence to treatment and quality of life
during hepatitis C therapy: A prospective, real-life, observational
study. Liver Int. 31:516–524. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ware JE, Snow KK, Kosinski M and Gandek B:
SF-36 Health Survey Manual and Interpretation Guide. The Health
Institute, New England Medical Center; Boston, MA: 1993
|
|
8
|
Aaronson NK, Acquadro C, Alonso J, Apolone
G, Bucquet D, Bullinger M, Bungay K, Fukuhara S, Gandek B and
Keller S: International quality of life assessment (IQOLA) project.
Qual Life Res. 1:349–351. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ware JE Jr and Gandek B: Overview of the
SF-36 health survey and the international quality of life
assessment (IQOLA) project. J Clin Epidemiol. 51:903–912. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fukuhara S, Bito S, Green J, Hsiao A and
Kurokawa K: Translation, adaptation, and validation of the SF-36
health survey for use in Japan. J Clin Epidemiol. 51:1037–1044.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fukuhara S and Suzukamo Y: Manual of
SF-36v2 Japanese version. Institute for Health Outcomes and Process
Evaluation Research; Kyoto: 2004
|
|
12
|
Foster GR, Goldin RD and Thomas HC:
Chronic hepatitis C virus infection causes a significant reduction
in quality of life in the absence of cirrhosis. Hepatology.
27:209–212. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Rodger AJ, Jolley D, Thompson SC, Lanigan
A and Crofts N: The impact of diagnosis of hepatitis C virus on
quality of life. Hepatology. 30:1299–1301. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Strauss E and Teixeira MC Dias: Quality of
life in hepatitis C. Liver Int. 26:755–765. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Younossi Z, Kallman J and Kincaid J: The
effects of HCV infection and management on health-related quality
of life. Hepatology. 45:806–816. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bonkovsky HL, Snow KK, Malet PF,
Back-Madruga C, Fontana RJ, Sterling RK, Kulig CC, Di Bisceglie AM,
Morgan TR, Dienstag JL, et al: Health-related quality of life in
patients with chronic hepatitis C and advanced fibrosis. J Hepatol.
46:420–431. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Teuber G, Schäfer A, Rimpel J, Paul K,
Keicher C, Scheurlen M, Zeuzem S and Kraus MR: Deterioration of
health-related quality of life and fatigue in patients with chronic
hepatitis C: Association with demographic factors, inflammatory
activity, and degree of fibrosis. J Hepatol. 49:923–929. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kumada H, Suzuki Y, Ikeda K, Toyota J,
Karino Y, Chayama K, Kawakami Y, Ido A, Yamamoto K, Takaguchi K, et
al: Daclatasvir plus asunaprevir for chronic HCV genotype 1b
infection. Hepatology. 59:2083–2091. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Spiegel BM, Younossi ZM, Hays RD, Revicki
D, Robbins S and Kanwal F: Impact of hepatitis C on health related
quality of life: A systematic review and quantitative assessment.
Hepatology. 41:790–800. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Björnsson E, Verbaan H, Oksanen A, Frydén
A, Johansson J, Friberg S, Dalgård O and Kalaitzakis E:
Health-related quality of life in patients with different stages of
liver disease induced by hepatitis C. Scand J Gastroenterol.
44:878–887. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kwan JW, Cronkite RC, Yiu A, Goldstein MK,
Kazis L and Cheung RC: The impact of chronic hepatitis C and
co-morbid illnesses on health-related quality of life. Qual Life
Res. 17:715–724. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kang SC, Hwang SJ, Lee SH, Chang FY and
Lee SD: Health-related quality of life and impact of antiviral
treatment in Chinese patients with chronic hepatitis C in Taiwan.
World J Gastroenterol. 11:7494–7498. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hollander A, Foster GR and Weiland O:
Health-related quality of life before, during and after combination
therapy with interferon and ribavirin in unselected Swedish
patients with chronic hepatitis C. Scand J Gastroenterol.
41:577–585. 2006. View Article : Google Scholar : PubMed/NCBI
|