Introduction
Ischemia/reperfusion (I/R)-induced liver damage is a
major complication following hemorrhagic shock, liver surgery and
transplantation (1). Therefore, the
development of effective strategies to treat hepatic I/R damage is
important. Recently, a number of mechanisms have been shown to be
involved in the process of I/R-induced injury in the liver,
including oxidative stress and hepatic cell apoptosis (2,3). These
mechanisms are supported by research demonstrating that the
inhibition of oxidative stress and hepatic cell apoptosis is
effective in preventing I/R-induced injury in the liver (4,5).
Tea polyphenols (TPs) are the primary active
ingredients in green tea and are strong antioxidants that exert a
significant free radical scavenging activity (6). A number of studies have demonstrated
that TP can protect against oxidative stress in a number of organs,
including the bones, liver and kidney (7–9);
Yokozawa et al (9) reported
that TP has a protective effect against renal damage caused by
oxidative stress. It has also been demonstrated that TP can improve
deficits in spatial cognitive ability resulting from cerebral
hypoperfusion (10). Furthermore, TP
has been observed to serve a protective role against apoptosis
(11), and Xue et al
(12) suggested that TP may
attenuate neurocognitive impairment caused by global cerebral I/R
injury via its anti-apoptotic properties. The role of TP in the
protection of liver tissue against I/R-induced damage has been
previously proposed. For instance, Zhong et al (13) demonstrated that green tea extract
containing polyphenolic free radical scavengers prevented
I/R-induced injury in the liver of rats. However, the specific
mechanism remains uncertain.
In the present study, the mechanism underlying the
protective effect of TPs against I/R-induced liver injury in mice
was investigated, in particular focusing on its anti-oxidative and
anti-apoptotic properties.
Materials and methods
Animals and ethical approval
The present study was approved by the Ethics
Committee of Xinxiang Central Hospital (Xinxiang, China). Each
experiment was performed in accordance with protocols set out by
the Guidelines for the Care and Use of Experimental Animals
(14). A total of 20 male C57BL/6
mice (Cavens Laboratory Animals Co., Ltd., (Changzhou, China), aged
12 weeks and weighing ~25 g, were used in the present study. Mice
were housed in a laminar flow, temperature-controlled (22±1°C),
pathogen-free environment with a 12-h light/dark cycle and ad
libitum access to food and water at the Experimental Animal
Center of Xinxiang Medical School. Mice were fasted for 24 h prior
to the experiments.
Pretreatment with TP
TP was purchased from Sigma-Aldrich (St. Louis, MO,
USA) and was dissolved in saline according to the manufacturer's
instructions. Mice were divided into four equal groups (n=5) as
follows: Saline-treated sham surgery mice (saline + sham);
TP-treated sham surgery mice (TP + sham); saline-treated I/R injury
mice (saline + I/R); and TP-treated I/R injury mice (TP + I/R).
Saline or TP (50 mg/kg) was orally administered 1 h prior to
surgery.
Induction of hepatic I/R injury
An intraperitoneal injection of pentobarbital (50
mg/kg; Kehaojia Biological Technology, Wuhan, China) was used to
anesthetize the animals. To induce I/R injury in the liver of the
mice, a transverse incision was made to the abdomen and a micro
clip (Hailunwentai, Shenzhen, China) was used to clamp the left
branches of the portal vein and hepatic artery for 30 min. Next,
the clamp was removed and the wound was closed. In the sham surgery
group, the same procedure was performed but the vessel was not
occluded. The liver tissue and blood of mice were collected 6 h
after the surgery.
Measurement of alanine
aminotransferase (ALT) and aspartate aminotransferase (AST) serum
activity
Blood was collected from the mice in each group. The
Mouse Alanine Aminotransferase ELISA kit (MAK052) and the Mouse
Aspartate Aminotransferase kit (MAK055; both Sigma-Aldrich) were
used to determine the activity of serum ALT and AST, respectively,
in accordance with the manufacturer's instructions.
Measurement of hepatic glutathione
(GSH)
Hepatic GSH and oxidized GSH (GSSG) levels were
measured using a GSH and GSSG Assay kit (Beyotime Institute of
Biotechnology, Shanghai, China). Following precipitation with 1%
picric acid (Jinhao, Shanghai, China), the level of glutathione
(GSH) was determined in liver homogenates using yeast-GSH
reductase, 5,5′-Dithio-bis(2-nitrobenzoic acid) and NADPH (both
Beyotime Institute of Biotechnology), and the absorbance was
recorded at a wavelength of 412 nm using an ELx800 microplate
reader (Biotek Instruments, Inc., Winooski, VT, USA), according to
the manufacturer's protocol. The expression of GSSG in the presence
of 2-vinylpyridine (Jinhao) was recorded using the same method. The
ratio of GSH:GSSG was then calculated.
Flow cytometry
Flow cytometry was used to determine cell apoptosis
using an Annexin-V-FITC Apoptosis Detection Kit I (BD Biosciences,
Franklin Lake, NJ, USA). Briefly, hepatic cells were washed twice
with cold PBS, and 106 cells were subsequently
resuspended in 200 µl binding buffer supplemented with 10 µl
Annexin-V-FITC and 5 µl propidium iodide-phycoerythrin for
incubation in the dark for 30 min. Following incubation, the cells
were supplemented with 300 µl binding buffer and analyzed using a
C6 flow cytometer (BD Biosciences).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from liver tissue using
TRIzol reagent (Thermo Fisher Scientific, Inc., Waltham, MA, USA)
according to the manufacturer's instructions. RT and qPCR detection
was performed using a SYBR Green RT PCR kit (Takara Bio, Inc.,
Otsu, Japan), according to the manufacturer's protocol. Reverse
transcription was performed at 16°C for 30 min, followed by an
incubation step at 42°C for 30 min and enzyme inactivation at 85°C
for 5 min. Negative control (no cDNA) and RT control (no reverse
transcription) were used. qPCR was performed to a final reaction
volume of 20 µl containing 0.5 µl cDNA, 10 µl PCR master mix
(Takara Bio, Inc.), 2 µl forward and reverse primers and 7.5 µl
H2O. PCR cycling conditions were as follows: 95°C for 5
min, followed by 45 cycles of denaturation at 95°C for 15 sec and
annealing/elongation at 60°C for 30 sec. Specific primers were
purchased from Sangon Biotech Co., Ltd., (Shanghai, China) as
follows: Inducible nitric oxide synthase (iNOS) sense,
5′GTTCTCAGCCCAACAATACAAGA'3, and anti-sense,
5′GTGGACGGGTCGATGTCAC'3; B-cell lymphoma 2 (Bcl-2) sense,
5′ATGCCTTTGTGGAACTATATGGC'3, and anti-sense,
5′GGTATGCACCCAGAGTGATGC'3; Bcl-2-associated X protein (Bax) sense,
5′TGAAGACAGGGGCCTTTTTG'3 and anti-sense, 5′AATTCGCCGGAGACACTCG'3;
and GAPDH sense, 5′AGGTCGGTGTGAACGGATTTG'3, and anti-sense,
5′TGTAGACCATGTAGTTGAGGTCA'3. GAPDH was used as an internal control.
The relative expression of mRNA was quantified using GraphPad Prism
version 4.0 (GraphPad Software, Inc., La Jolla, CA, USA) and the
2−ΔΔCq method (15).
Western blot analysis
Total protein was extracted from the liver tissues
in each group using a radioimmunoprecipitation assay solution
(Sigma-Aldrich). The protein concentration was determined using a
Bradford DC protein assay (Bio-Rad Laboratories, Inc., Hercules,
CA, USA). To determine the expression level, protein (20 µg) was
separated using 10% sodium dodecyl sulfate-polyacrylamide gel
electrophoresis, transferred to a polyvinylidene difluoride (PVDF)
membrane (Thermo Fisher Scientific, Inc.) and incubated in
Tris-buffered saline with Tween 20 (TBST; Sigma-Aldrich) and 50 g/l
skimmed milk at room temperature for 3 h. The PVDF membrane was
then incubated at room temperature for 3 h with the following
rabbit monoclonal primary antibodies (all from Abcam, Cambridge,
MA, USA): Anti-iNOS (1:100; ab15323), anti-Bax (1:50; ab32503),
anti-cytochrome c (1:100; ab133504), anti-Bcl-2 (1:200;
ab32124) or anti-GAPDH (1:200; ab8245). Next, the PVDF membranes
were washed with TBST three times and then incubated with a mouse
anti-rabbit secondary antibody (1:20,000; ab99697; Abcam) at room
temperature for 1 h. An enhanced chemiluminescence kit (Pierce
Biotechnology, Rockford, IL, USA) was used to perform
chemiluminescent detection. Results were quantified using ImageJ
software (National Institutes of Health, Bethesda, MA, USA).
Measurement of caspase-3 activity
The activity of caspase-3 was determined using a
Caspase-3 Colorimetric Assay kit (BioVision, Inc., Milpitas, CA,
USA), according to the manufacturer's instructions. Protein (20 µg)
from liver tissues was incubated in the solution buffer provided
with the kit at room temperature for 30 min. Next, 200 µM
N-acetyl-Asp-Glu-Val-Asp-7-amino-(4-trifluoromethyl)-coumarin was
added and the samples were incubated at 37°C for 2 h. The
absorbance was measured spectrophotometrically at 400 nm using an
ELx800 microplate reader.
Statistical analysis
The mean ± standard error of the data was calculated
and analyzed using one-way analysis of variance. SPSS version 17.0
(SPSS, Inc., Chicago, IL, USA) was used to perform statistical
analyses. P<0.05 was considered to indicate a statistically
significant difference.
Results
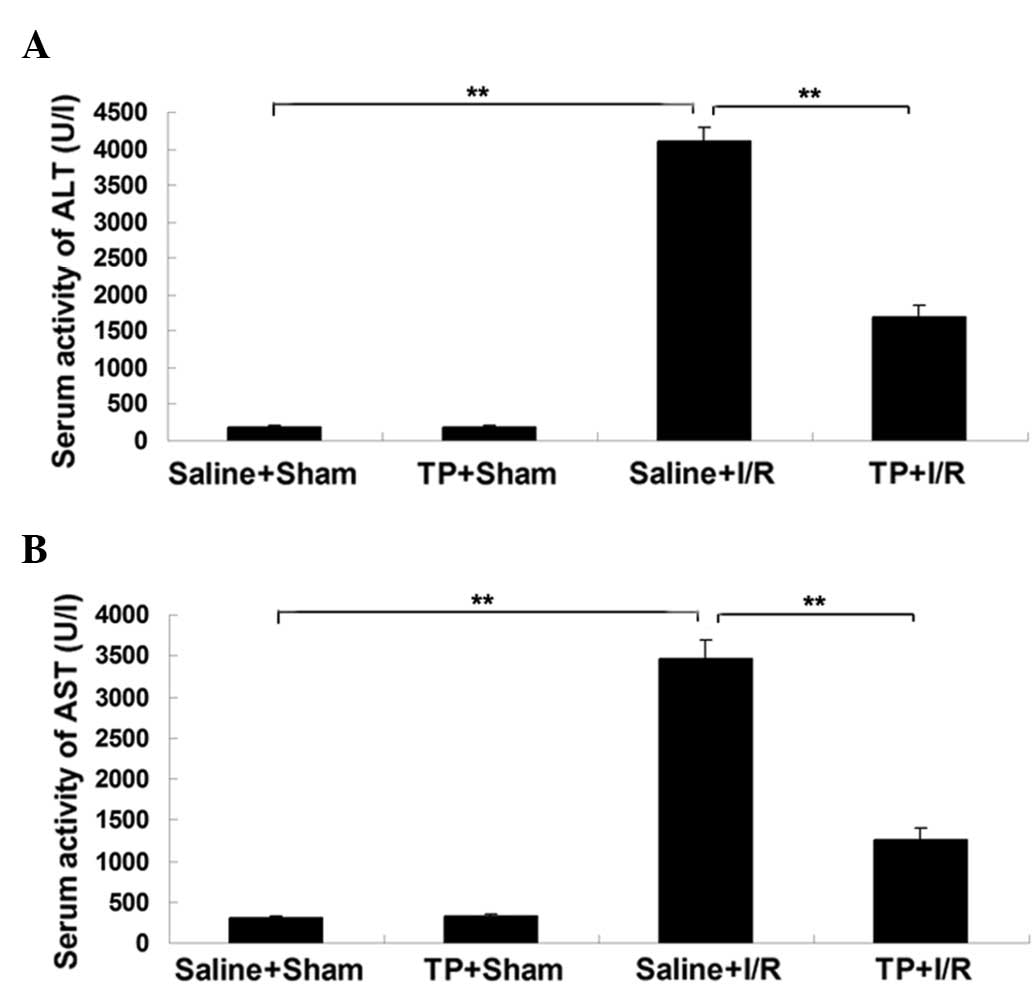
Pretreatment with TP attenuates the
upregulation of serum ALT and AST activity in mice with I/R-induced
liver injury
To evaluate the extent of hepatic injury in mice,
the activity of serum ALT and AST was measured in each group. As
presented in Fig. 1A, the serum
activity of ALT was significantly upregulated in the saline + I/R
group in comparison with the saline + sham group (P<0.01),
suggesting that the liver was damaged by the I/R-induced injury.
However, this increase was significantly attenuated by
pre-treatment with TP (P<0.01; Fig.
1A). There was no significant difference in the serum activity
of ALT between the saline + sham group and the TP + sham group.
Similar results were observed in the activity of
serum AST. As presented in Fig. 1B,
the serum activity of AST was significantly increased in the saline
+ I/R group in comparison with the saline + sham group (P<0.01),
which was markedly attenuated by pre-treatment with TP (P<0.01).
By contrast, no significant difference in the serum activity of AST
was observed between the saline + sham and TP + sham groups. Based
on these observations, it can be suggested that pretreatment with
TP attenuates I/R-induced liver injury in mice.
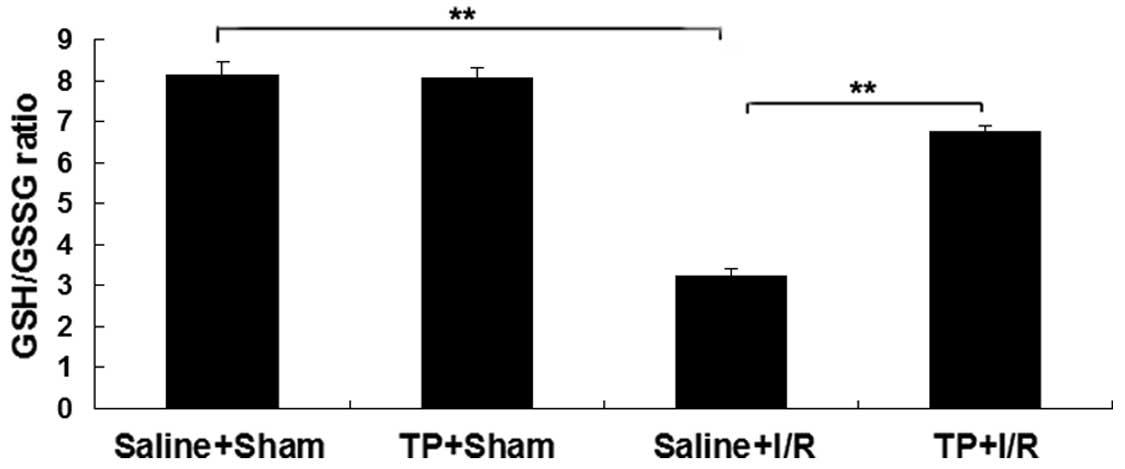
Pretreatment with TP attenuates the
decease in GSH/GSSG ratio in mice with I/R-induced liver
injury
The content of GSH in the liver of mice was observed
in each group. As presented in Fig.
2, the GSH/GSSG ratio in the saline + I/R group was
significantly decreased in comparison with the saline + sham group
(P<0.01), suggesting that the liver was injured by I/R. However,
this downregulation was attenuated in the TP + I/R group in
comparison with the saline + I/R group (P<0.01; Fig. 2), suggesting that pretreatment with
TP attenuated the I/R-induced liver injury in mice. However, no
statistically significant difference in the GSH/GSSG ratio was
detected between the saline + sham group and the TP + sham
group.
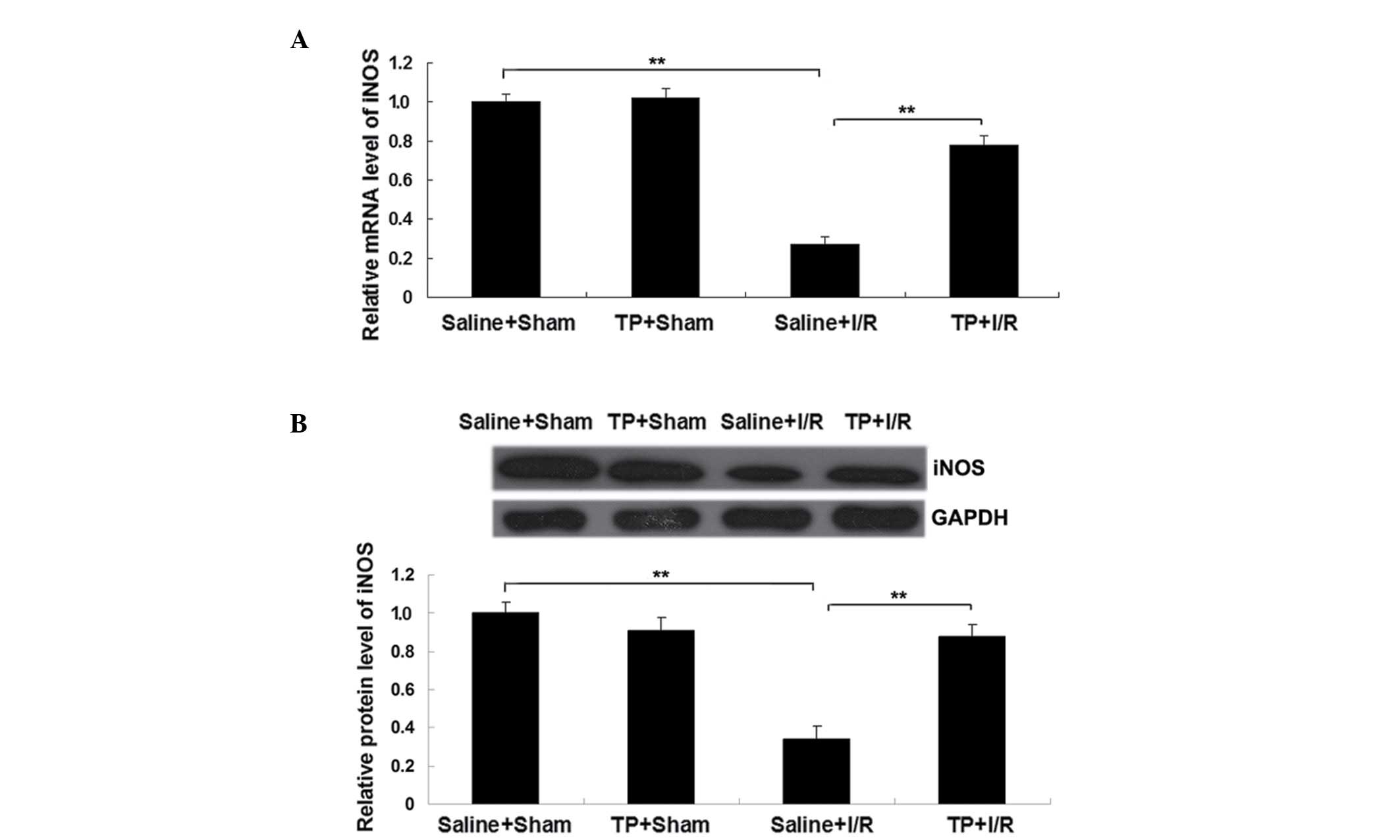
Pretreatment with TP suppresses the
downregulation of iNOS in mice with I/R-induced liver injury
iNOS is known to participate in a host's defense
against oxidative damage (16);
therefore, the mRNA and protein expression of iNOS in each group
was analyzed. As demonstrated in Fig. 3A
and B, the mRNA and protein expression levels of iNOS were
significantly decreased in the saline + I/R group in comparison
with the saline + sham group (P<0.01); however, this
downregulation was significantly attenuated by pretreatment with TP
(P<0.01). Furthermore, there was no significant difference in
the iNOS levels between the saline + sham group and TP + sham
groups.
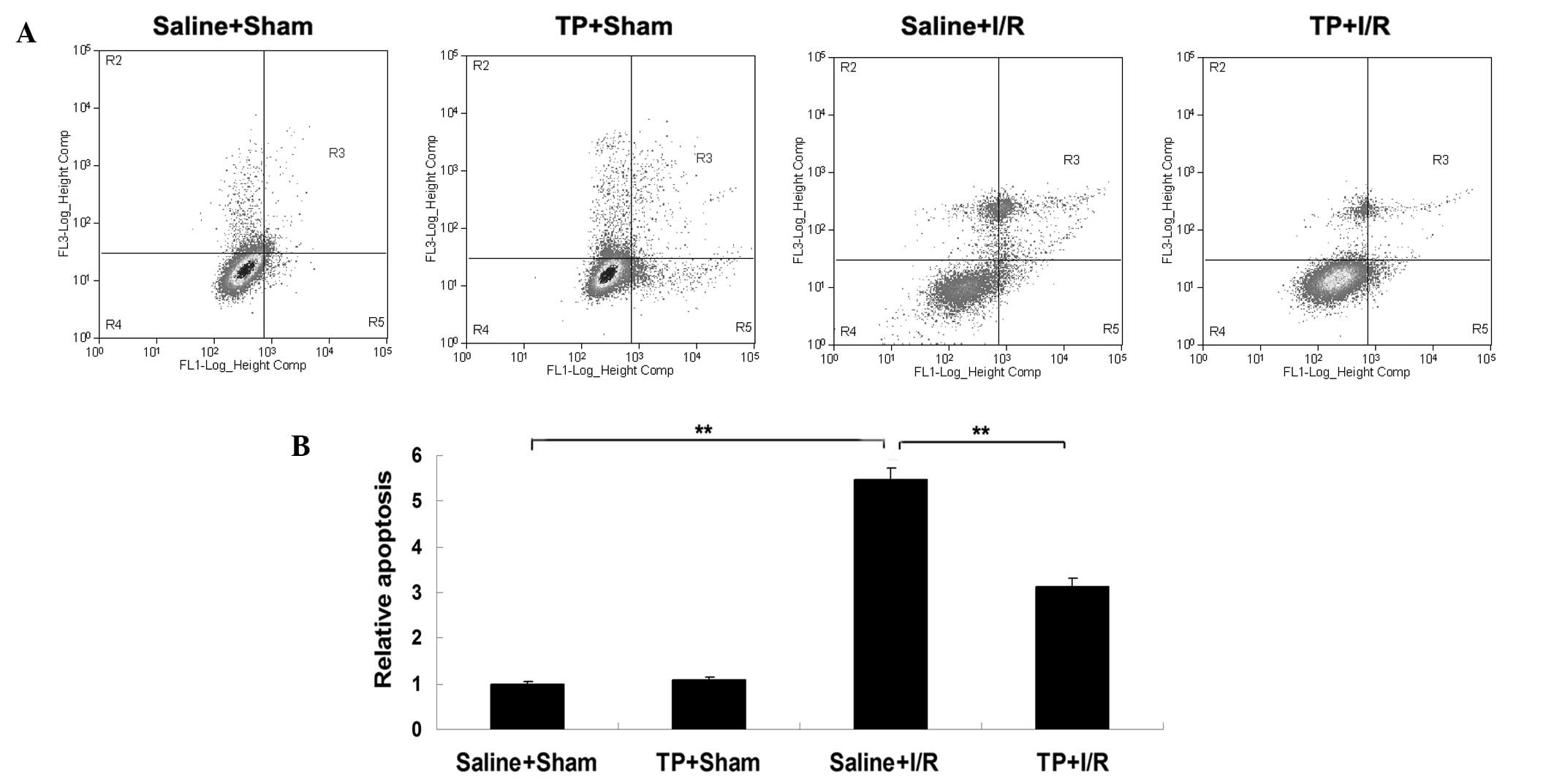
Pretreatment with TP attenuates
I/R-induced hepatic cell apoptosis in mice
The level of apoptosis in liver tissues in each
group was analyzed. As presented in Fig.
4, the level of apoptosis was significantly upregulated in the
liver tissues of mice in the saline + I/R group in comparison with
the saline + sham group (P<0.01), indicating that I/R injury
induced cell apoptosis in the liver of mice. However, the level of
apoptosis was significantly reduced in the TP + I/R group in
comparison with the saline + I/R group (P<0.01; Fig. 4), suggesting that pretreatment with
TP attenuated I/R-induced hepatic cell apoptosis in mice. However,
there was no significant difference in the level of apoptosis
between the saline + sham and TP + sham groups.
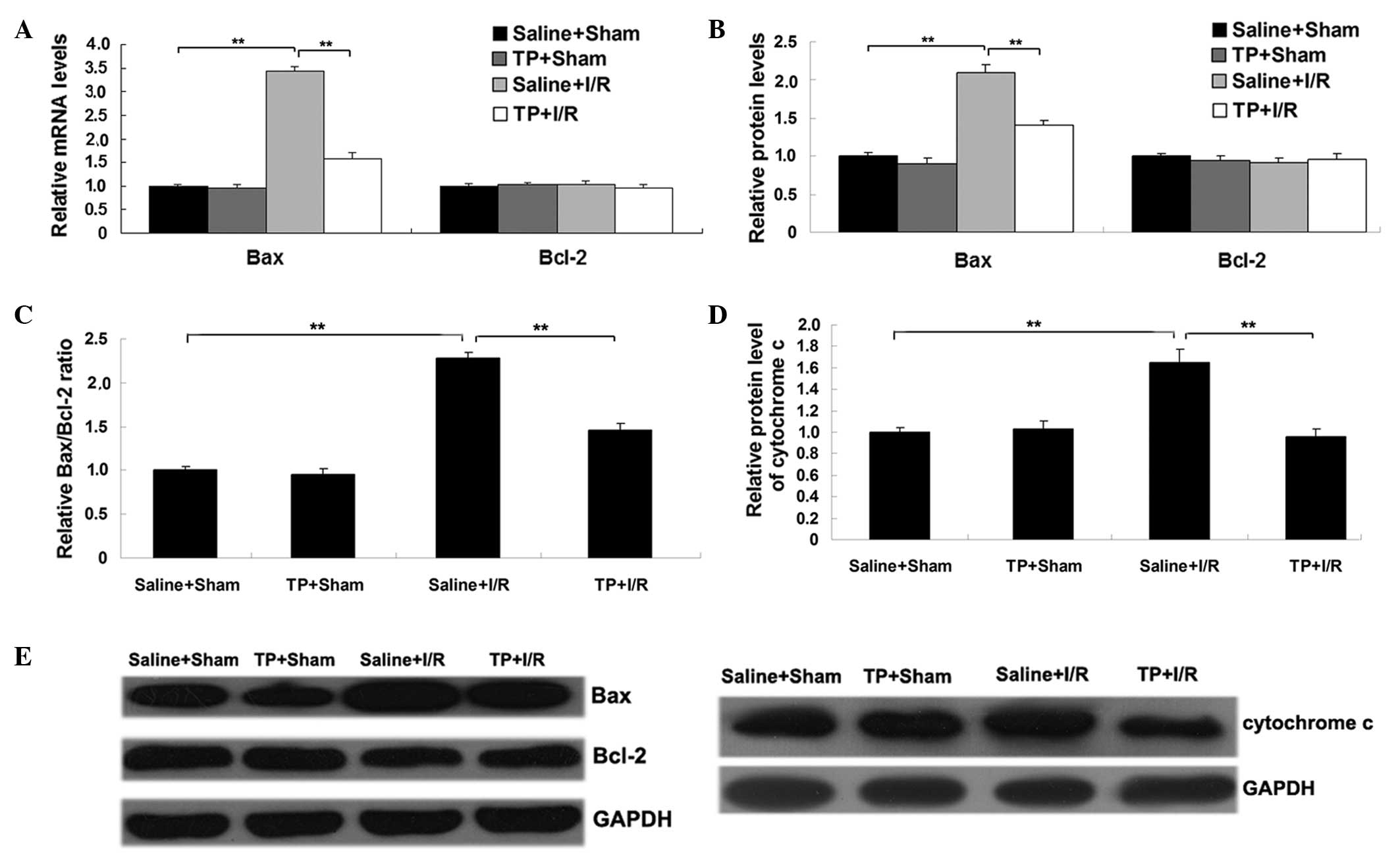
The mRNA and protein expression levels of two key
apoptosis-associated proteins, Bcl-2 and Bax, were also analyzed in
each group. As demonstrated in Fig. 5A
and B, the mRNA and protein levels of pro-apoptotic Bax were
significantly increased in the saline + I/R group in comparison
with the saline + sham group (P<0.01). However, this
upregulation was markedly attenuated by the pretreatment with TP
(P<0.01; Fig. 5A and B). Although
the mRNA and protein expression levels of Bcl-2 were not
significantly different between groups (Fig. 5A and B), the Bax/Bcl-2 ratio was
significantly increased in the saline + I/R group in comparison
with that in the saline + sham group (P<0.01; Fig. 5C), and was significantly attenuated
by pretreatment with TP (P<0.01). Similar findings were observed
in the protein expression levels of cytochrome c. In
addition, cytochrome c expression levels were significantly
increased in the saline + I/R group in comparison with those in the
saline + sham group (P<0.01; Fig.
5D), which was significantly attenuated by pretreatment with TP
(P<0.01).
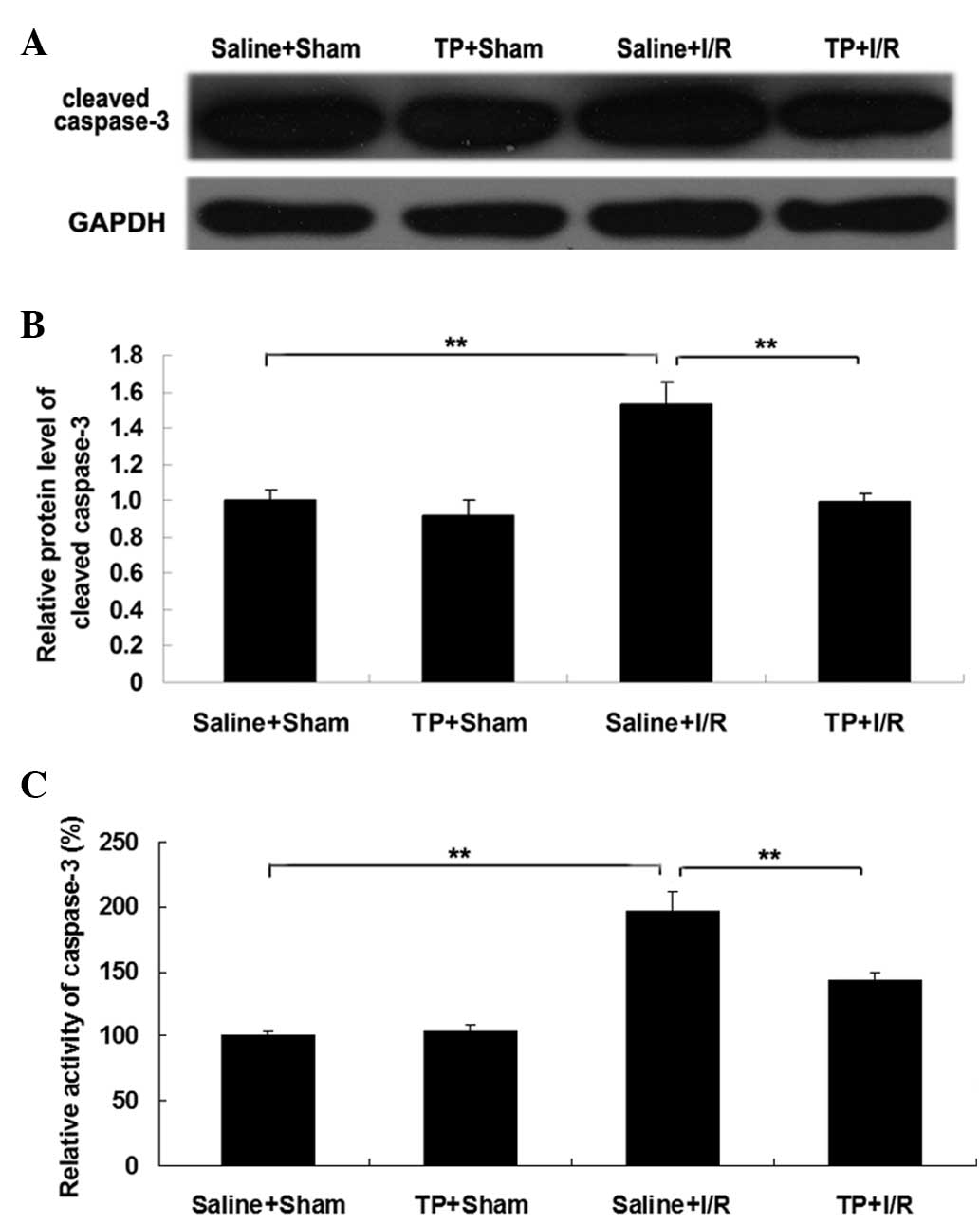
Furthermore, the expression levels and activity of
caspase-3 in the liver were analyzed in each group. As presented in
Fig. 6A and B, the protein
expression level of cleaved caspase-3 was significantly upregulated
in the saline + I/R group compared with those in the saline + sham
group (P<0.01), suggesting that the activation of caspase-3 is
involved in I/R-induced liver cell apoptosis. However, pretreatment
with TP significantly attenuated I/R-induced upregulation of
cleaved caspase-3 expression in liver tissues in mice (P<0.01;
Fig. 6A and B). Consistent with
these data, the activity of caspase-3 was also significantly
upregulated in the saline + I/R group compared with the saline +
sham group (P<0.01), which was also significantly attenuated by
pretreatment with TP (P<0.01). Taken together these findings
suggested that TP may at least partially protect against hepatic
I/R injury in mice, through the inhibition of the expression of
cytokine inducible nitric oxide synthase in liver tissues, and the
apoptosis of liver cells.
Discussion
TPs have been shown to protect against carbon
tetrachloride and lipopolysaccharide-induced liver injury, hepatic
I/R injury and liver fibrosis (13,17–19).
However, the precise mechanism underlying TPs protective effect
against I/R-induced liver injury remains uncertain. In the present
study, it was demonstrated that TP protected against I/R-induced
liver injury in mice using anti-oxidative and anti-apoptotic
properties.
The activities of serum ALT and AST are important
indicators of oxidative damage in liver tissues (20,21). In
the current study, it was demonstrated that the upregulation of ALT
and AST activity following I/R injury was attenuated by
pretreatment with TP in mice. Furthermore, GSH serves as an
antioxidant in removing reactive oxygen in human tissue, while GSSG
can be converted to GSH by glutathione reductase (22). In the present study, it was observed
that the administration of TP effectively attenuated the
I/R-induced decrease in the GSH/GSSG ratio.
It has been reported that overexpression of iNOS can
protect against hepatic I/R injury by modulating oxidative stress
(23). Therefore, the mRNA and
protein expression levels of iNOS were investigated in the
different groups of the current study. The results demonstrated
that pretreatment with TP attenuated the downregulation of iNOS in
I/R-induced injured liver tissues in mice. Based on these results,
it can be suggested that TP protects against I/R-induced liver
injury by inhibiting oxidative damage. In addition, I/R can induce
cell apoptosis in a number of organs including the liver, and the
inhibition of cell apoptosis effectively attenuates I/R-induced
liver damage (24,25). The present study revealed that
pretreatment with TP attenuated I/R-induced liver cell
apoptosis.
A number of proteins, including Bax, Bcl-2,
cytochrome c and caspase-3, have been demonstrated to serve
key roles in I/R-induced cell apoptosis. Bax is an important member
of the Bcl-2 family and can promote cell apoptosis via a
mitochondrial-mediated apoptosis pathway (26). Bcl-2 inhibits cell endoplasmic
reticulum Ca2+ release, lipid peroxide formation and
free radical production, and thus plays a suppressive role in cell
apoptosis (27). However, Bax can
bind to Bcl-2 and inhibit its anti-apoptotic activity (28). In the present study, although the
expression level of Bcl-2 presented no significant changes
following I/R-induced injury, Bax expression levels and the
Bax/Bcl-2 ratio were significantly upregulated in I/R-induced
injured liver tissues, which was significantly attenuated by
pretreatment with TP.
When cell apoptosis occurs, the mitochondrial
membrane potential collapses and cytochrome c moves into the
cytosol (29); therefore, the
expression level of cytochrome c was analyzed in the cytosol
of each group in the present study. The results demonstrated that
cytochrome c expression was significantly upregulated in
I/R-induced liver tissues, which was significantly attenuated by
pretreatment with TP.
Caspase-3 is a key member of the
cysteine-aspartate-specific protease family, and has been
demonstrated to function as an ultimate enforcer during cell
apoptosis. Upregulated expression, in addition to increased
activation, of caspase-3 has been reported in apoptotic cells
(30,31). In addition, the upregulation of
caspase-3 has been reported in I/R-induced hepatic injury,
indicating that caspase-mediated cell apoptosis serves a crucial
role in I/R-induced organ damage (32). In the present study, the expression
level and activity of caspase-3 was examined in each group and it
was observed that pretreatment with TP significantly attenuated the
expression and activity of caspase-3 in I/R injured liver tissues
in mice. Based on these observations, it can be suggested that TP
can attenuate I/R-induced liver cell apoptosis by inhibiting the
upregulation of Bax, caspase-3, and the release of cytochrome
c. Therefore, the results of the present study demonstrated
that TP has a protective effect against hepatic I/R injury in mice
via its anti-oxidative function, which inhibits the expression of
cytokine inducible nitric oxide synthase in liver tissues.
Furthermore, TP was also capable of inhibiting the I/R-induced
apoptosis of liver cells via the downregulation of pro-apoptotic
BAX and upregulation of anti-apoptotic Bcl2.
In conclusion, the present study demonstrated that
oral administration of TP can attenuate I/R-induced hepatic injury
via the inhibition of oxidative damage and liver cell apoptosis.
Therefore, TP may be a potential candidate for treating hepatic
injury.
References
|
1
|
Mendes-Braz M, Elias-Miró M,
Jiménez-Castro MB, Casillas-Ramírez A, Ramalho FS and Peralta C:
The current state of knowledge of hepatic ischemia-reperfusion
injury based on its study in experimental models. J Biomed
Biotechnol. 2012:2986572012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhuonan Z, Sen G, Zhipeng J, Maoyou Z,
Linglan Y, Gangping W, Cheng J, Zhongliang M, Tian J, Peijian Z and
Kesen X: Hypoxia preconditioning induced HIF-1α promotes glucose
metabolism and protects mitochondria in liver I/R injury. Clin Res
Hepatol Gastroenterol. 39:610–619. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Trocha M, Merwid-Ląd A, Chlebda E,
Sozański T, Pieśniewska M, Gliniak H and Szeląg A: Influence of
ezetimibe on selected parameters of oxidative stress in rat liver
subjected to ischemia/reperfusion. Arch Med Sci. 10:817–824. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bae UJ, Yang JD, Ka SO, Koo JH, Woo SJ,
Lee YR, Yu HC, Cho BH, Zhao HY, Ryu JH, et al: SPA0355 attenuates
ischemia/reperfusion-induced liver injury in mice. Exp Mol Med.
46:e1092014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sheng M, Zhou Y, Yu W, Weng Y, Xu R and Du
H: Protective effect of Berberine pretreatment in hepatic
ischemia/reperfusion injury of rat. Transplant Proc. 47:275–282.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rehman H, Krishnasamy Y, Haque K, Thurman
RG, Lemasters JJ, Schnellmann RG and Zhong Z: Green tea polyphenols
stimulate mitochondrial biogenesis and improve renal function after
chronic cyclosporin a treatment in rats. PLoS One. 8:e650292014.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Shen CL, Chyu MC and Wang JS: Tea and bone
health: Steps forward in translational nutrition. Am J Clin Nutr.
98(Suppl 6): 1694S–1699S. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Clifford MN, van der Hooft JJ and Crozier
A: Human studies on the absorption, distribution, metabolism, and
excretion of tea polyphenols. Am J Clin Nutr. 98(Suppl 6):
1619S–1630S. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yokozawa T, Noh JS and Park CH: Green tea
polyphenols for the protection against renal damage caused by
oxidative stress. Evid Based Complement Alternat Med.
2012:8459172012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Xu Y, Zhang JJ, Xiong L, Zhang L, Sun D
and Liu H: Green tea polyphenols inhibit cognitive impairment
induced by chronic cerebral hypoperfusion via modulating oxidative
stress. J Nutr Biochem. 21:741–748. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang M and Lei YX: Effects of tea
polyphenols on proliferation and apoptosis of cadmium-transformed
cells. Int J Clin Exp Med. 8:3054–3062. 2015.PubMed/NCBI
|
|
12
|
Xue R, Wu G, Wei X, Lv J, Fu R, Lei X,
Zhang Z, Li W, He J, et al: Tea polyphenols may attenuate the
neurocognitive impairment caused by global cerebral
ischemia/reperfusion injury via anti-apoptosis. Nutr Neurosci. Nov
20–2014.(Epub ahead of print). PubMed/NCBI
|
|
13
|
Zhong Z, Froh M, Connor HD, Li X,
Conzelmann LO, Mason RP, Lemasters JJ and Thurman RG: Prevention of
hepatic ischemia-reperfusion injury by green tea extract. Am J
Physiol Gastrointest Liver Physiol. 283:G957–G964. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Institute of Laboratory Animal Resources
(US). Committee on Care, Use of Laboratory Animals, and National
Institutes of Health (US). Division of Research Resources, . Guide
for the care and use of laboratory animals. 8th. National Academies
Press; Washington, DC: 2011, PubMed/NCBI
|
|
15
|
Mahale A, Othman MW, Al Shahwan S, Al
Jadaan I, Owaydha O, Khan Z and Edward DP: Altered expression of
fibrosis genes in capsules of failed Ahmed glaucoma valve implants.
PLoS One. 10:e01224092015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li YN, Wang XJ, Li B, Liu K, Qi JS, Liu BH
and Tian Y: Tongxinluo inhibits cyclooxygenase-2, inducible nitric
oxide synthase, hypoxia-inducible factor-2α/vascular endothelial
growth factor to antagonize injury in hypoxia-stimulated cardiac
microvascular endothelial cells. Chin Med J (Engl). 128:1114–1120.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cui Y, Yang X, Lu X, Chen J and Zhao Y:
Protective effects of polyphenols-enriched extract from Huangshan
Maofeng green tea against CCl4-induced liver injury in
mice. Chem Biol Interact. 220:75–83. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yuan GJ, Gong ZJ, Sun XM, Zheng SH and Li
X: Tea polyphenols inhibit expressions of iNOS and TNF-alpha and
prevent lipopolysaccharide-induced liver injury in rats.
Hepatobiliary Pancreat Dis Int. 5:262–267. 2006.PubMed/NCBI
|
|
19
|
Li YM, Zhang XG, Zhou HL, Chen SH, Zhang Y
and Yu CH: Effects of tea polyphenols on hepatic fibrosis in rats
with alcoholic liver disease. Hepatobiliary Pancreat Dis Int.
3:577–579. 2004.PubMed/NCBI
|
|
20
|
Zhong Z and Lemasters JJ: Role of free
radicals in failure of fatty liver grafts caused by ethanol.
Alcohol. 34:49–58. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cetinkunar S, Tokgoz S, Bilgin BC, Erdem
H, Aktimur R, Can S, Erol HS, Isgoren A, Sozen S and Polat Y: The
effect of silymarin on hepatic regeneration after partial
hepatectomy: Is silymarin effective in hepatic regeneration? Int J
Clin Exp Med. 8:2578–2585. 2015.PubMed/NCBI
|
|
22
|
Korge P, Calmettes G and Weiss JN:
Increased reactive oxygen species production during reductive
stress: The roles of mitochondrial glutathione and thioredoxin
reductases. Biochim Biophys Acta. 1847:514–525. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tao X, Wan X, Xu Y, Xu L, Qi Y, Yin L, Han
X, Lin Y and Peng J: Dioscin attenuates hepatic
ischemia-reperfusion injury in rats through inhibition of
oxidative-nitrative stress, inflammation and apoptosis.
Transplantation. 98:604–611. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Guo Y, Hu B, Huang H, Tsung A, Gaikwad NW,
Xu M, Jiang M, Ren S, Fan J, Billiar TR, et al: Estrogen
sulfotransferase is an oxidative stress responsive gene that
gender-specifically affects liver ischemia/reperfusion injury. J
Biol Chem. 290:14754–14764. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yan Y, Li G, Tian X, Ye Y, Gao Z, Yao J,
Zhang F and Wang S: Ischemic preconditioning increases
GSK-3β/β-catenin levels and ameliorates liver ischemia/reperfusion
injury in rats. Int J Mol Med. 35:1625–1632. 2015.PubMed/NCBI
|
|
26
|
Renault TT, Teijido O, Antonsson B, Dejean
LM and Manon S: Regulation of Bax mitochondrial localization by
Bcl-2 and Bcl-x(L): Keep your friends close but your enemies
closer. Int J Biochem Cell Biol. 45:64–67. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Shamas-Din A, Kale J, Leber B and Andrews
DW: Mechanisms of action of Bcl-2 family proteins. Cold Spring Harb
Perspect Biol. 5:a0087142013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Renault TT and Manon S: Bax: Addressed to
kill. Biochimie. 93:1379–1391. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bernardi P and Rasola A: Calcium and cell
death: The mitochondrial connection. Subcell Biochem. 45:481–506.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
McIlwain DR, Berger T and Mak TW: Caspase
functions in cell death and disease. Cold Spring Harb Perspect
Biol. 5:a0086562013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hua P, Liu J, Tao J, Liu J and Yang S:
Influence of caspase-3 silencing on the proliferation and apoptosis
of rat bone marrow mesenchymal stem cells under hypoxia. Int J Clin
Exp Med. 8:1624–1633. 2015.PubMed/NCBI
|
|
32
|
Qin Y, Hoek TL Vanden, Wojcik K, Anderson
T, Li CQ, Shao ZH, Becker LB and Hamann KJ: Caspase-dependent
cytochrome c release and cell death in chick cardiomyocytes after
simulated ischemia-reperfusion. Am J Physiol Heart Circ Physiol.
286:H2280–H2286. 2004. View Article : Google Scholar : PubMed/NCBI
|