Introduction
Thyrotropin releasing hormone (TRH) is produced in
medial neurons of the paraventricular nucleus (PVN) of the
hypothalamus. The secretion of TRH stimulates the release of
thyroid stimulating hormone (TSH) from the pituitary gland, which
travels to the thyroid via the blood where it stimulates the
secretion of thyroid hormone (TH). TH then acts on the hypothalamus
and pituitary to exert negative feedback. In addition to TRH, a
number of hypothalamic proteins can regulate the secretion of TSH,
including somatostatin (SST) (1).
Additionally, deiodinase, which is an enzyme affecting the
concentration of T3 (triiodothyronine) and T4 (thyroxine) tissue,
is important in thyroid-mediated signal transduction (2).
The hypothalamic-pituitary-thyroid (HPT) axis is
important in cell metabolism, oxygen consumption, tissue growth,
maturation and differentiation as well as in the regulation of body
fat and the carbohydrate metabolism (3). Additionally, the HPT axis has a
prominent role in human stress reaction systems (4–6).
Advances in the field of human neuroendocrinology
have revealed that two thirds of diseases are directly or
indirectly associated with the body's stress response, thus drawing
a greater focus on the negative effects of stress on human health
(7). For example, there is data
demonstrating that psychological stress can significantly impede
immune responses (8), and intense
stress can impair animal learning and memory capacity (9). A sudden serious illness, which often
elicits a variety of acute stress, is often associated with low
levels of blood T3 (low T3 syndrome) (10). Moreover, a number of scholars have
revealed that low levels of T3 are an independent risk factor for
poor prognosis and mortality caused by heart disease (11). Furthermore, it has previously been
demonstrated that combinations of T3 and antidepressants can
significantly enhance the treatment of refractory depression
(12). However, current
investigations of the association between stress and thyroid
hormone levels in the peripheral blood have generated mixed
results, indicating a slight increase or no change in thyroid
hormone blood levels in response to mild stress (psychological
stress-slow-binding experiments) (13,14), or
a decrease following high stress (such as electric shock
stimulation) (6,15,16).
Some researchers have concluded that such changes reflect the
sensitivity of the HPT axis to the stress strength and intensity
(17,18). Additionally, studies have revealed
that TRH mRNA levels in the PVN change following stress (15,16,19), and
thus stress responses may partially act through the regulation of
TRH expression in the central nervous system.
In the classical model of stress, cortisol hormone
levels exhibit significant increases, and a number of studies have
revealed that increased cortisol can influence pituitary TSH
release (20,21). However, there are studies suggesting
that cortisol exerts effects on the hypothalamus (1,20).
Furthermore, glucocorticoid receptors are discovered in the TRH
neurons of the PVN, and glucocorticoid response elements have been
identified on the TRH gene (22).
The present study aimed to establish bilateral
adrenalectomy Wistar rats by artificially blocking the effect of
cortisol, and sought to investigate the influence of acute stress
on the HPT axis by measuring alterations of blood T4, T3, TSH and
pituitary TSHβ mRNA levels, as well as TRH expression levels in the
PVN.
Materials and methods
Animals
A total of 50 (6–8-week-old) specific-pathogen-free
grade male Wistar rats, weighing 190–210 g were purchased from
Vital River Company (Beijing, China) and housed under controll
temperature and light conditions (23±2°C; 12-h light/dark cycle)
with ad libitum access to standard rat chow and water. The
present study conformed to the guidelines outlined by the Animal
Care and Use Research Committee of China Medical University.
Starting 1 week prior to the experiment, the animals were handled
for 5 min every day to accustom the rats to the experimenters.
Then, the rats were randomly divided into the following two groups:
The surgical group (without adrenal glands) and the non-surgical
group (with adrenal glands). These two groups were then divided
into five groups, where each group consisted of 5 animals. Surgery
was performed as previously described (23,24).
Experiments were all performed between 8:00 and 12:00 am. Group 1
consisted of blank controls where the animals were decapitated
without subjection to stress, and their blood samples were taken.
Group 2 were forced to swim in a water bath set to 26°C for 10 min
and were then immediately decapitated and their blood samples
collected. Group 3 were forced to swim in a water bath set at 26°C
for 10 min and after 2 h these animals were decapitated and their
blood samples obtained. Group 4 were forced to swim in a water bath
set at 26°C for 10 min and after 12 h were subjected to
decapitation and their blood samples collected. Group 5 were forced
to swim in a water bath set at 26°C for 10 min and then decapitated
after 24 h and their blood samples obtained. The brains were
quickly removed and frozen in −40°C isopentane (Sangon Biotech,
Shanghai, China) and stored at −70°C. Blood samples were
immediately centrifuged at 956 × g at 0°C for 3–5 min and
the plasma stored at −20°C.
Serum hormone measurements
Peripheral serum T3, T4 and TSH levels were measured
by chemiluminescence (Immulite; Siemens Healthcare, Ltd., Surrey,
UK), according to the manufacturer's instructions.
Immunohistochemical staining of
pituitary TSHβ
Pituitary tissue was removed from the −70°C freezer,
equilibrated for ≥30 min (to prevent a sudden change of the
temperature, which would affect the structure of the organization),
sliced into 10 µm sections, mounted on poly-lysine coated slides
and transported on dry ice to be stored in the −70°C freezer.
Immunohistochemistry sections were removed from the
−70°C freezer and fixed in 4% paraformaldehyde and
phosphate-buffered saline (PBS) (pH=7.4) at room temperature for 1
h, rinsed three times in 0.01 M PBS for 3 min each time, soaked in
3% H2O2 for 10 min to block endogenous
peroxidase and then rinsed again three times in 0.01 M PBS for 3
min each time. The sections were then incubated with a rabbit
anti-TRH primary antibody (1:100 dilution; bs-1053R; BIOSS,
Beijing, China) at 37°C for 90 min and then washed three times in
0.01 M PBS for 3 min each time. Sections were subsequently covered
with drops of polymer helper reagent from the horseradish
peroxidase-conjugated secondary antibody kit (PV9003 ready-to-use
type; ZSGB-Bio, Beijing, China), incubated at 4°C for 30 min and
rinsed three times in 0.01 M PBS for 2 min each time. Following
this, sections were incubated with poly-horseradish peroxidase
anti-goat IgG from the PV9003 reagent box (read-to-use type) at 4°C
for 30 min and rinsed three times in 0.01 M PBS for 2 min each
time. 3,3′-diaminobenzidine solution (BIOSS) was prepared according
to the manufacturer's instructions. The sections were developed
with chromogen (BIOSS) for 3 min and rinsed under running tap water
in order to terminate the reaction. They were then stained with
hematoxylin and eosin for 30 sec, rinsed in running tap water until
the water was colorless, immersed twice in hydrochloric acid
alcohol, incubated in blocking solution for 5 min and then mounted
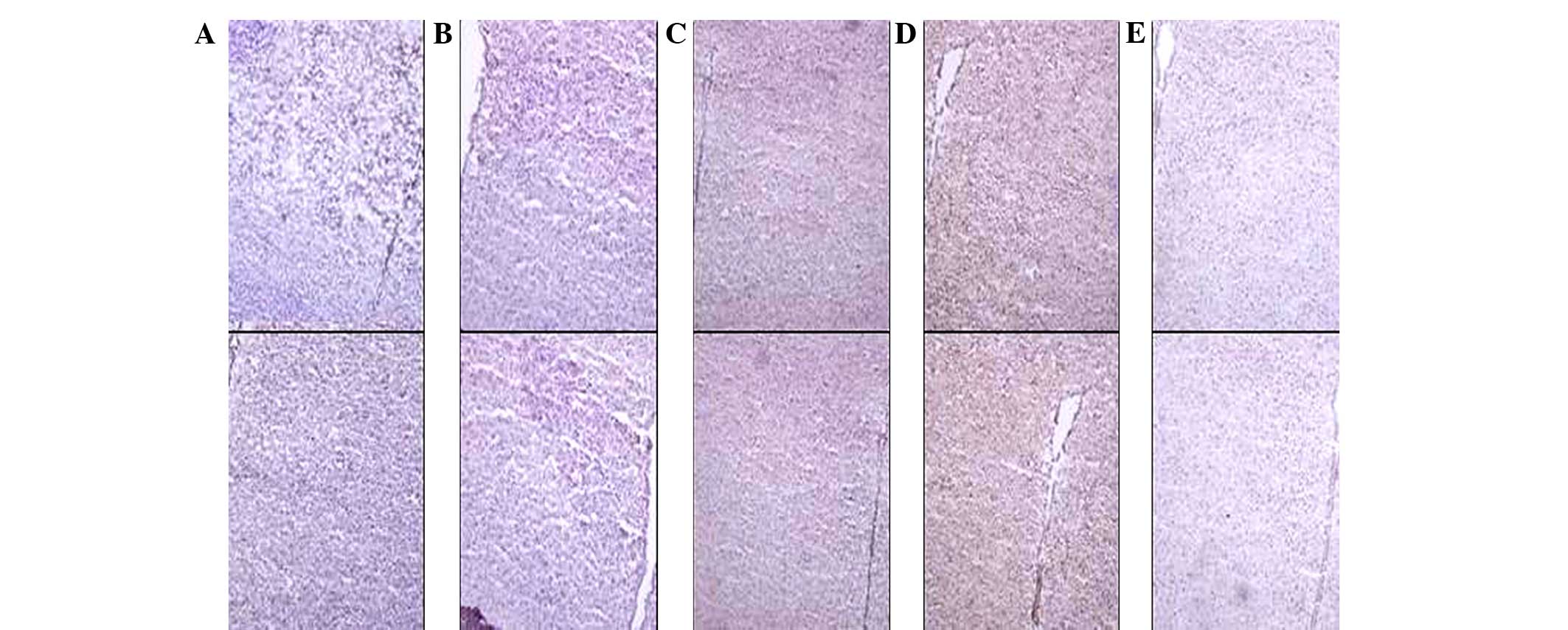
with 50% glycerogelatin. Staining results were quantitatively
analyzed under an image analysis system (MetaMorph/DP10/Bx41;
Olympus Corporation, Tokyo, Japan) at a magnification of ×400. The
average optical density of positive staining of the pituitary
tissues was calculated as follows: Five sections were selected from
each group; from each slice three microscopic pictures were
randomly captured at a magnification of ×400. The average
integrated optical density (IOD) of these 15 microscopic pictures
was calculated as the measured value of this group.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) measurement of pituitary TSHβ
mRNA
RNA extraction
Total RNA from the Wistar rat pituitary was
extracted using TRIzol (TaKaRa Bio, Inc., Otsu, Japan) according to
the manufacturer's instructions. Frozen rat pituitary tissues were
removed from −70°C and homogenized in 1 ml TRIzol reagent on ice.
The homogenates were then pipetted 20 times using 1 ml sterile
syringes and transferred to a new 1.5 ml centrifuge tube.
Homogenates were pipetted repeatedly until there were no apparent
precipitate in the lysates, and were then incubated at room
temperature for 5 min.
Purity and concentration of RNA
A total of 2 µl RNA solution was added to 198 µl
DEPC-treated water and 260 and 280 nm optical density (OD) values
were determined using a UV spectrophotometer. RNA samples with an
OD260/OD280 ratio >1.8 were used in the studies. The RNA
concentration was calculated as (µg/ml)=OD260 × 40 × dilution
factor, and the solutions were divided into small aliquots and
stored at −70°C until further use.
cDNA synthesis
Total RNA was diluted to 100 ng/µl and cDNA was
synthesized by reverse transcription (RT) using PrimeScript RT
Reagent kit (RR036A), according to the manufacturer's instructions
(TaKaRa Bio, Inc.). The RT kit contained the following reagents:
2.0 µl 5X primescript buffer; 0.5 µl oligo dT primer (50 µM); 0.5
µl random 6-mers (100 µM); 0.5 µl primescript RT enzyme mix; 1.5 µl
RNase-free H2O; and 5.0 µl total RNA. The reagents were
mixed and centrifuged at 3,824 × g for 3–5 sec at room
temperature and RT was performed at 37°C for 15 min. Next, the
inactivation of RT was performed at 85°C for 5 sec. The reaction
tube was then placed on ice immediately after the end of the
reaction, and the cDNA was stored at −70°C or directly used for the
next step, qPCR. The volume of cDNA added did not exceed 1/10 of
the total volume of the total qPCR reaction system (v/v).
Assay design
TSHβ and GAPDH sequences were obtained from the Gene
Bank database and Primer version 5.0 (TaKaRa Bio, Inc.) was used to
design the primers. Primers were synthesized and purified by TaKaRa
Bio, Inc., and are shown in Table I.
A reference gene is included as an internal standard to correct for
sample to sample variations in RT-qPCR. Furthermore, GAPDH was
selected as the endogenous control in the present study. The
primers were dissolved in double-distilled water to a concentration
of 100 µmol/µl and aliquots were stored at −20°C. Next, the primers
were further diluted to 10 µmol/µ1 for the next step of the
experiments. The preparation of the qPCR reaction was performed
according to the SYBR Green qPCR protocol. The qPCR reaction was
performed on a LightCycler 480 Real-Time PCR System (Roche
Diagnostics GmbH, Basel, Switzerland) with the following programs:
Program 1 (denaturation), 95°C/30 sec and 20°C/sec for 1 cycle;
program 2 (PCR reaction), 95°C for 5 sec and 20°C/sec, 59°C for 30
sec and 20°C/sec for 40 cycles; and program 3 (melting curve), 95°C
for 0 sec and 20°C/sec, 65°C for 15 sec and 20°C/sec, and 95°C for
0 sec and 0.1°C/sec.
 | Table I.Primer sequences. |
Table I.
Primer sequences.
| Protein | Genes | Genebank accession
number | Primer | Amplification
length |
|---|
| TSHβ | Tshb | NM _013116.1 | Sense
GTGCCTACTGCCTGACCATCAA | 557 bp |
|
|
|
| Antisense
AGCAACATGGTGTGGGCATC |
|
| GAPDH |
Gapdh | NC_005103.2 | Sense
TGGTGAAGGTCGGTGTGAAC | 123 bp |
|
|
|
| Antisense
CCATGTAGTTGAGGTCAATGAAGG |
|
Following the reaction, a qPCR amplification curve
was established and the following requirements were met: The
melting curve had a single peak and the standard curve had an
R2 value ≥0.98. Moreover, the amplification efficiency
was E ≥2.0, and the negative control showed no fluorescence signal.
In addition, the final result, or gene correction value, was equal
to the quantitative results of the target gene/quantitative results
of GAPDH. LightCycler 480 Rotor-Gene Real-Time Analysis Software
6.0 was used with the option set as ‘Advanced Relative
Quantitation’ (senior relative quantification) to analyze the
changes in mRNA expression levels.
Statistical analysis
Data processing and analysis was performed using
SPSS version 16.0 statistical software (SPSS, Inc., Chicago, IL,
USA). Data are presented as the mean + standard deviation. Data
were evaluated by one-way analysis of variance and the
independent-samples t test was performed within groups, and
evaluated by factorial design. P<0.05 was considered to indicate
a statistically significant difference.
Results
Serum levels of T3, T4 and TSH
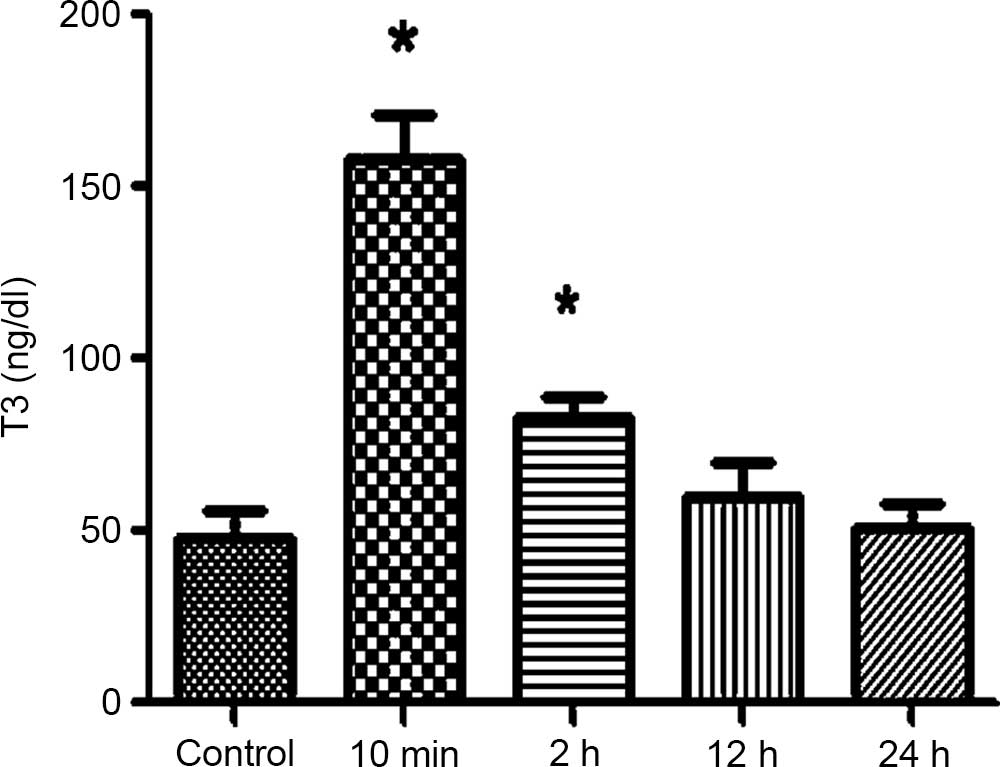
Following the forced swimming in the surgical 10 min
and 2 h groups, the serum T3 and T4 levels were gradually increased
and significantly different compared with the control group
(P<0.05). The serum T3 and T4 levels in the 10 min group reached
a peak and gradually decreased over time. In the non-surgical
group, the serum T3 and T4 showed no significant differences
compared with the control group.
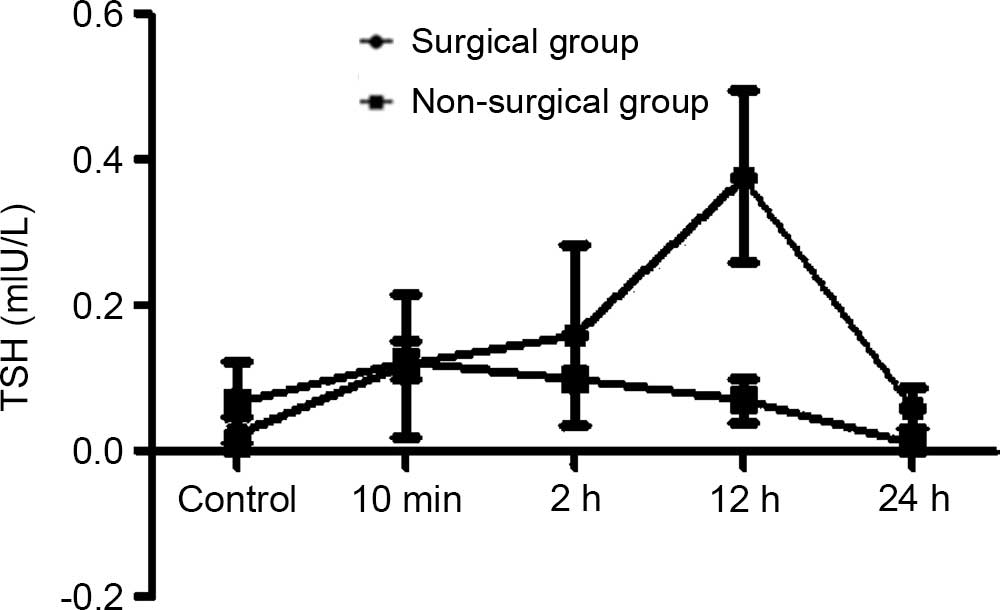
In the surgical group, the serum TSH was gradually
increased in the 10 min, 2 h and 12 h groups and reached a peak in
the 12 h group; a significant difference were observed compared
with the control group at 12 h (P<0.05). In the non-surgical
group, the serum TSH was slight elevated but no significant
difference was observed compared with the control group.
Furthermore, the trend of the serum T3, T4 and TSH levels in the
surgical group was markedly different compared with the
non-surgical group (Figs. 1–9).
Expression levels of rat pituitary
TSHβ mRNA
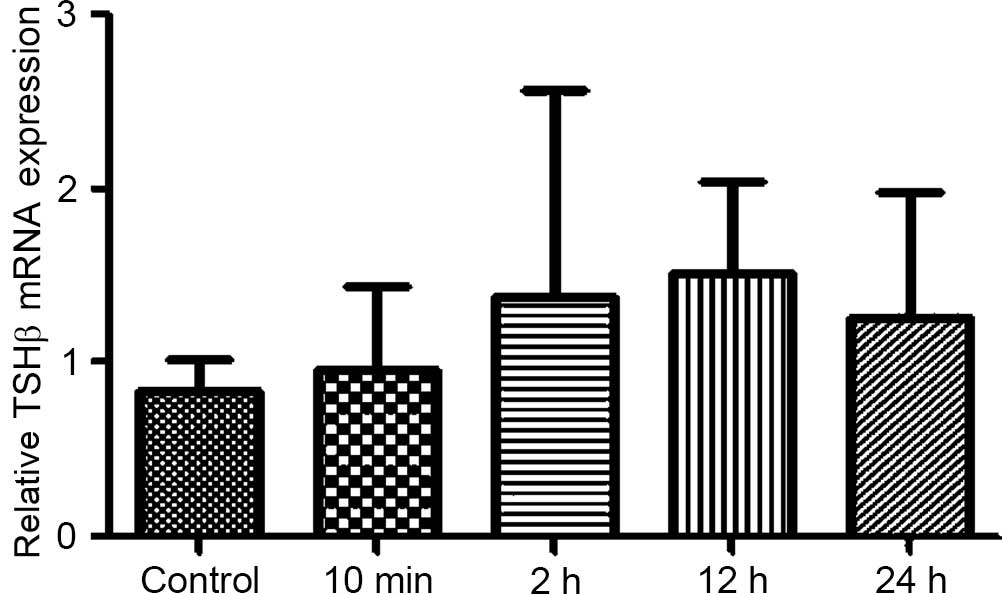
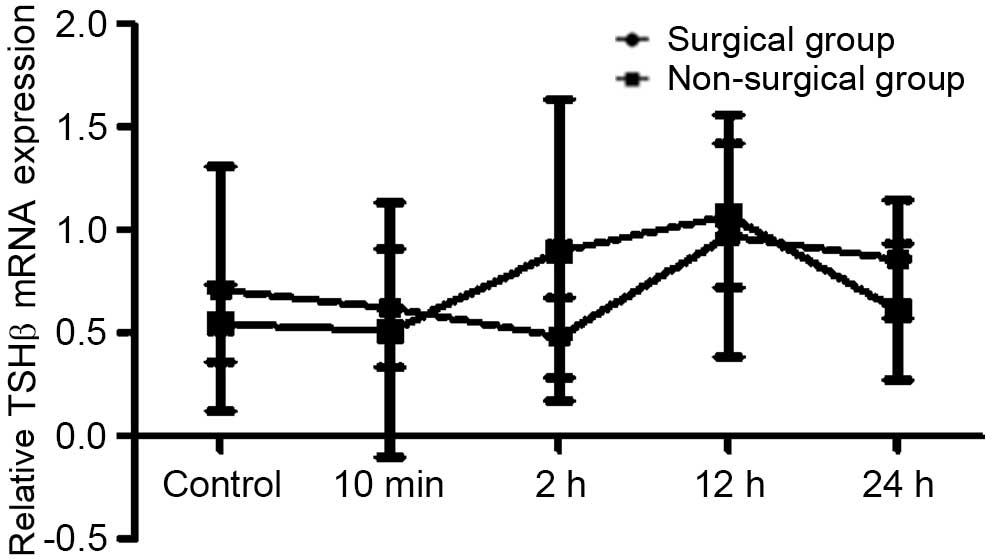
In order to determine whether forced swimming stress
can affect expression levels of TSHβ mRNA in rat pituitary tissue,
RT-qPCR was used to analyze the pituitary tissue TSHβ mRNA
expression levels.
The option of Advanced Relative Quantitation was set
in order to analyze TSHβ mRNA levels in rat pituitary tissue. The
results revealed that after 10 min of forced swimming in the
surgical and non-surgical groups, Wistar rat pituitary TSHβ mRNA
expression levels increased in all of the groups, but these
increases were no significantly different compared with the control
group. Furthermore, the index in the surgical group changed
significantly compared with the non-surgical group (Figs. 10–12).
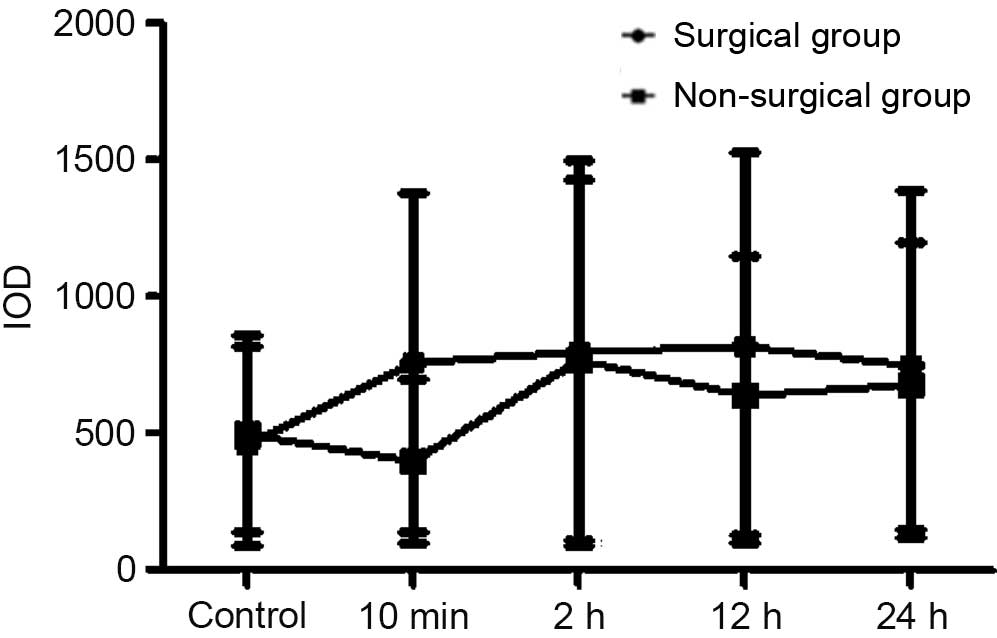
Hypothalamus TRH protein levels
As demonstrated by immunohistochemical staining of
frozen sections, 10 min of forced swimming immediately triggered
increases in pituitary TRH expression levels (presented as IOD).
TRH expression levels significantly increased 10 min after forced
swimming in the surgical and the non-surgical groups. TRH
expression levels significantly increased 10 min after forced
swimming in the surgical group. In addition, there was no
significant difference between the surgical and non-surgical groups
(Figs. 13–17).
Discussion
Thyroid hormone is important in the endocrine system
and serves a role in maintaining homeostasis and development.
Furthermore, it is intimately involved in the body's stress
response. In response to stress, hormone secretion and metabolic
homeostasis imbalances may induce nervous system, circulatory
system, endocrine system and mental disorders, as well as lead to
physical and mental diseases, such as Graves' disease and
depression (25,26). Through studies of thyroid function
changes in response to stress, it is possible to predict the impact
of stress on the human body and what can be expected from
intervention, and elucidate the function of the thyroid hormone and
the mechanisms by which the body adapts to environmental
changes.
It has previously been demonstrated that 10 min of
forced swimming can immediately increase glucocorticoid secretion
significantly by the hypothalamic-pituitary-adrenal (HPA) axis
(27). However, by 2 h after stress,
the glucocorticoid levels return to normal. It has been indicated
that a high-dose of glucocorticoids suppresses serum TSH in a
healthy individual and was controlled at the level of the
hypothalamus (28). The present
study aimed to establish bilateral adrenalectomy Wistar rats in
order to block the effect of cortisol. A forced swim stress was
applied to rats to examine whether it had the same effects on the
physiological function of the HPT axis as on the HPA axis. It was
revealed that 10 min of swimming stress immediately elevated the
peripheral blood levels of T3, T4 and TSH or pituitary TSH levels
in the surgical group, and that these were markedly different
compared with the non-surgical group. These data indicate that the
response of the HPT axis to forced swimming is different to that of
the HPA axis, as it is a prolonged reaction and can last for an
extended period of time.
Typically, the effects of thyroid hormone are
observed several days later and can last for a number of weeks.
However, a recent study revealed that thyroid hormones may cause
acute effects within hours. For example, significant changes in
brain-derived neurotrophic factor mRNA in the hippocampus of rats
intraperitoneally injected with T3 have been observed within 2 h
(29). Additionally, food intake
increases within 2 h of a subcutaneous injection with T3,
regardless of the energy consumption status (30). In addition, the effects of T3 on
hippocampal neurons have been demonstrated (31). The acute effects of thyroid hormones
may function through cell receptors in order to control gene
expression, but may act through other mechanisms (32).
In the present study, serum T3, T4 and TSH levels
were concurrently elevated 10 min after forced swimming in the
surgical group, suggesting that forced swimming stress can increase
the blood levels of thyroid hormones. As mentioned earlier, a
recent study has revealed that moderate stressful stimuli (such as
psychological and chronic restraints) can cause alterations in rat
serum thyroid hormone levels (33),
which is similar to the results of this experiment. In order to
further address the mechanism of stress response, RT-qPCR was used
to analyze the expression levels of pituitary TSHβ mRNA.
The increase in pituitary mRNA expression levels in
the surgical group in the current study demonstrated that the
effects on the HPT axis of stress are a possible source of TSH
production. In addition, the level of TRH expression increased in
the surgical group. The trend in the surgical group was more
evident compared with that in the non-surgical group. This suggests
that there are direct or indirect connections between the HPT and
HPA axes, which perhaps organize the core elements, such as energy
mobilization. These complex and intertwined axes regulate the body
in order to adapt to complex and stressful situations (34). Glucocorticoids, as a product of the
HPA axis, can inhibit the HPT axis at the level of the hypothalamus
and pituitary. Furthermore, intravitreal corticosteroids inhibit
the level of TRH mRNA expression on the hypothalamic PVN (35) and inhibit peripheral conversion of T4
to T3 (36). In addition, a number
of in vitro studies have provided evidence that
glucocorticoids stimulate the production of TSH (37). By contrast, HPT can affect the HPA
axis. The aforementioned evidence has proves that there is a trend
between the surgical and the non-surgical groups. A study by
Helmreich et al (34)
revealed that chronic mild stress in the HPA axis and the
hypothalamic arcuate nucleus agouti-related protein (AGRP) are most
likely involved in the regulation of the HPT axis (34). However, with regard to acute stress,
AGRP, which is involved in the HPT axis, has not yet been
reported.
A positive correlation between stress and the
increase of PVN TRH mRNA levels has previously been reported
(38). Various types of stress will
produce different effects on the HPT axis (39), thus indicating that besides TRH,
there are other neurological proteins or feedback mechanisms that
can affect the HPT axis, and TRH activity change is not necessarily
accompanied by changes in mRNA levels. Processing of the TRH
molecule can change its activity (40), for example in the ependyma located at
the base of the hypothalamus (41),
the TRH-specific poly-l-proline type II can directly alter the
biological activity of TRH (42).
Further studies of these proteins as well as deiodinase and SST may
further reveal their role.
The present study aimed to investigate the acute
effects of forced swimming stress on the HPT axis of rats. It was
revealed that peripheral T3, T4 and TSH levels increased, and an
increased expression of pituitary TSH was observed in the 12-h
surgical group. A previous study by Helmreich et al
(34) reports that in mice that were
given repeated and inescapable foot electrical stimulation for 14
days consecutively experienced a significant drop in their plasma
T3 and T4 levels, whereas their TRH mRNA levels in the hypothalamic
PVN did not change. In addition, they discovered that TRH mRNA
levels were closely associated with plasma glucocorticoid levels,
HPT composition and weight changes. Furthermore, it was identified
that T3 are associated with AGRP mRNA levels, and that T4 are
associated with plasma glucocorticoid levels and HPT composition
change; the levels of T3 were significantly reduced 2 h later under
inescapable stress conditions. These observations are in contrast
with the results of the present study, but this discrepancy may be
due to a difference in stress paradigms. In the study by Helmreich
et al (34), an iterative
chronic stress was used, and the impact of stress on the HPT axis
was measured for a number of days or weeks. What the authors did
not measure was the change in the HPT following acute stress, for
example after the first inescapable foot HPT electrical
stimulation. The present study had a stronger focus on acute stress
and employed a physical and psychological double stress-forced
swimming stress, with the effects of the stress measured in minutes
to hours. Acute stress was shown to lead to an increase in thyroid
hormones and TSH levels, and the response of the HPT axis to acute
stress was delayed but lasted longer than that of the HPA axis.
As the majority of the previous literature has
reported, most stresses caused by exogenous noxious stimuli can
increase the thyroid hormone level in the short term, but in the
long term they lead to a reduction (18,35,37).
This is consistent with the staging of the stress response reported
by Selye (43). The mechanism by
which stress causes changes in thyroid function is unclear.
However, a number of studies have reported that there are numerous
stress factors, including extreme cold or heat, that can directly
act through the central temperature sensors to control HPT, TRH and
TSH release, leading to an increase in thyroid hormone secretion
(32,44). Acute stress primarily acts through
the locus coeruleus-sympathetic-adrenal medullary system to elicit
a response, and thus excite the sympathetic nervous system, speed
up adrenal medulla function and increase the concentration of
plasma catecholamine to stimulate thyroid hormone secretion. It is
suggested that thyroid hormone reduction, particularly T3, caused
by long-term stress are closely related to HPA. Overall, the
effects of chronic stress on the HPT axis function is an area that
requires further investigation and research.
In conclusion, swim stress increases the blood
levels of T3, T4, TSH and the TSHβ mRNA expression at the pituitary
in adrenalectomized Wistar rats, and this is associated with the
HPT axis. Furthermore, the significant difference between the index
of surgery between the non-surgical and surgical groups suggests
that the adrenal glands have a role in the correlation between
stress induced by forced swimming and variation in HPT levels.
Acknowledgements
The authors thank Ms. Chenling Fan and Ms. Hong Wang
(Liaoning Provincial Key Laboratory of Endocrine Diseases) for
their excellent technical assistance. In addition, the authors
thank all colleagues and students who contributed to the present
study. The research was supported by Dr Weiping Teng's scientific
research funds (grant no. 2011225023).
References
|
1
|
Haugen BR: Drugs that suppress TSH or
cause central hypothyroidism. Best Pract Res Clin Endocrinol Metab.
23:793–800. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gereben B, Zavacki AM, Ribich S, Kim BW,
Huang SA, Simonides WS, Zeöld A and Bianco AC: Cellular and
molecular basis of deiodinase-regulated thyroid hormone signaling.
Endocr Rev. 29:898–938. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wrutniak-Cabello C, Casas F and Cabello G:
Thyroid hormone action in mitochondria. J Mol Endocrinol. 26:67–77.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Servatius RJ, Natelson BH, Moldow R,
Pogach L, Brennan FX and Ottenweller JE: Persistent neuroendocrine
changes in multiple hormonal axes after a single or repeated
stressor exposures. Stress. 3:263–274. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Helmreich DL, Crouch M, Dorr NP and
Parfitt DB: Peripheral triiodothyronine (T(3)) levels during
escapable and inescapable footshock. Physiol Behav. 87:114–119.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kilburn-Watt E, Banati RB and Keay KA:
Altered thyroid hormones and behavioural change in a sub-population
of rat following chronic constriction injury. J Neuroendocrinol.
22:960–970. 2010.PubMed/NCBI
|
|
7
|
Sabban EL and Kvetnanský R:
Stress-triggered activation of gene expression in catecholaminergic
systems: Dynamics of transcriptional events. Trends Neurosci.
24:91–98. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Han Mei-Jun and Ma Dian-Li: Change of
immune function in psychological stress. Zhong Guo Lin Chuang Kang
Fu. 10:189–192. 2006.(In Chinese).
|
|
9
|
Hölscher C: Stress impairs performance in
spatial water maze learning tasks. Behav Brain Res. 100:225–235.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mebis L and Van den Berghe G: Thyroid axis
function and dysfunction in critical illness. Best Pract Res Clin
Endocrinol Metab. 25:745–757. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Iervasi G, Pingitore A, Landi P, Raciti M,
Ripoli A, Scarlattini M, L'Abbate A and Donato L: Low-T3 syndrome:
A strong prognostic predictor of death in patients with heart
disease. Circulation. 107:708–713. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Shelton RC: The use of antidepressants in
novel combination therapies. J Clin Psychiatry. 64(Suppl 2):
S14–S18. 2003.
|
|
13
|
Armario A, Castellanos JM and Balasch J:
Effect of acute and chronic psychogenic stress on corticoadrenal
and pituitary-thyroid hormones in male rats. Horm Res. 20:241–245.
1984. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Turakulov YKh, Burikhanov RB, Patkhitdinov
PP and Myslitskaya AI: Influence of immobilization stress on the
levels of thyroid hormones. Neurosci Behav Physiol. 24:462–464.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cizza G, Brady LS, Esclapes ME, Blackman
MR, Gold PW and Chrousos GP: Age and gender influence basal and
stress-modulated hypothalamic-pituitary-thyroidal function in
Fischer 344/N rats. Neuroendocrinology. 64:440–448. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kondo K, Harbuz MS, Levy A and Lightman
SL: Inhibition of the hypothalamic-pituitary-thyroid axis in
response to lipopolysaccharide is independent of changes in
circulating corticosteroids. Neuroimmunomodulation. 4:188–194.
1997.PubMed/NCBI
|
|
17
|
Helmreich DL and Tylee D: Thyroid hormone
regulation by stress and behavioral differences in adult male rats.
Horm Behav. 60:284–291. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Fliers E, Korbonits M and Romijn JA:
Hypothalamic-pituitary hormones during critical illness: A dynamic
neuroendocrine response. Clin Neuroendocrinol. 124:115–126.
2014.
|
|
19
|
Légrádi G, Emerson CH, Ahima RS, Flier JS
and Lechan RM: Leptin prevents fasting-induced suppression of
prothyrotropin-releasing hormone messenger ribonucleic acid in
neurons of the hypothalamic paraventricular nucleus. Endocrinology.
138:2569–2576. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Roelfsema F, Pereira AM, Biermasz NR,
Frolich M, Keenan DM, Veldhuis JD and Romijn JA: Diminished and
irregular TSH secretion with delayed acrophase in patients with
Cushing's syndrome. Eur J Endocrinol. 161:695–703. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hangaard J, Andersen M, Grodum E,
Koldkjaer O and Hagen C: Pulsatile thyrotropin secretion in
patients with Addison's disease during variable glucocorticoid
therapy. J Clin Endocrinol Metab. 81:2502–2507. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bruhn TO, McFarlane MB, Deckey JE and
Jackson IM: Analysis of pulsatile secretion of thyrotropin and
growth hormone in the hypothyroid rat. Endocrinology.
131:2615–2621. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Su Y, van der Spek R, Foppen R, Kwakkel J,
Fliers E and Kalsbeek A: Effects of adrenalectomy on daily gene
expression rhythms in the rat suprachiasmatic and paraventricular
hypothalamic nuclei and in white adipose tissue. Chronobiol Int.
32:211–224. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Silva EJ, Vendramini V, Restelli A,
Bertolla RP, Kempinas WG and Avellar MC: Impact of adrenalectomy
and dexamethasone treatment on testicular morphology and sperm
parameters in rats: insights into the adrenal control of male
reproduction. Andrology. 2:835–846. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Matos-Santos A, Nobre EL, Costa JG,
Nogueira PJ, Macedo A, Galvão-Teles A and de Castro JJ:
Relationship between the number and impact of stressful life events
and the onset of Graves' disease and toxic nodular goitre. Clin
Endocrinol. 55:15–19. 2001. View Article : Google Scholar
|
|
26
|
Slavich GM and Irwin MR: From stress to
inflammation and major depressive disorder: a social signal
transduction theory of depression. Psychol Bull. 140:774–815. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Jiang YQ, Kawashima H, Iwasaki Y, Uchida
K, Sugimoto K and Itoi K: Differential effects of forced
swim-stress on the corticotrophin-releasing hormone and vasopressin
gene transcription in the parvocellular division of the
paraventricular nucleus of rat hypothalamus. Neurosci Lett.
358:201–204. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Haugen BR: Drugs that suppress TSH or
cause central hypothyroidism. Best Pract Res Clin Endocrinol Metab.
23:793–800. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sui L, Ren WW and Li BM: Administration of
thyroid hormone increases reelin and brain-derived neurotrophic
factor expression in rat hippocampus in vivo. Brain Res. 1313:9–24.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kong WM, Martin NM, Smith KL, Gardiner JV,
Connoley IP, Stephens DA, Dhillo WS, Ghatei MA, Small CJ and Bloom
SR: Triiodothyronine stimulates food intake via the hypothalamic
ventromedial nucleus independent of changes in energy expenditure.
Endocrinology. 145:5252–5258. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Caria MA, Dratman MB, Kow LM, Mameli O and
Pavlides C: Thyroid hormone action: Nongenomic modulation of
neuronal excitability in the hippocampus. J Neuroendocrinol.
21:98–107. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Davis PJ, Leonard JL and Davis FB:
Mechanisms of nongenomic actions of thyroid hormone. Front
Neuroendocrinol. 29:211–218. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Guo TY, Liu LJ, Xu L, Zhang JC, Li SX,
Chen C, He LG, Chen YM, Yang HD, Lu L and Hashimoto K: Alterations
of the daily rhythms of HPT axis induced by chronic unpredicted
mild stress in rats. Endocrine. 48:1–7. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Helmreich DL, Parfitt DB, Lu XY, Akil H
and Watson SJ: Relation between the hypothalamic-pituitary-thyroid
(HPT) axis and the hypothalamic-pituitary-adrenal (HPA) axis during
repeated stress. Neuroendocrinology. 81:183–192. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kakucksa I, Qi Y and Lechan RM: Changes in
adrenal status affect hypothalamic thyrotropin-releasing hormone
gene expression in parallel with corticotropin-releasing hormone.
Endocrinology. 136:2795–2802. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Bianco AC, Nunes MT, Hell NS and Maciel
RM: The role of glucocorticoids in the stress-induced reduction of
extrathyroidal 3,5,3′-triiodothyronine generation in rats.
Endocrinology. 120:1033–1038. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Luo LG, Bruhn T and Jackson IM:
Glucocorticoids stimulate thyrotropin-releasing hormone gene
expression in cultured hypothalamic neurons. Endocrinology.
136:4945–4950. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lechan RM and Fekete C: The TRH neuron: A
hypothalamic integrator of energy metabolism. Prog Brain Res.
153:209–235. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Herman JP and Cullinan WE: Neurocircuitry
of stress: Central control of the hypothalamic-adrenocortical axis.
Trends Neurosci. 20:78–84. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Perello M, Friedman T, Paez-Espinosa V,
Shen X, Stuart RC and Nillni EA: Thyroid hormones selectively
regulate the posttranslational processing of
prothyrotropin-releasing hormone in the paraventricular nucleus of
the hypothalamus. Endocrinology. 147:2705–2716. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Heuer H, Schäfer MK and Bauer K: The
thyrotropin-releasing hormone-degrading ectoenzyme: The third
element of the thyrotropin-releasing hormone-signaling system.
Thyroid. 8:915–920. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Sánchez E, Vargas MA, Singru PS, Pascual
I, Romero F, Fekete C, Charli JL and Lechan RM: Tanycyte
pyroglutamyl peptidase II contributes to regulation of the
hypothalamic-pituitary-thyroid axis through glial-axonal
associations in the median eminence. Endocrinology. 150:2283–2291.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Selye H: A syndrome produced by diverse
nocuous agents. Nature. 10:230–231. 1998.
|
|
44
|
Wang J and Zhang J: The effect of cold
stress on the HPT axis of chicken. Zhong Guo Chu Mu Za Zhi.
44:39–42. 2008.(In Chinese).
|