Introduction
Osteoarthritis (OA) affects ~10% of the world
population >60 years old; it is difficult to treat joint disease
and it is associated with a heavy financial burden on families and
society (1). In developed countries,
the cost of OA treatment represents 1.0–2.5% of the gross domestic
product per year (2). To date, the
treatment of OA is primarily based on symptom management, such as
the use of non-steroidal anti-inflammatory drugs (NSAIDs) to
relieve pain (3). However, few
treatments have the proven ability to delay OA progression
(4).
Gene therapy may be a useful method to delay OA
progression (5), and it has been
studied for nearly 20 years (6). The
transferred gene could deliver gene products to the local area of
the joint in a sustained manner, which has fewer extra-articular
adverse effects (7). Currently, the
most prominent transgenes used in OA gene therapy are transforming
growth factor β1 (TGF-β1) (8) and
insulin-like growth factor-1 (IGF-1) (9), which primarily promote the regeneration
of cartilage. However, small interfering RNA (siRNA), which could
silence the effect of specific mRNA (10), may be another important tool in OA
gene therapy. The present study aimed to explore the biological
effect of Wnt5a-specific siRNA in OA.
It is understood that OA results from cartilage
degeneration. Specifically, the destruction of collagen type II
(COL2) in the cartilage matrix is an important initiating factor
for cartilage degeneration and OA progression. COL2 forms the
skeletal structure of cartilage, which provides structural and
biochemical support (11). As a
result of the poor self-repair ability of cartilage, preventing
COL2 degradation in cartilage injury is the key factor in
inhibiting further cartilage degeneration and OA progression
(12). Interleukin (IL)-1β is the
most important proinflammatory cytokine in the pathophysiology of
OA, which serves a key role in COL2 degradation (13,14). It
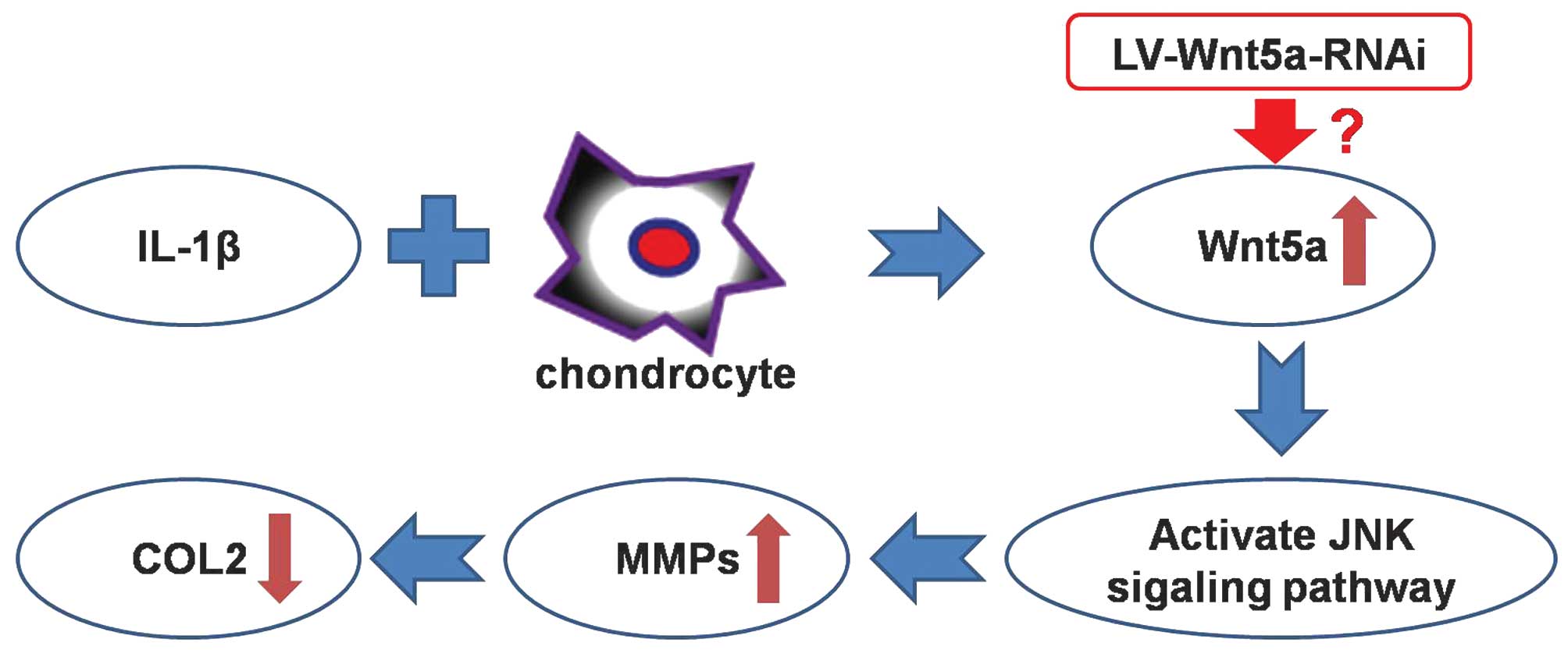
has been reported that IL-1β could upregulate the Wnt5a protein,
and the Wnt5a protein could activate the Jun amino-terminal kinase
(JNK) signaling pathway, inducing the upregulation of matrix
metalloproteinases (MMPs) (15,16).
Ultimately, the MMPs cause COL2 degradation and destruction, thus
inducing OA. That is to say, the Wnt5a protein is the core site of
IL-1β-induced COL2 degradation in chondrocytes (Fig. 1). Consequently, Wnt5a mRNA can be
chosen as a therapeutic target of siRNA.
The present study aimed to silence Wnt5a mRNA with
lentiviral vector-mediated Wnt5a-specific siRNA (LV-Wnt5a-RNAi) to
prevent COL2 degradation. OA-like chondrocyte injury was mimicked
using IL-1β in vitro, and LV-Wnt5a-RNAi was used to
transfect the OA-like chondrocytes. Following this, the efficiency
of Wnt5a mRNA silencing was determined. Finally, the expression of
COL2 proteins were determined to assess whether the silencing of
Wnt5a mRNA can prevent COL2 degradation in vitro.
Materials and methods
Articular chondrocyte culture and
identification
Chondrocytes from Sprague-Dawley rats (n=24 rats;
age, 9 weeks; weight, 180±12 g) were harvested according to a
previous study (17). These rats
were fed with standard laboratory food (containing 1.56% calcium,
0.8% phosphorus and 800 IU/kg vitamin D) and kept under a 12 h
light/dark cycle at 24°C and 50% humidity. After sacrifice with an
overdose of anesthetic (12 ml/kg 20% urethane; Hengyuan Biological
Technology Co., Ltd., Shanghai, China), the cartilage was sliced
into small pieces from the femoral trochlea and digested with 2.0
mg/ml type II collagenase (Invitrogen; Thermo Fisher Scientific,
Inc., Waltham, MA, USA) at 37°C for 4 h. Then, the digest solution
was filtered with 200 mesh-filtrating screen to remove large
fragments. The collected chondrocytes were seeded into culture
dishes and cultured with Dulbecco's modified Eagle's medium (DMEM;
Hyclone, GE Healthcare Life Sciences, Logan, UT, USA) supplemented
with 15% fetal bovine serum (Hyclone), and 50 U/ml penicillin and
streptomycin. At 90% confluence, the chondrocytes were passaged;
chondrocytes of passage 2 were used, as in a previous study
(18). All animal experiments were
approved by the ethics committee of Shandong Provincial Hospital
Affiliated to Shandong University, China, and complying with the
‘Guide for the Care and Use of Laboratory Animals’ published by the
National Academy Press (NIH Publication no. 85–23, revised
1996).
To verify that the cultured cells were chondrocytes,
the Toluidine Blue staining for sulfated glycosaminoglycans (GAGs)
and immunohistochemical staining for COL2 were performed according
to previous studies, respectively (19,20). The
GAGs and COL2 were specific markers of hyaline cartilage.
To mimic the OA-like chondrocyte injury in
vitro, IL-1β was used, as previously described (21). The chondrocytes of passage 2 were
cultivated with serum-free DMEM for 24 h, and stimulated with
medium containing 10 ng/ml IL-1β for 0, 1, 6, 12 or 24 h,
respectively, as previously described (22).
To assess whether the OA-like chondrocytes were
successfully generated, reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) was performed to determine the
gene expression of Wnt5a at different time points. Briefly, total
RNA was harvested from chondrocytes of different groups using 1 ml
TRIzol reagent (Takara Bio, Inc., Otsu, Japan). Subsequently, 1 µg
total RNA was reverse transcribed in each group using PrimeScript
RT Reagent Kit with gDNA Eraser (Takara Bio, Inc., Otsu, Japan),
under incubation conditions of 37°C for 15 min, followed by 85°C
for 5 sec. The primer sequences of Wnt5a and β-actin are reported
in Table I. RT-qPCR was performed on
an Applied Biosystems 7300 Real-Time PCR System (Applied
Biosystems; Thermo Fisher Scientific, Inc.) with the amplification
program as follows: Initial denaturation at 95°C for 10 min;
followed by denaturation at 95°C for 5 sec, annealing at 55°C for
30 sec and extension at 72°C for 30 sec for a total of 40 cycles;
and a final extension at 60°C for 1 min. The total PCR reaction
volume was 15 µl, including 1 µl cDNA, 7.5 µl SYBR®
Green PCR Master Mix (Toyobo Co., Ltd., Osaka, Japan), 1 µl of each
primer and 5.5 µl sterile water. The expression of Wnt5a relative
to β-actin was calculated with the 2−∆∆Cq method
(23) using SPSS v. 19.0 software
(IBM SPSS, Armonk, NY, USA). This procedure was performed in
triplicate for each gene, with each reaction also performed without
reverse transcriptase as a negative control.
 | Table I.Primer sequence of reverse
transcription-quantitative polymerase chain reaction. |
Table I.
Primer sequence of reverse
transcription-quantitative polymerase chain reaction.
| Gene | Primer sequence |
|---|
| Wnt5a |
|
|
Forward |
5′-TGTGGTTTAATGGTGCCTGA-3′ |
|
Reverse |
5′-TTCGTCGTGCTCAAGGTATG-3′ |
| β-actin |
|
|
Forward |
5′-CTAAGGCCAACCGTGAAAAG-3′ |
|
Reverse |
5′-AACACAGCCTGGATGGCTAC-3′ |
To verify that the OA-like chondrocytes were viable
and could be used in the present study, Live-Dead staining was
performed according to the manufacturer's instructions (Invitrogen;
Thermo Fisher Scientific, Inc.) and as previously described
(24). Briefly, the OA-like
chondrocytes were cultivated with reagent containing 2 mM calcein
AM and 4 mM ethidium homodimer-1 for 30 min at 37°C. After washing
with phosphate-buffed saline (PBS), the specimens were assessed
using a fluorescence microscope with 488 or 568 nm excitation. Live
cells were colored green by calcein AM, while dead cells were
colored red by ethidium homodimer-1.
Lentiviral vector packaging and
transfection
The siRNA of Wnt-5a was designed and packaged into
the LV-Wnt5a-RNAi by Genechem Co., Ltd. (Shanghai, China). The
operation process of lentiviral vector formation followed the
recommendations of the manufacturer.
In Wnt-5a mRNA silencing, the passage 2 chondrocytes
were stimulated for 6 h with IL-1β as previously described
(25). The OA-like chondrocytes were
divided into three groups: Group I, incubated with complete DMEM
for 7 days; group II, incubated with complete DMEM supplemented
with empty lentiviral vector for 7 days; group III, incubated with
complete DMEM supplemented with LV-Wnt5a-RNAi for 7 days. The
concentration of empty lentiviral vector in group II was the same
with the concentration of LV-Wnt5a-RNAi in group III, which ensured
that the multiplicity-of-infection (MOI) was 50.
Western blotting analysis of COL2
Western blotting analysis was performed to determine
whether the silencing of Wnt5a mRNA could prevent COL2 degradation
in OA-like chondrocytes. Protein was harvested from the cells using
lysis buffer (Beyotime Institute of Biotechnology, Haimen, China)
and the protein concentration was determined using a BCA protein
assay kit (Pierce Biotechnology, Inc., Rockford, IL, USA). Protein
(30 µg from each sample) was run on 10% sodium dodceyl
sulfate-polyacrylamide gels and electrotransferred onto
nitrocellulose membranes. Subsequently, the membranes were blocked
with 5% bovine serum albumin (BSA; Sigma-Aldrich; Merck Millipore,
Darmstadt, Germany) for 1 h at room temperature. The blots were
probed with monoclonal mouse anti-collagen II (cat. no. CP18;
Calbiochem; Merck Millipore; 1:300 dilution) and monoclonal mouse
anti-GAPDH (cat. no. AG019; Beyotime Institute of Biotechnology,
Haimen, China, 1:500 dilution) antibodies overnight at 4°C, then
incubated with a 1:4,000 dilution of goat anti-mouse, conjugated to
horseradish peroxidase (HRP) (cat. no. sc-2005; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA; 1:1,000 dilution) for 1 h at
room temperature. The blots were visualized using the enhanced
chemiluminescence method according to the manufacturer's
recommendations (EMD Millipore, Billerica, MA, USA). GAPDH was used
as an internal control. Finally, the band density values were
quantified with ImageJ v. 1.48 software (www.imagej.nih.gov/ij/).
Immunohistochemical analysis of
COL2
To determine the COL2 protein expression in OA-like
chondrocytes following the silencing of Wnt5a mRNA,
immunohistochemical staining was performed, according to the
previous report (26). Briefly, the
chondrocytes of the three groups were fixed with 4%
paraformaldehyde and blocked with 5% BSA in PBS. Then, the samples
were incubated overnight at 4°C with anti-collagen II antibody (as
with the Western blot). After three PBS washes, the cells were
immersed in polyclonal goat anti-mouse secondary antibody (cat. no.
PV-9002, Polink-2 plus Polymer HRP Detection System; Zhongshan
Goldenbridge Co. Ltd., Beijing, China) at 37°C for 1 h. Following
another round of washing, 3,3′-diaminobenzidine reagent (Zhongshan
Golden Bridge Biotechnology Co., Ltd., Beijing, China) was used to
determine the immunoactivity, and then the cell nuclei were
counterstained with hematoxylin. The samples were observed under a
light microscope, and the integrated optical density of different
images were analyzed with Image-Pro Plus version 6.0 software
(Media Cybernetics, Inc., Rockville, MD, USA).
Statistical analysis
All experiments were performed in triplicate, and
the results were analyzed using one-way analysis of variance,
followed by Tukey's test. SPSS software version 19.0 (IBM SPSS,
Armonk, NY, USA) was used to perform the statistical analysis. The
data were presented as mean ± standard deviation. P<0.05 were
considered to indicate a statistically significant difference.
Results
Identification of chondrocytes
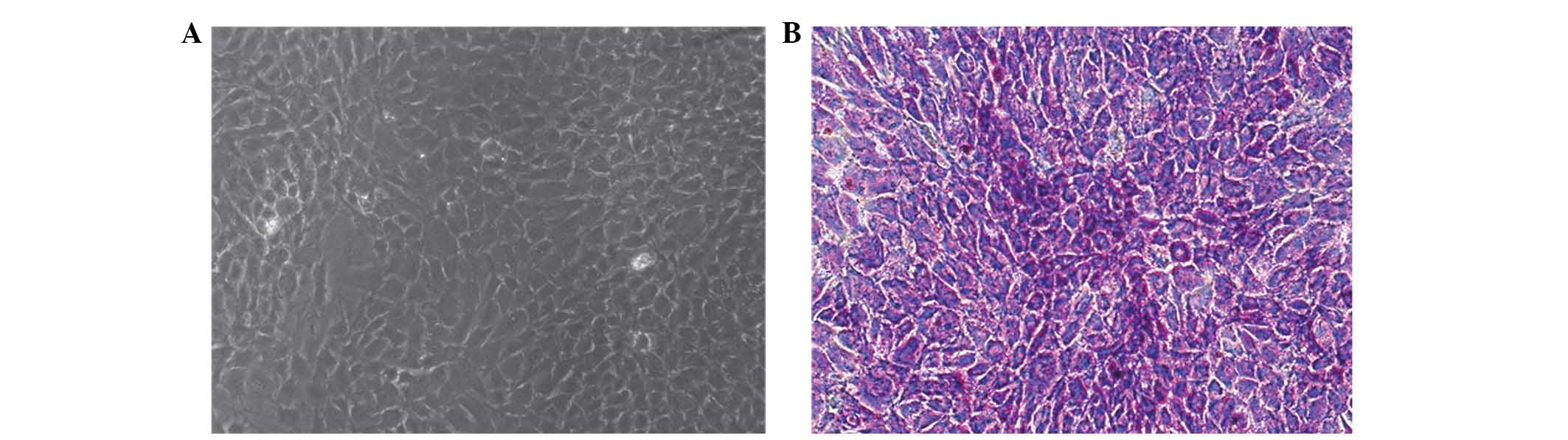
As shown in Fig. 2A,
the cultured cells of passage 2 were not elongated, which indicated
that the cultured cells were chondrocytes. Furthermore, the
Toluidine Blue staining confirmed that the passage 2 cells were
chondrocytes (Fig. 2B).
Consequently, the chondrocytes were successfully cultured, which
resulted in approval for the follow-up study.
Properties and bioactivity of OA-like
chondrocytes
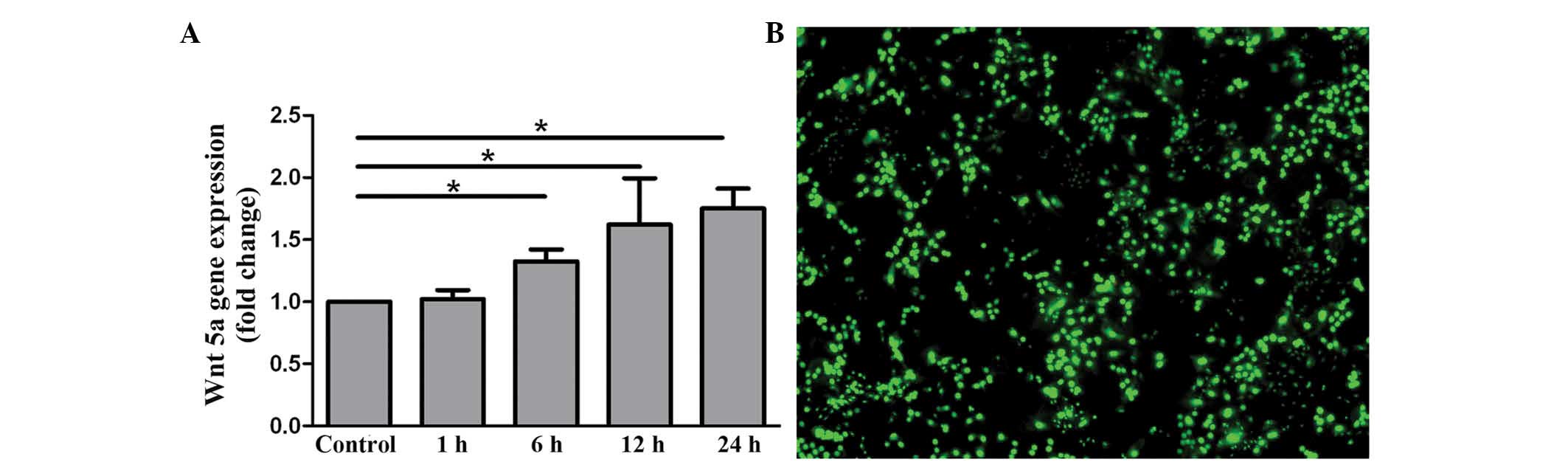
RT-qPCR was performed to determine whether the
OA-like chondrocytes were successfully induced with IL-1β. The data
in Fig. 3A illustrates that the mRNA
of Wnt5a was upregulated in a time-dependent manner. The results
indicate that the OA-like chondrocytes were successfully induced
with IL-1β.
The bioactivity of OA-like chondrocytes were
assessed using Live-Dead staining. As shown in Fig. 3B, although the inflammatory reaction
was activated in the OA-like chondrocytes due to the stimulation of
IL-1β, the cells remained viable. From these data, it can be
concluded that the OA-like chondrocytes stimulated with IL-1β for 6
h could be used in subsequent studies of Wnt5a silencing.
LV-Wnt5a-RNAi transfected chondrocytes
and Wnt5a mRNA silencing
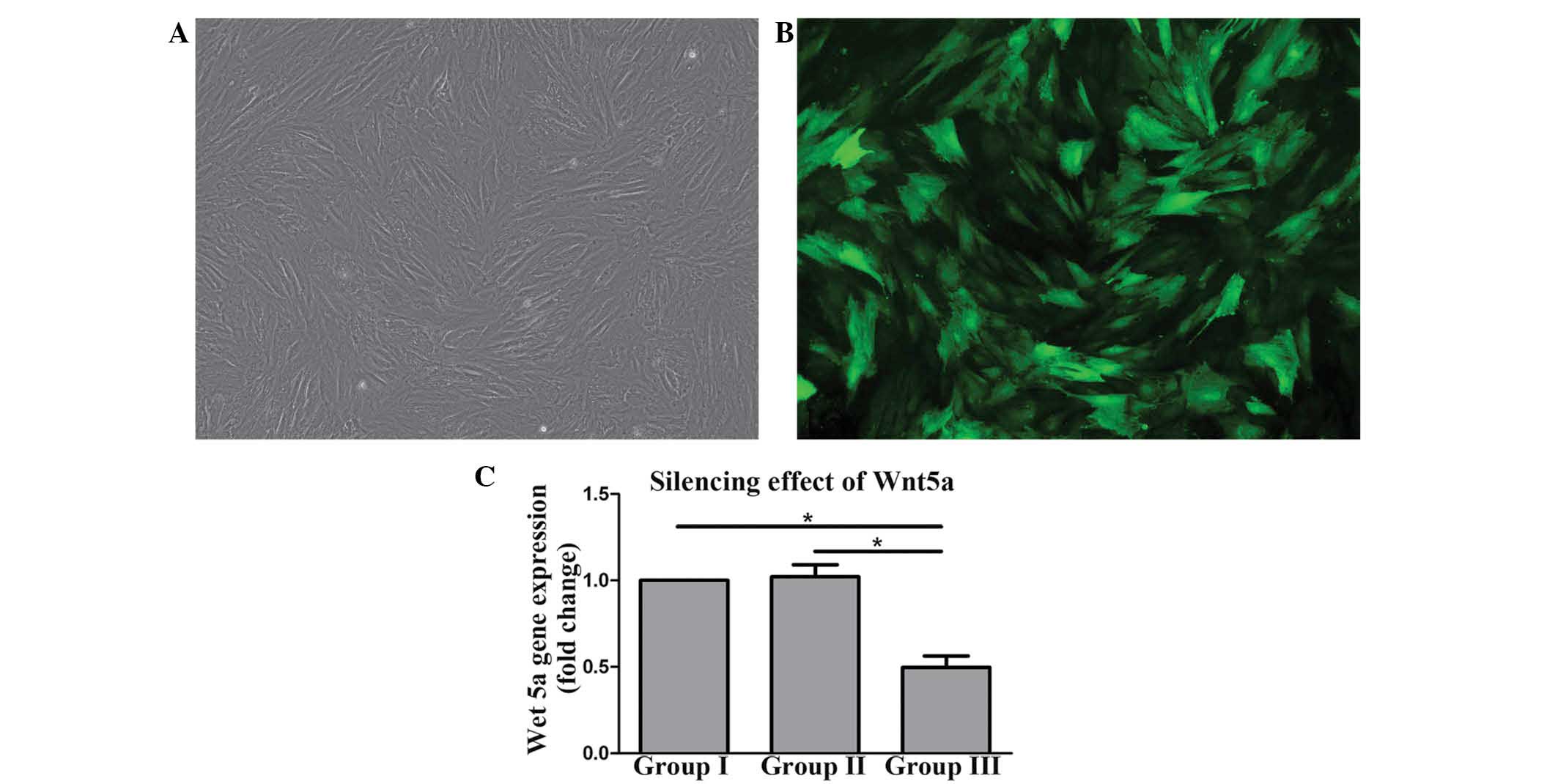
After 7 days of incubation, the LV-Wnt5a-RNAi could
be transfected into the OA-like chondrocytes with MOI = 50
(Fig. 4A), as determined by
fluorescence microscope (Fig.
4B).
RT-qPCR demonstrated that Wnt5a mRNA in group III
was significantly lower compared with that in groups I and II
(P<0.05), while there was no significant difference between
groups I and II (Fig. 4C). In
conclusion, the LV-Wnt5a-RNAi used in group III could silence the
Wnt5a mRNA expressed by the OA-like chondrocytes.
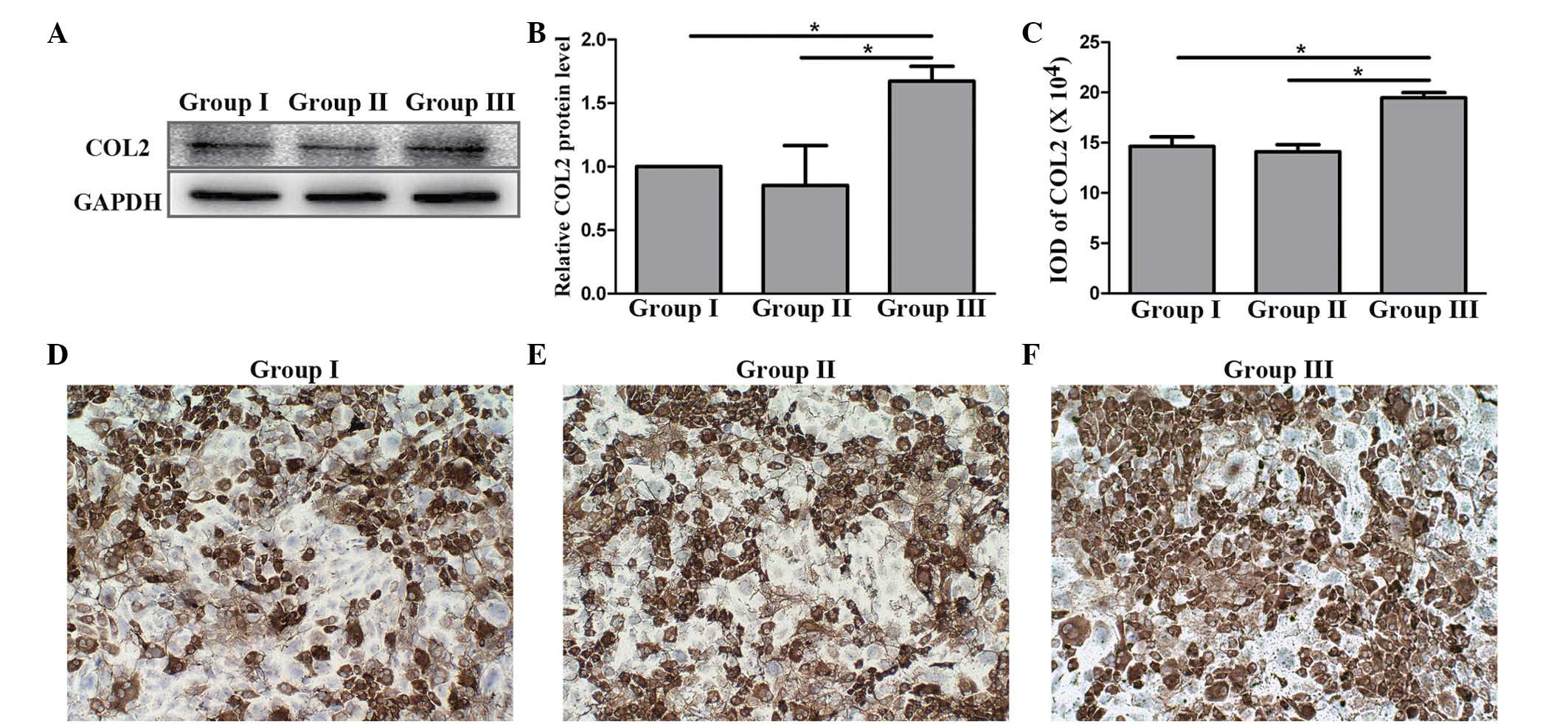
Preventing COL2 degradation by
silencing Wnt5a mRNA
To determine whether the silencing of Wnt5a by
LV-Wnt5a-RNAi could prevent COL2 degradation, COL2 proteins
expressed in the three groups were assessed using western blotting
and immunohistochemical analysis. The results of western blotting
indicated that the COL2 content in group III was significantly
higher compared with that in groups I and II (P<0.05; Fig. 5A and B), while no significant
difference were observed between groups I and II. In the
immunohistochemical analysis, the immunoactivity of group III was
the highest among the three groups (Fig.
5C-F); this is consistent with the results of western blotting
analysis. From these data, it can be concluded that the silencing
of Wnt5a mRNA may prevent COL2 protein degradation.
Discussion
OA is a degenerative joint disease which affects a
large number of individuals worldwide (1). In developed countries, the cost of OA
treatment is about 1.0–2.5% of gross domestic product per year
(2). To date, no highly effective
drug can delay OA progression, because the existing treatment of OA
is primarily based on symptom management, such as the use of NSAIDs
to relieve pain (7). Gene therapy
may be a useful method to delay OA progression, which has been
studied for >20 years (6). The
gene therapy of OA could be more effective and less expensive than
the existing method, and be associated with fewer extra-articular
adverse effects (10). Currently,
the most prominent gene therapy of OA is the transgene of TGF-β1
(8) and IGF-1 (9), which primarily promote the regeneration
of cartilage. However, siRNA may be another useful tool of gene
therapy in OA and may silence the biological effects of specific
mRNA (10). For example, Chen et
al (27) used the adenoviral
vector-mediated nuclear factor-κB p65-specific siRNA to alleviate
inflammation of the synovium in OA.
It is understood that IL-1β is the most important
proinflammatory cytokine in the pathophysiology of OA. IL-1β may
upregulate the Wnt5a protein, and therefore activate the JNK
signaling pathway to increase the expression of MMPs. MMPs result
in the degradation and destruction of COL2, thus inducing OA
(15,16). That is to say, the Wnt5a protein is
the core site for IL-1β-induced COL2 degradation in OA.
Consequently, the silencing of Wnt5a mRNA was chosen as the
therapeutic target of Wnt5a-specific siRNA to prevent COL2
degradation in the present study.
The Wnt5a-specific siRNA was packaged in a
lentiviral vector to improve the transfection efficiency. Previous
studies have reported that the lentiviral vector is an effective
siRNA delivery system, which can protect the enclosed siRNA and
transport the siRNA to targeted cells (10). In the current study, green
fluorescence could be observed in the majority of the chondrocytes,
as shown in Fig. 4B, which indicated
that the transfection efficiency of LV-Wnt5a-RNAi was excellent and
the MOI used was appropriate.
The Wnt5a mRNA was silenced at least in part by
LV-Wnt5a-RNAi, since the expression of Wnt5a mRNA in group III was
significantly lower compared with that in groups I and II (Fig. 4C). With the action of LV-Wnt5a-RNAi,
the Wnt5a mRNA becomes the component of RNA-induced silencing
complexes (28). As a result, the
Wnt5a mRNA is silenced and loses its biological activity.
To further explore whether silencing Wnt5a mRNA with
LV-Wnt5a-RNAi can prevent COL2 degradation, the synthesis of COL2
was determined in the three groups. As shown in Fig. 5, the content of COL2 in group III was
significantly higher compared with that in groups I and II. These
results illustrate that the silencing of Wnt5a may prevent the
degradation of COL2, the underlying mechanism being the silencing
of Wnt5a reducing the synthesis of Wnt5a protein. The decrease of
Wnt5a protein may reduce the activation of the JNK sigaling
pathway, further inducing the downregulation of MMPs (15,16).
Consequently, the silencing of Wnt5a may protect COL2 from
degradation in vitro, which may be a useful method of
treating OA. Further animal experiments should be performed in
future studies to fully assess the protection of COL2 by the
silencing of Wnt5a mRNA.
In conclusion, the present constructed
LV-Wnt5a-RNAi, which is siRNA of Wnt-5a packaged into a lentiviral
vector. The LV-Wnt5a-RNAi could successfully silence the mRNA of
Wnt5a. This silencing of Wnt5a mRNA may prevent the degradation of
COL2, which is the key component in cartilage matrix. Therefore,
LV-Wnt5a-RNAi may be a useful tool to prevent the progression of
OA.
Acknowledgements
The present work was supported by grants from the
National Natural Science Foundation of China (grant no. 30672115)
and the Science and Technology Development Plan of Shandong
Province (grant no. 2012GSF21809).
References
|
1
|
Ni GX, Li Z and Zhou YZ: The role of small
leucine-rich proteoglycans in osteoarthritis pathogenesis.
Osteoarthr Cartil. 22:896–903. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hiligsmann M, Cooper C, Arden N, Boers M,
Branco JC, Brandi M Luisa, Bruyère O, Guillemin F, Hochberg MC,
Hunter DJ, et al: Health economics in the field of osteoarthritis:
An expert's consensus paper from the European Society for Clinical
and Economic Aspects of Osteoporosis and Osteoarthritis (ESCEO).
Semin Arthritis Rheum. 43:303–313. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cutolo M, Berenbaum F, Hochberg M, Punzi L
and Reginster JY: Commentary on recent therapeutic guidelines for
osteoarthritis. Semin Arthritis Rheum. 44:611–617. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Glyn-Jones S, Palmer AJ, Agricola R,
Prince AJ, Vincent TL, Weinans H and Carr AJ: Osteoarthritis.
Lancet. 386:376–387. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Evans CH and Huard J: Gene therapy
approaches to regenerating the musculoskeletal system. Nat Rev
Rheumatol. 11:234–242. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bandara G, Robbins PD, Georgescu HI,
Mueller GM, Glorioso JC and Evans CH: Gene transfer to
synoviocytes: Prospects for gene treatment of arthritis. DNA Cell
Biol. 11:227–231. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Evans CH, Ghivizzani SC and Robbins PD:
Getting arthritis gene therapy into the clinic. Nat Rev Rheumatol.
7:244–249. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ivkovic A, Pascher A, Hudetz D, Maticic D,
Jelic M, Dickinson S, Loparic M, Haspl M, Windhager R and Pecina M:
Articular cartilage repair by genetically modified bone marrow
aspirate in sheep. Gene Ther. 17:779–789. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Haupt JL, Frisbie DD, McIlwraith CW,
Robbins PD, Ghvizzani S, Evans CH and Nixon AJ: Dual transduction
of insulin-like growth factor-I and interleukin-1 receptor
antagonist protein controls cartilage degradation in an
osteoarthritic culture model. J Orth Res. 23:118–126. 2005.
View Article : Google Scholar
|
|
10
|
Shi Q, Zhang XL, Dai KR, Benderdour M and
Fernandes JC: siRNA therapy for cancer and non-lethal diseases such
as arthritis and osteoporosis. Expert Opin Biol Ther. 11:5–16.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gao Y, Liu S, Huang J, Guo W, Chen J,
Zhang L, Zhao B, Peng J, Wang A, Wang Y, et al: The ECM-cell
interaction of cartilage extracellular matrix on chondrocytes.
BioMed Res Int. 2014:6484592014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tchetina EV, Squires G and Poole AR:
Increased type II collagen degradation and very early focal
cartilage degeneration is associated with upregulation of
chondrocyte differentiation related genes in early human articular
cartilage lesions. J Rheumatol. 32:876–886. 2005.PubMed/NCBI
|
|
13
|
Li Z, Meng D, Li G, Xu J, Tian K and Li Y:
Celecoxib combined with diacerein effectively alleviates
osteoarthritis in rats via regulating JNK and p38MAPK signaling
pathways. Inflammation. 38:1563–1572. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mabey T and Honsawek S: Cytokines as
biochemical markers for knee osteoarthritis. J Orthop. 6:95–105.
2015.
|
|
15
|
Ge X, Ma X, Meng J, Zhang C, Ma K and Zhou
C: Role of Wnt-5A in interleukin-1beta-induced matrix
metalloproteinase expression in rabbit temporomandibular joint
condylar chondrocytes. Arthritis Rheum. 60:2714–2722. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ryu JH and Chun JS: Opposing roles of
WNT-5A and WNT-11 in interleukin-1beta regulation of type II
collagen expression in articular chondrocytes. J Biol Chem.
281:22039–22047. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yu DG, Ding HF, Mao YQ, Liu M, Yu B, Zhao
X, Wang XQ, Li Y, Liu GW, Nie SB, et al: Strontium ranelate reduces
cartilage degeneration and subchondral bone remodeling in rat
osteoarthritis model. Acta Pharmacol Sin. 34:393–402. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang PY and Tsai WB: Modulation of the
proliferation and matrix synthesis of chondrocytes by dynamic
compression on genipin-crosslinked chitosan/collagen scaffolds. J
Biomater Sci Polym Ed. 24:507–519. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yu L, Weng Y, Shui X, Fang W, Zhang E and
Pan J: Multipotent Adult Progenitor Cells from Bone Marrow
Differentiate into Chondrocyte-Like Cells. J Arthroplasty.
30:1273–1276. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tekari A, Luginbuehl R, Hofstetter W and
Egli RJ: Chondrocytes expressing intracellular collagen type II
enter the cell cycle and co-express collagen type I in monolayer
culture. J Orthop Res. 32:1503–1511. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chen WP and Wu LD: Chlorogenic acid
suppresses interleukin-1β-induced inflammatory mediators in human
chondrocytes. Int J Clin Exp Pathol. 7:8797–8801. 2014.PubMed/NCBI
|
|
22
|
Tao R, Wang S, Xia X, Wang Y, Cao Y, Huang
Y, Xu X, Liu Z, Liu P, Tang X, et al: Pyrroloquinoline Quinone
Slows Down the Progression of Osteoarthritis by Inhibiting Nitric
Oxide Production and Metalloproteinase Synthesis. Inflammation.
38:1546–1555. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Xu HG, Zhang XH, Wang H, Liu P, Wang LT,
Zuo CJ, Tong WX and Zhang XL: Intermittent Cyclic Mechanical
Tension-Induced Calcification and downregulation of ankh gene
expression of end plate chondrocytes. Spine. 37:1192–1197. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lu J, Sun Y, Ge Q, Teng H and Jiang Q:
Histone deacetylase 4 alters cartilage homeostasis in human
osteoarthritis. BMC Musculoskelet Disord. 15:4382014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wu PC, Tsai CL, Gordon GM, Jeong S,
Itakura T, Patel N, Shi S and Fini ME: Chondrogenesis in scleral
stem/progenitor cells and its association with form-deprived myopia
in mice. Mol Vision. 21:138–147. 2015.
|
|
27
|
Chen LX, Lin L, Wang HJ, Wei XL, Fu X,
Zhang JY and Yu CL: Suppression of early experimental
osteoarthritis by in vivo delivery of the adenoviral
vector-mediated NF-kappaBp65-specific siRNA. Osteoarthritis
Cartilage. 16:174–184. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hammond SM, Bernstein E, Beach D and
Hannon GJ: An RNA-directed nuclease mediates post-transcriptional
gene silencing in Drosophila cells. Nature. 404:293–296. 2000.
View Article : Google Scholar : PubMed/NCBI
|