Introduction
The hepatitis C virus (HCV) is considered to be the
primary cause of chronic hepatitis, liver cirrhosis and
hepatocellular carcinoma (HCC) (1),
and has a worldwide incidence of 3% (2–4). Based
on China's National Seroprevalence Survey (years 1992–1995), China
has a slightly higher incidence of ~3.2% (5). Diagnosis of HCV is based on the
detection of anti-HCV antibodies and HCV RNA in the serum or liver
(6,7).
Approximately 50–85% of patients with HCV develop a
chronic infection with persistent viremia (8). A previous study demonstrated that, at
20 years after infection, 10–20% of these patients develop
cirrhosis and 1–3% develop HCC (9).
However, patients who achieved a sustained viral response (SVR)
after treatment showed low rates of disease progression and
end-stage liver disease at 35 years after infection (10).
The HCV genotype is thought to serve a role in
determining the disease course and prognosis of hepatitis C, and
has been shown to be associated with the sensitivity and
specificity of diagnostic tests for hepatitis C (11). HCV genotype 1 is the most prevalent
worldwide accounting for 83.4 million cases, which represents 46.2%
of all HCV cases (12). The HCV 1b
genotype has been found in 31.3% of chronic hepatitis cases, 50% of
liver cirrhosis cases and 57.1% of HCC cases (13). Genotype affects the HCV treatment
outcome. Pegylated interferon α (PEG IFNα) administered once weekly
in combination with ribavirin was effective in 85% of patients with
HCV infected with genotypes 2 or 3, but only in 45% of patients
infected with genotypes 1 and 4 (14). Genotype 1 patients who are treated
with telapravir and boceprevir in combination with PEG IFNα were
shown to have increased SVR compared with patients treated with PEG
IFNα alone (15,16). Transfusion of blood and blood
products represents the primary transmission route for HCV in
China. The wide-spread implementation of standardized HCV detection
techniques in 1993 resulted in a significant decrease in the number
of new infections via blood transfusions (17). However, since most
transfusion-acquired HCV infections in China occurred between the
years 1990 and 1996, individuals infected during that period have
been infected with HCV for ~20 years, and progression of fibrosis
puts them at risk of HCC (18).
Although the natural history of HCV infection and
HCV infection routes have been extensively studied, there is
limited information describing the association between the
progression of HCV infection and the HCV infection route. Since
blood transfusion remains an important mode of HCV infection, it is
important to understand the progression of the disease in patients
with HCV infected via blood transfusion. The molecular mechanisms
underlying the progression of chronic HCV infection to cirrhosis
and HCC remain largely unclear. Based on the clinical experience at
the First Affiliated Hospital of Zhengzhou University (Zhengzhou,
China_, a 20-year duration of HCV infection marks an important time
point at which to evaluate disease progression. However, disease
progression in patients with HCV has not been studied
systematically in patients who were infected with HCV via
transfusions twenty years earlier. In this systematic,
retrospective cohort study, the progression of disease in patients
who were infected with HCV through blood transfusions, and who did
not receive antivirus therapy, is investigated.
Materials and methods
Study participants
The present study was a systematic retrospective
analysis of 804 consecutive patients with chronic HCV infection who
were admitted to the First Affiliated Hospital of Zhengzhou
University between January 2011 and December 2013. Inclusion
criteria were as follows: i) Patients infected with HCV via blood
transfusion prior to 1996, and who did not receive antivirus
treatment; ii) patients positive for anti-HCV antibodies and for
HCV viral load. Routine screening of blood products for HCV using
techniques and kits with better specificity and sensitivity was
implemented in China in 1996. Therefore, the year 1996 was
established as the cut-off point for transfusion history. Patients
who had a history of blood transfusion prior to 1996, who were
positive for HCV RNA and who had no other risk factors for HCV,
were diagnosed with transfusion-associated hepatitis C. Factors
such as drug abuse, hemodialysis and sexual exposure were excluded
prior to concluding that HCV infection occurred via blood
transfusion. Reasons for transfusion included bleeding associated
with childbirth, ectopic pregnancy, surgery (breast, lung and
thyroid cancer or benign lesions), trauma or bleeding disorders. No
age-related issues were noted. The quantity of blood transfused was
not recorded. Among HCV patients screened for inclusion in the
present study, 43.7% had no history of blood transfusion. Exclusion
criteria, which were established to rule out other possible causes
of HCV infection, were as follows: i) Patients with other potential
causes of liver disease concurrent with HCV (such as hepatitis B
surface antigen-positive patients, patients with hepatitis A,
hepatitis E, drug-induced liver injury and EB virus infection); ii)
excess alcohol consumption (i.e., consuming >50 g of alcohol per
day); iii) infection with human immunodeficiency virus (HIV) or
presence of autoimmune or metabolic liver disease. In addition,
patients who had received multiple transfusions were excluded.
Demographic and clinical characteristics, including age, gender,
time of infection and alcohol consumption, were collected from
patient records. In addition, information regarding known risk
factors, such as intravenous drug use, travel history, acupuncture,
sexual habits and incarceration, was obtained from patient medical
records. When the information was not available in the medical
records, a telephone follow-up was performed. Body mass index data
were not collected.
The HCV genotype was identified in 53.6% of the
enrolled study patients. For the purpose of comparative analysis,
patients with cirrhosis or HCC were classified as the serious
development group (SD group) and the remaining patients with
chronic hepatitis were classified as the hepatitis group (H group).
Patients in the SD group were subdivided further into those with
compensated cirrhosis, decompensated cirrhosis and HCC.
Ethical considerations
The internal review board of the First Affiliated
Hospital of Zhengzhou University reviewed and approved the study
protocol. Patients' records were reviewed retrospectively without
patient identity so informed consent was waived for this study.
Laboratory and clinical evaluation of
HCV infection
Serum samples were collected from the patients and
the presence of anti-HCV antibodies was determined using
commercially a available third generation ELISA kit (cat. no.
1707-12; Diagnostic Automation, Inc., Calabasas, CA, USA) according
to the manufacturer's instructions. Seroreactive samples were
tested for HCV RNA by quantitative polymerase chain reaction (PCR)
using an ABI-7500 Fast Real-Time PCR System (Applied Biosystems;
Thermo Fisher Scientific, Inc., Waltham, MA, USA). The detection
range of HCV RNA was >500 IU/ml, and since HCV RNA values did
not show normal distribution and there was a large skewing of the
data, logarithmic transformations were performed.
Diagnosis of cirrhosis was based on imaging
indicators, such as irregular liver shape, presence of liver
parenchyma nodules, presence of widened portal vein, splenomegaly
or hypersplenism, or liver biopsy results which showed the
formation of false lobules. Diagnosis of decompensated cirrhosis
was based on the presence of cirrhosis, bleeding in the esophageal
varices, ascites and hepatic encephalopathy. The diagnosis of HCC
was based on pathological, histological and clinical criteria, and
included evaluation of clinical manifestations, serum AFP levels
and imaging features of HCC obtained through dynamic
contrast-enhanced computed tomography or magnetic resonance imaging
(MRI), which was performed in all patients. Among the typical
imaging features noted were heterogeneous enhancement of the
space-occupying lesion in the liver during the arterial phase of
Multi-Detector-Row Computed Tomography and (or) dynamic
contrast-enhanced MRI, which disappears during the venous phase or
lag phase. Twenty-three patients had follow-up pathological
confirmation of HCC diagnosis. Diagnosis of cirrhosis included the
following: i) Indicators based on imaging, such as irregular liver
shape, liver parenchyma nodule, widening of portal vein diameter
and splenomegaly; ii) hypersplenism; and iii) liver biopsies
revealing formation of false lobules. A total of 33 patients had
biopsy results and the remaining patients were diagnosed based on
the first and second criteria described above.
HCV genotyping
Direct sequencing of products generated by nested
reverse transcription (RT)-PCR was performed using the VERSANT HCV
Genotype Assay (Siemens Healthcare Diagnostics, Tarrytown, NY,
USA), according to the manufacturer's instructions. Viral RNA was
extracted using TRIzol (Thermo Fisher Scientific, Inc.) and the
first-round amplification was performed by RT-PCR under the
following cycling conditions: 42°C for 30 min; denaturation at 94°C
for 3 min; followed by 25 cycles of 94°C for 10 sec, 55°C for 20
sec and 72°C for 30 sec. The product from the first round
amplification (1 µl) was used as the template for the second-round
PCR amplification under the following cycling conditions: 94°C for
5 min; followed by 25 cycles of 94°C for 10 sec, 55°C for 20 sec
and 72°C for 30 sec. After digestion of the excess dNTPs and
primers in the reaction mix with shrimp alkaline phosphatase (SAP),
the PCR product was sequenced using the ABI 3130 DNA Sequencer
(DIAN Medical Diagnostic Center Co., Ltd., Hangzhou, China), and
the data were analyzed using genotyping software (www.ncbi.nlm.nih.gov/projects/genotyping/formpage.cgi)
of the National Center for Biotechnology Information.
Statistical analysis
Continuous variables are presented as the mean ±
standard deviations. Independent t test was performed to compare
the differences between two groups. One-way analysis of variance
with Bonferroni post-hoc test was performed to compare the
differences between more than two groups. Categorical variables are
presented as counts and percentages, with chi-square tests or
Fisher's exact tests for group comparison, appropriately. Logistic
regression models were performed to detect the risk factors for
patients with serious, progressive hepatitis disease or with HCC.
Factors with P<0.05 in univariate analyses were included in the
multivariate model. P<0.05 was considered to indicate a
statistically significant difference. The statistical analyses were
performed using SAS version 9.2 software (SAS Inc., Cary, NC,
USA).
Results
Demographic and clinical
characteristics of patients in the chronic hepatitis and serious
development groups
Of the 804 subjects included in this study, 564
(70.15%) were included in the H group and 240 (29.85%) were
included in the SD group. The study population comprised 333 males
and 471 females with a mean age of 47.96 years. Fourteen subjects
were infected with HCV prior to the year 1980; 179 subjects were
infected during the years 1981–1990, and 607 subjects were infected
during 1990 and 1996. Data for the year of HCV infection were
missing in 4 cases. A total of 431 patients had diagnostic
information for genotype. Genotype analysis showed that 301
subjects had genotype 1b, 124 subjects had genotype 2a, 4 subjects
had genotype 1a, 1 subject had genotype 2b and 1 subject had
genotype 6a. Genotype information was not available for 373
patients. The mean age for blood transfusion was 26.6 years, the
mean duration of HCV infection was 21.38 years and the mean value
of log HCV RNA was 14.9 (Table
I).
 | Table I.Demographic and clinical
characteristics of patients by group. |
Table I.
Demographic and clinical
characteristics of patients by group.
|
| Total (n=804) | H group
(n=564) | SD group
(n=240) | P-value |
|---|
| Gender |
|
|
| 0.141 |
|
Male | 333 (41.42%) | 243 (43.09%) | 90 (37.5%) |
|
|
Female | 471 (58.58%) | 321 (56.91%) | 150 (62.5%) |
|
| Age (years) | 47.96±12.42 | 44.71±11.81 | 55.61±10.3 |
<0.001a |
| Age at
blood transfusion, years | 26.6±12.25 | 23.56±11.62 | 33.73±10.66 |
<0.001a |
| Comorbidity |
|
|
|
|
|
Diabetes | 15 (1.87%) | 12 (2.13%) | 3 (1.25%) | 0.527 |
|
Hypertension | 10 (1.24%) | 8 (1.42%) | 2 (0.83%) | 0.482 |
| HCV
combined HBV | 10 (1.24%) | 3 (0.53 %) | 7 (2.92%) | 0.942 |
| HCV infected
year |
|
|
| 0.08 |
|
≤1980 | 14 (1.75%) | 7 (1.25%) | 7 (2.93%) |
|
|
1981–1990 | 179 (22.38%) | 118 (21.03%) | 61 (25.52%) |
|
|
>1990 | 607 (75.88%) | 436 (77.72%) | 171 (71.55%) |
|
| HCV infected
duration, years | 21.38±4.03 | 21.15±3.76 | 21.88±4.56 | 0.029a |
| HCV
RNA, ×107 | 2.75±27 | 3.40±32 | 1.19±3.23 | 0.106 |
| log HCV
RNA | 14.9±2.29 | 15.12±2.28 | 14.37±2.24 |
<0.001a |
|
Genotypeb |
|
|
| 0.555 |
| Type
1b | 301 (69.84%) | 240 (70.80%) | 61 (66.30%) |
|
| Type
2a | 124 (28.77%) | 95 (28.02%) | 29 (31.52%) |
|
| Type
1a | 4 (0.93%) | 2 (0.59%) | 2 (2.17%) |
|
| Type
2b | 1 (0.23%) | 1 (0.29%) | 0 (0%) |
|
| Type
6a | 1 (0.23%) | 1 (0.29%) | 0 (0%) |
|
| Platelets,
103/µl | – | 143.86±58.90 | 59.37±35.47 | 0.001 |
| WBC,
103/µl | – | 4.77±1.81 | 4.16±2.25 | 0.004 |
| Prothrombin time,
sec | – | 9.89±1.32 | 12.74±2.80 |
<0.001a |
| Albumin, g/l | – | 42.14±5.04 | 35.06±5.97 |
<0.001a |
| Alanine
transaminase, U/l | – | 48.11±60.68 | 51.88±41.79 | 0.245 |
| Aspartate, U/l | – | 40.42±34.58 | 72.20±53.02 |
<0.001a |
| γ-glutamyl
transferase, U/l | – | 43.00±47.08 | 63.00±49.52 | 0.002 |
| Total bilirubin,
mg/l | – | 13.25±10.26 | 24.32±29.09 |
<0.001a |
The mean age of patients in the SD group was
significantly higher compared with that of patients in the H group
at the time of observation in this study (55.61 vs. 44.71 years;
P<0.001). Patients in the SD group had a significantly higher
mean age at the time of blood transfusion compared with patients in
the H group (33.73 vs. 23.56 years, P<0.001). The mean duration
of HCV infection was significantly longer in the SD group compared
with that in the H group (21.88 vs. 21.15 years; P=0.029). The mean
log HCV RNA value was significantly higher in the H group compared
with that in the SD group (15.12 vs. 14.37; P<0.001). No
significant differences were noted between the H and SD groups with
regard to gender, HCV infected year, HCV RNA levels and genotypes
(P>0.05). In addition, there was no significant difference
between the two groups with regard to the incidence of diabetes and
hypertension, which were the major co-morbidities in these
patients. All study patients were screened for HBV. However,
co-infection was rare, and only ~1% of patients were co-infected
with HBV (Table I).
The mean platelet count (PLT), WBC count and albumin
(ALB) levels of SD group patients were significantly lower in the
SD group compared with those in the H group (PLT, 59.37 vs. 143.86,
respectively, P=0.001; WBC, 4.16 vs. 4.77, respectively, P=0.004;
ALB, 35.06 vs. 42.14, respectively, P<0.001). The mean
prothrombin time (PT), aspartate transaminase (AST), γ-glutamyl
transferase (GGT) and total bilirubin (TB) of SD group patients
were significantly higher compared with those of H group patients
(PT, 12.74 vs. 9.89, P<0.001; AST, 72.2 vs. 40.42, P<0.001;
GGT, 63.00 vs. 43.00, P=0.002; TB, 24.32 vs. 13.25, P<0.001)
(Table I).
Risk factors in the serious
development group
Risk factors associated with the SD group are shown
in Table II. Univariate logistic
regression analysis revealed that age at blood transfusion,
duration of HCV infection and log HCV RNA values were all
significant risk factors in the SD group compared with the H group
(P<0.001, P=0.02 and P<0.001, respectively). There was a
significant increase in risk with every year's increase in age
(OR=1.09, P<0.001) (data not shown), with every year's increase
in age at the time of blood transfusion (OR=1.09, P<0.001) and
with every year's increase in the duration of HCV infection
(OR=1.05, P=0.02). However, the log HCV RNA value significantly
decreased with disease progression (OR=0.87, P<0.001) (Table II).
 | Table II.Risk factors for the serious
development group. |
Table II.
Risk factors for the serious
development group.
|
| Univariate | Multivariate |
|---|
|
|
|
|
|---|
|
| OR (95% CI) | P-value | OR (95% CI) | P-value |
|---|
| Gender (ref =
female) | 0.79
(0.58–1.08) | 0.142 |
|
|
| Age at blood
transfusion | 1.09
(1.07–1.10) |
<0.001a | 1.1
(1.08–1.11) |
<0.001a |
| HCV infected
duration | 1.05
(1.01–1.08) | 0.02a | 1.09
(1.05–1.14) |
<0.001a |
| log HCV RNA | 0.87
(0.81–0.93) |
<0.001a |
|
|
| Genotype (ref =
type 2) | 0.92
(0.57–1.49) | 0.746 |
|
|
Factors with P<0.05 in univariate analyses,
including age at the time of blood transfusion and duration of HCV
infection, were included in the multivariate logistic regression
model. log HCV RNA values were excluded since log HCV RNA is a
time-dependent variable. After adjusting for duration of HCV
infection and log HCV RNA values, the risk of progression in SD
group patients increased with every year's increase in age at the
time of blood transfusion (OR=1.1, P<0.001). After adjusting for
age at the time of blood transfusion and log HCV RNA values, the
risk of progression in SD group patients increased with every
year's increase in the duration of HCV infection (OR=1.09,
P<0.001) (Table II).
Demographic and clinical
characteristics of the SD group
The distribution of patients in the SD group is
presented in Table III. Of the 240
patients in the SD group, 157 had compensated cirrhosis, 54 had
decompensated cirrhosis and 29 had HCC. The mean age and age at the
time of blood transfusion were both significantly higher in the HCC
group compared with the compensated cirrhosis and decompensated
cirrhosis groups (age, 61.34 vs. 54.89 and 54.61, P≤0.013; age at
blood transfusion, 39.59 vs. 32.68 and 33.63, P≤0.043). No
significant differences were found between groups in gender, year
of HCV infection, duration of HCV infection, HCV RNA levels, log
HCV RNA values and genotype studies (P>0.05; Table III).
 | Table III.Characteristics of patients in the
serious development group by disease type and progression. |
Table III.
Characteristics of patients in the
serious development group by disease type and progression.
|
| Compensated
cirrhosis (n=157) | Decompensated
cirrhosis (n=54) | HCC (n=29) | P-value |
|---|
| Gender |
|
|
| 0.105 |
|
Male | 54 (34.39%) | 20 (37.04%) | 16 (55.17%) |
|
|
Female | 103 (65.61%) | 34 (62.96%) | 13 (44.83%) |
|
| Age, years | 54.89±10.19 | 54.61±9.76 |
61.34±10.34a,b | 0.005 |
| Age at blood
transfusion, years | 32.68±10.5 | 33.63±10.35 |
39.59±10.55a,b | 0.005 |
| HCV infected
yearc |
|
|
| 0.593 |
|
≤1980 | 7 (4.49%) | 0 (0%) | 0 (0%) |
|
|
1981–1990 | 40 (25.64%) | 14 (25.93%) | 7 (24.14%) |
|
|
>1990 | 109 (69.87%) | 40 (74.07%) | 22 (75.86%) |
|
| HCV infection
duration, years | 22.22±5.07 | 20.98±3.29 | 21.76±3.36 | 0.226 |
| HCV RNA,
×107 | 1.13±2.64 | 1.69±5.14 | 0.65±1.27 | 0.355 |
| log HCV RNA | 14.51±2.2 | 14.13±2.31 | 14.06±2.33 | 0.428 |
|
Genotypec |
|
|
| 0.934 |
| Type
1 | 55 (69.62%) | 10 (66.67%) | 7 (77.78%) |
|
| Type
2 | 24 (30.38%) | 5 (33.33%) | 2 (22.22%) |
|
Risk factors for HCC in the SD
group
Risk factors for HCC in SD patients are shown in
Table IV. Univariate logistic
regression analysis showed that male gender and age at the time of
blood transfusion were significant risk factors for HCC among SD
patients with progressive disease (OR=2.35, P<0.037; and
OR=1.07, P=0.002, respectively) (Table
IV).
 | Table IV.Risk factors for hepatocellular
carcinoma in the serious development group. |
Table IV.
Risk factors for hepatocellular
carcinoma in the serious development group.
|
| Univariate | Multivariate |
|---|
|
|
|
|
|---|
|
| OR (95% CI) | P-value | OR (95% CI) | P-value |
|---|
| Gender (ref =
female) | 2.35
(1.05–5.24) | 0.037a | 2.48
(1.09–5.65) | 0.031a |
| Age at blood
transfusion | 1.07
(1.02–1.11) | 0.002a | 1.07
(1.02–1.11) | 0.002a |
| HCV infected
year | 0.98
(0.89–1.07) | 0.631 |
|
|
| log HCV RNA | 0.92
(0.77–1.09) | 0.322 |
|
|
| Genotype (ref =
type 2) | 1.53 (0.3–7.9) | 0.613 |
|
|
Male gender and age at transfusion were included in
multivariate logistic regression analysis. Since there was a high
correlation between variables of age at the time of observation and
age at the time of blood transfusion, these variables were
separated into two models. Multivariate analysis showed that males
had a higher risk of developing HCC than females (OR=2.48,
P=0.031); after adjusting for gender, the risk of HCC increased
with every year's increase in age at the time of blood transfusion
(OR=1.07, P=0.002) (Table IV).
Prevalence of hepatitis and
progressive disease within different age groups
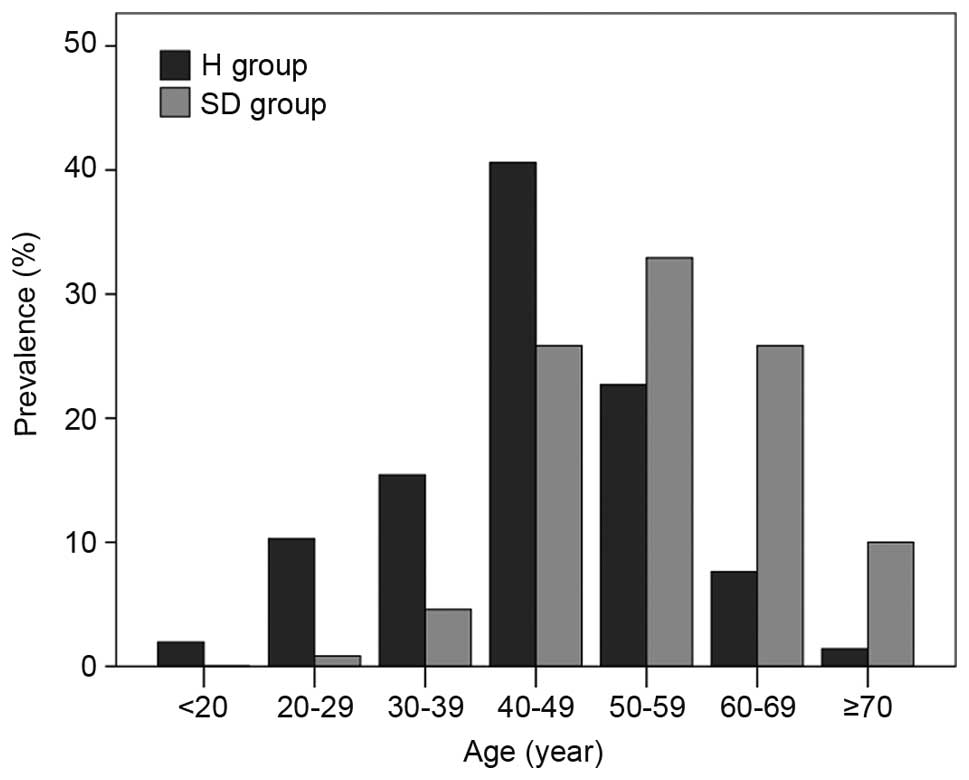
Fig. 1 depicts the
prevalence of hepatitis (H group patients) and disease progression
(SD group patients) in the different age groups (40–49, 50–59 and
60–69 years). Prevalence was highest in H group patients at 40–49
years old and SD group patients at 50–59 years old. A significant
difference was found in age between patients in the H and SD groups
(P<0.001; Fig. 1).
Prevalence of compensated cirrhosis,
decompensated cirrhosis and HCC within different age groups
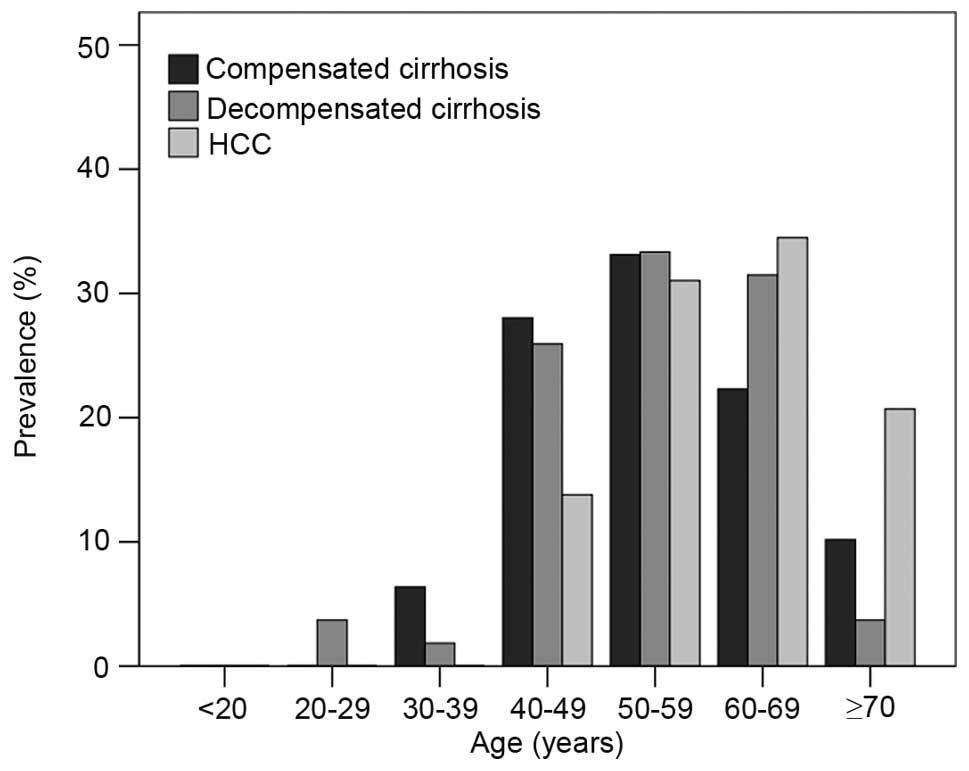
Fig. 2 depicts the
prevalence of compensated cirrhosis, decompensated cirrhosis and
HCC in the different age groups (40–49, 50–59 and 60–69 years). The
highest prevalence of both compensated and decompensated cirrhosis
was seen in the 50–59 year-old age group and the highest prevalence
of HCC was seen in the 60–69 year-old age group. Significant
differences were found between different age groups in the
prevalence of compensated cirrhosis, decompensated cirrhosis and
HCC (P<0.001; Fig. 2).
Discussion
The detection of HCV antibodies prior to blood
transfusion has been mandatory in China since 1993, and routine HCV
screening using highly specific and sensitive kits has been
implemented since 1996. It is therefore likely that patients who
received transfusion prior to this time period, and who were HCV
positive, had transfusion-associated HCV. The present study
investigated disease progression in patients with blood
transfusion-acquired HCV who had not received antivirus therapy.
Significant differences were identified in the age at the time of
transfusion-acquired HCV infection, duration of HCV infection, age
at the time of observation and HCV RNA load at the time of
observation between those with disease progression to fibrosis and
HCC (SD group) and those with chronic hepatitis without disease
progression (H group). The two groups did not differ significantly
in HCV genotype. Male gender and age at the time of transfusion
were significant risk factors for HCC in these patients. Patients
in the SD group who had developed liver cirrhosis and HCC were
significantly older at the time of transfusion and had a longer
mean duration of HCV infection compared to patients in the H group
without fibrosis or HCC. Based on clinical experience and results
of the present study, it can be proposed that a 20-year duration of
HCV infection marks a critical time point at which to evaluate
disease progression in patients with transfusion-acquired HCV.
Despite enormous progress in the treatment of HCV
over the past two decades, almost a third of treated non-responders
who fail to achieve SVR are at risk for disease progression
(19). It has been suggested that
the mode of HCV acquisition does not significantly impact the
outcome of the disease (20).
Nevertheless, a number of studies have investigated risk factors
for disease progression in HCV patients, regardless of mode of
transmission, and results have varied. Age at the time of
infection, alcohol consumption and gender were shown to be primary
risk factors of disease progression, and the median duration of
infection associated with progression to cirrhosis was 30 years
(21). However, a number of clinical
trials investigating the incidence of cirrhosis have ignored the
duration of infection, transmission route, alcohol consumption, and
other associated factors, such as HIV co-infection in patients with
HCV (22–25). In addition, numerous clinical trials
failed to perform continuous, long-term observation of disease
progression, resulting in significant differences in the rate of
disease progression between various clinical trials (22–25). One
report showed that progression of mild fibrosis occurred over a
median interval of 52.5 months, while another reported that
progression of fibrosis occurred in patients with mild chronic HCV
within 5–10 years after infection and was associated with age,
alcohol consumption and inflammatory activity (26,27).
Although children with chronic HCV showed no significant histologic
progression of disease at 5 years after infection, almost a third
of the children showed increased severity of fibrosis (28). A long-term study of HCV infections
acquired at birth and followed for 35 years showed a slow disease
progression and mild outcome (29).
Results from another long-term study showed that cirrhosis in
transfusion-acquired HCV patients was significantly associated with
age at the time of infection and disease activity (30).
The progression to cirrhosis is often clinically
silent, and some patients are not diagnosed with HCV until they
present with complications of end-stage liver disease or HCC
(31). Approximately 10–20% of
patients developed cirrhosis and 1–3% of them developed HCC after
20 years of HCV infection (9). In
the current study, based on clinical observations, patients with
HCV gradually progressed to severe liver disease after ~20 years of
HCV infection, and the progression seemed more rapid after 20
years, suggesting that 20 years is an important time point for
evaluation. Since the majority of cases of HCV infection in China
today were transfusion-acquired more than twenty years earlier, it
can be suggested that patients with a 20-year duration of HCV
infection are at a critical time point in terms of disease
progression and must be evaluated for signs of progression.
It was previously reported that more than half of
chronic patients with HCV had a history of transfusion, with a mean
interval of 10 years, 21.2 years and 29 years between the time of
transfusion and the time of diagnosis of chronic HCV, cirrhosis and
HCC, respectively (32). Another
study showed that 15.3% of patients with post-transfusion chronic
HCV died from liver failure or HCC (33). These data suggested a slow,
sequential progression of HCV infection acquired following
transfusion. The lack of large-scale screening for hepatitis C in
China's blood product industry prior to 1993 resulted in a large
number of transfusion-related HCV infections.
Results of the present study indicated that patients
in the SD group with progressive disease were significantly older
at the time of observation compared with patients in the H group
with chronic hepatitis and no obvious progression. In addition
patients in the SD group were significantly older at the time of
blood transfusion and had a longer mean duration of HCV infection
compared with patients in the H group. In this study, duration of
infection signified the time between the onset of HCV infection and
the time at which the case was closed. Age at the time of
transfusion, duration of infection and log HCV RNA values were
significant risk factors for serious development. The present study
showed that a majority of HCV-positive patients with HCC had also
been diagnosed with cirrhosis. This is in contrast with HBV-related
HCC, in which cirrhosis is present in a majority of cases (73–85%),
but HBV infection is known to progress to HCC in the absence of
cirrhosis (34).
Data from the present study showed that male gender
and age at the time of blood transfusion were significant risk
factors for HCC in patients with transfusion-acquired HCV. However,
other studies have suggested that females may be at higher risk for
HCC. Approximately 62.5% of asymptomatic patients with
post-transfusion HCV who underwent biopsy for cirrhosis were
female, and the median duration of infection was 21.5 years
(35). However, that study reported
a low progression to cirrhosis (20%), possibly because subjects
were middle-aged, asymptomatic and infected at a young age. In
contrast, a recent study in Italy by Zavaglia et al
(36) of 248 previously transfused
patients with HCV infection agreed with the data in the current
study and showed that age at transfusion and male gender were
independent predictors of HCC development; in fact, age at
transfusion was shown to affect the risk for decompensation. In the
present study, although gender was not a risk factor for the
progression of liver disease, among those who showed the
development of progressive disease (SD group), males had a higher
risk of progression to HCC compared with females. This suggested
that sex hormones may serve a role in HCV-related HCC. This finding
of gender disparity is similar to results of previous studies
(37,38). The Shimizu and Ito (39) study has suggested that estrogens
protect against oxidative stress in liver injury and hepatic
fibrosis.
Importantly, the present study demonstrated that
patients with cirrhosis and HCC had significantly lower HCV RNA
titers compared with patients with chronic hepatitis. This may
possibly be due to hepatocyte damage induced by HCV replication and
the immune response, resulting in necrosis of a large number of
liver cells and a subsequent decrease in viral replication. A
previous study indicated that HCV RNA in serum tended to increase
with the progression of histopathological changes in the liver
(40), while other data have
demonstrated that patients with chronic active hepatitis had
significantly higher HCV RNA titers compared with patients with
chronic persistent hepatitis and those with cirrhosis or HCC
(41). Although the data in the
current study demonstrated a correlation between HCV RNA titers in
patients with chronic hepatitis, cirrhosis and HCC, the HCV RNA
titers reported in this study represent the titers at the time of
patient enrollment, and not over the entire course of the
disease.
A number of studies have investigated the role of
HCV genotype in determining the severity and outcome of disease in
HCV-infected patients (42).
Genotype 1b was shown to be more prevalent among patients with
decompensated cirrhosis and HCC (2,11,43). In
addition, HCV genotype 1b was shown to influence the risk of HCC in
patients with cirrhosis (44–46).
However, other studies concluded that there was no significant
association between HCV genotype and occurrence of cirrhosis
(47,48). In the present study, although not
statistically significant, a higher number of patients with HCC had
genotype 1, compared with genotype 2, and genotype was not a
significant risk factor for HCV disease progression in the selected
geographic area. In future studies, the aim is to investigate
further whether HCV genotypes serve a role in the pathogenicity of
HCV disease in China.
Results of the present study are limited by several
factors. First, this was a retrospective study, which precludes
inferring direct causation. The retrospective nature of the study
also precluded access to direct evidence of HCV-positive donor
status. Secondly, the study focused on the progression of HCV
infection and risk factors associated with transfusion-acquired
disease, and did not investigate other risk factors for
progression, such as obesity, insulin resistance, organ
transplantation and tobacco smoking. In addition, the study did not
collect data on the number of patients with HIV-positive status,
although all enrolled patients were HIV negative. Additionally,
almost half the patients were not genotyped, either due to economic
considerations, or because their physicians did not recommend it
(possibly because the majority of Chinese patients are known to
have genotype 1b) (49). Samples
which were genotyped were grouped into the SD or H groups based on
disease severity. The number of samples in the two groups was
therefore not the same, and it is not possible to make a definitive
conclusion regarding the influence of genotypes on outcomes. In
addition, it is acknowledged that a study based on anti-HCV and
HCV-RNA seropositivity may introduce bias regarding higher
progression rates. Future large multicenter prospective cohort
studies are necessary to confirm the findings of the present study
and to further define the role of HCV genotypes in the outcomes of
Chinese patients with transfusion-acquired HCV infection.
In conclusion, Chinese patients with HCV aged >50
years, who were infected for more than 20 years with
transfusion-acquired HCV, showed significant disease progression.
There was a positive correlation between the duration of HCV
infection and the possibility of progressing to chronic hepatitis.
Male gender and age at the time of transfusion were significant
risk factors for HCC development. The exponential progression of
transfusion-acquired HCV makes this disease a serious challenge for
infected patients in the third and fourth decades after infection.
Although the present study did not demonstrate a significant
correlation between genotype and disease progression, the results
will be particularly helpful for clinical nursing staff and
physicians to predict the progression of transfusion-acquired HCV
infection based on patients' gender and age at the time of
transfusion-acquired infection and duration of infection.
References
|
1
|
Alter MJ: Epidemiology of hepatitis C
virus infection. World J Gastroenterol. 13:2436–2441. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Irshad M, Mankotia DS and Irshad K: An
insight into the diagnosis and pathogenesis of hepatitis C virus
infection. World J Gastroenterol. 19:7896–7909. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lauer GM and Walker BD: Hepatitis C virus
infection. N Engl J Med. 345:41–52. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pybus OG, Barnes E, Taggart R, Lemey P,
Markov PV, Rasachak B, Syhavong B, Phetsouvanah R, Sheridan I,
Humphreys IS, et al: Genetic history of hepatitis C virus in East
Asia. J Virol. 83:1071–1082. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Xia GLLC, Cao HL, Bi SL, Zhan MY, Su CA,
Nan JH and Qi XQ: Prevalence of hepatitis B and C virus infections
in the general Chinese population. Results from a nationwide
cross-sectional seroepidemiologic study of hepatitis A, B, C, D and
E virus infections in China. Int Hepatol Commun. 5:62–73. 1996.
View Article : Google Scholar
|
|
6
|
Kato N, Yokosuka O, Hosoda K, Ito Y, Ohto
M and Omata M: Detection of hepatitis C virus RNA in acute non-A,
non-B hepatitis as an early diagnostic tool. Biochem Biophys Res
Commun. 192:800–807. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yuki N, Hayashi N, Ohkawa K, Hagiwara H,
Oshita M, Katayama K, Sasaki Y, Kasahara A, Fusamoto H and Kamada
T: The significance of immunoglobulin M antibody response to
hepatitis C virus core protein in patients with chronic hepatitis
C. Hepatology. 22:402–406. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
TCLDA: IDaPDB, . Prevention and treatment
of hepatitis C. Chin J Hepatol. 12:194–198. 2004.
|
|
9
|
Ding JYX and Zhang HM: Analysis on testing
of elderly patients with hepatitis C. Lab Med Clin. 17:1474–1475.
2009.
|
|
10
|
Wiese M, Fischer J, Lobermann M, Göbel U,
Grüngreiff K, Güthoff W, Kullig U, Richter F, Schiefke I, Tenckhoff
H, et al: East German HCV Study Group: Evaluation of liver disease
progression in the German hepatitis C virus (1b)-contaminated
anti-D cohort at 35 years after infection. Hepatology. 59:49–57.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zein NN: Clinical significance of
hepatitis C virus genotypes. Clin Microbiol Rev. 13:223–235. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Messina JP, Humphreys I, Flaxman A, Brown
A, Cooke GS, Pybus OG and Barnes E: Global distribution and
prevalence of hepatitis C virus genotypes. Hepatology. 61:77–87.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Utama A, Budiarto BR, Monasari D, Octavia
TI, Chandra IS, Gani RA, Hasan I, Sanityoso A, Miskad US, Yusuf I,
et al: Hepatitis C virus genotype in blood donors and associated
liver disease in Indonesia. Intervirology. 51:410–416. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Fried MW, Shiffman ML, Reddy KR, Smith C,
Marinos G, Gonçales FL Jr, Häussinger D, Diago M, Carosi G,
Dhumeaux D, et al: Peginterferon alfa-2a plus ribavirin for chronic
hepatitis C virus infection. N Engl J Med. 347:975–982. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kieffer TL, Sarrazin C, Miller JS, Welker
MW, Forestier N, Reesink HW, Kwong AD and Zeuzem S: Telaprevir and
pegylated interferon-alpha-2a inhibit wild-type and resistant
genotype 1 hepatitis C virus replication in patients. Hepatology.
46:631–639. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kieffer TL, Kwong AD and Picchio GR: Viral
resistance to specifically targeted antiviral therapies for
hepatitis C (STAT-Cs). J Antimicrob Chemother. 65:202–212. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Allain JP, Thomas I and Sauleda S: Nucleic
acid testing for emerging viral infections. Transfus Med.
12:275–283. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Xu GG, Wu SM, Zhou XQ, Zhang QB, Kang LY,
Zhou XM, Jiang Y, Qi X and Ren XJ: Clinical characteristics of 638
patients infected with hepatitis C virus by post blood transfusion,
in Shanghai. Zhonghua Liu Xing Bing Xue Za Zhi. 32:388–391.
2011.(In Chinese). PubMed/NCBI
|
|
19
|
Dabbouseh NM and Jensen DM: Future
therapies for chronic hepatitis C. Nat Rev Gastroenterol Hepatol.
10:268–276. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Rerksuppaphol S, Hardikar W and Dore GJ:
Long-term outcome of vertically acquired and post-transfusion
hepatitis C infection in children. J Gastroenterol Hepatol.
19:1357–1362. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Poynard T, Bedossa P and Opolon P: Natural
history of liver fibrosis progression in patients with chronic
hepatitis C. The OBSVIRC, METAVIR, CLINIVIR and DOSVIRC groups.
Lancet. 349:825–832. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Conjeevaram HS, Fried MW, Jeffers LJ,
Terrault NA, Wiley-Lucas TE, Afdhal N, Brown RS, Belle SH,
Hoofnagle JH, Kleiner DE, et al: Peginterferon and ribavirin
treatment in African American and Caucasian American patients with
hepatitis C genotype 1. Gastroenterology. 131:470–477. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ferenci P, Fried MW, Shiffman ML, Smith
CI, Marinos G, Goncales FL Jr, Häussinger D, Diago M, Carosi G,
Dhumeaux D, et al: Predicting sustained virological responses in
chronic hepatitis C patients treated with peginterferon alfa-2a (40
KD)/ribavirin. J Hepatol. 43:425–433. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jensen DM, Marcellin P, Freilich B,
Andreone P, Di Bisceglie A, Brandão-Mello CE, Reddy KR, Craxi A,
Martin AO, Teuber G, et al: Re-treatment of patients with chronic
hepatitis C who do not respond to peginterferon-alpha2b: A
randomized trial. Ann Intern Med. 150:528–540. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Shiffman ML, Di Bisceglie AM, Lindsay KL,
Morishima C, Wright EC, Everson GT, Lok AS, Morgan TR, Bonkovsky
HL, Lee WM, et al: Hepatitis C Antiviral Long-Term Treatment
Against Cirrhosis Trial Group.: Peginterferon alfa-2a and ribavirin
in patients with chronic hepatitis C who have failed prior
treatment. Gastroenterology. 126:1015–1023; discussion 1947. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Alberti A, Benvegnù L, Boccato S, Ferrari
A and Sebastiani G: Natural history of initially mild chronic
hepatitis C. Dig Liver Dis. 36:646–654. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Boccato S, Pistis R, Noventa F, Guido M,
Benvegnù L and Alberti A: Fibrosis progression in initially mild
chronic hepatitis C. J Viral Hepat. 13:297–302. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Mohan P, Barton BA, Narkewicz MR,
Molleston JP, Gonzalez-Peralta RP, Rosenthal P, Murray KF, Haber B,
Schwarz KB and Goodman ZD: Evaluating progression of liver disease
from repeat liver biopsies in children with chronic hepatitis C: A
retrospective study. Hepatology. 58:1580–1586. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Casiraghi MA, De Paschale M, Romanò L,
Biffi R, Assi A, Binelli G and Zanetti AR: Long-term outcome (35
years) of hepatitis C after acquisition of infection through mini
transfusions of blood given at birth. Hepatology. 39:90–96. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Minola E, Prati D, Suter F, Maggiolo F,
Caprioli F, Sonzogni A, Fraquelli M, Paggi S and Conte D: Age at
infection affects the long-term outcome of transfusion-associated
chronic hepatitis C. Blood. 99:4588–4591. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chen SL and Morgan TR: The natural history
of hepatitis C virus (HCV) infection. Int J Med Sci. 3:47–52. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kiyosawa K, Sodeyama T, Tanaka E, Gibo Y,
Yoshizawa K, Nakano Y, Furuta S, Akahane Y, Nishioka K, Purcell RH,
et al: Interrelationship of blood transfusion, non-A, non-B
hepatitis and hepatocellular carcinoma: Analysis by detection of
antibody to hepatitis C virus. Hepatology. 12:671–675. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Tong MJ, el-Farra NS, Reikes AR and Co RL:
Clinical outcomes after transfusion-associated hepatitis C. N Engl
J Med. 332:1463–1466. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Salhab M and Canelo R: An overview of
evidence-based management of hepatocellular carcinoma: A
meta-analysis. J Cancer Res Ther. 7:463–475. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Reggiardo MV, Fay F, Tanno M,
Garcia-Camacho G, Bottaso O, Ferretti S, Godoy A, Guerrita C, Paez
M, Tanno F, et al: Natural history of hepatitis C virus infection
in a cohort of asymptomatic post-transfused subjects. Ann Hepatol.
11:658–666. 2012.PubMed/NCBI
|
|
36
|
Zavaglia C, Silini E, Mangia A, Airoldi A,
Piazzolla V, Vangeli M, Stigliano R, Foschi A, Mazzarelli C and
Tinelli C: Prognostic factors of hepatic decompensation and
hepatocellular carcinoma in patients with transfusion-acquired HCV
infection. Liver Int. 34:e308–e316. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Akiyama T, Mizuta T, Kawazoe S, Eguchi Y,
Kawaguchi Y, Takahashi H, Ozaki I and Fujimoto K: Body mass index
is associated with age-at-onset of HCV-infected hepatocellular
carcinoma patients. World J Gastroenterol. 17:914–921. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Farinati F, Sergio A, Giacomin A, Di Nolfo
MA, Del Poggio P, Benvegnu L, Rapaccini G, Zoli M, Borzio F,
Giannini EG, et al: Italian Liver Cancer group: Is female sex a
significant favorable prognostic factor in hepatocellular
carcinoma? Eur J Gastroenterol Hepatol. 21:1212–1218. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Shimizu I and Ito S: Protection of
estrogens against the progression of chronic liver disease. Hepatol
Res. 37:239–247. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kato N, Yokosuka O, Hosoda K, Ito Y, Ohto
M and Omata M: Quantification of hepatitis C virus by competitive
reverse transcription-polymerase chain reaction: Increase of the
virus in advanced liver disease. Hepatology. 18:16–20. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Mita E, Hayashi N, Kanazawa Y, Hagiwara H,
Ueda K, Kasahara A, Fusamoto H and Kamada T: Hepatitis C virus
genotype and RNA titer in the progression of type C chronic liver
disease. J Hepatol. 21:468–473. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Ripoli M and Pazienza V: Impact of HCV
genetic differences on pathobiology of disease. Expert Rev Anti
Infect Ther. 9:747–759. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Silini E, Bono F, Cividini A, Cerino A,
Bruno S, Rossi S, Belloni G, Brugnetti B, Civardi E, Salvaneschi L,
et al: Differential distribution of hepatitis C virus genotypes in
patients with and without liver function abnormalities. Hepatology.
21:285–290. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Kobayashi M, Tanaka E, Sodeyama T,
Urushihara A, Matsumoto A and Kiyosawa K: The natural course of
chronic hepatitis C: A comparison between patients with genotypes 1
and 2 hepatitis C viruses. Hepatology. 23:695–699. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Silini E, Bottelli R, Asti M, Bruno S,
Candusso ME, Brambilla S, Bono F, Iamoni G, Tinelli C, Mondelli MU
and Ideo G: Hepatitis C virus genotypes and risk of hepatocellular
carcinoma in cirrhosis: A case-control study. Gastroenterology.
111:199–205. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Takada A, Tsutsumi M, Zhang SC, Okanoue T,
Matsushima T, Fujiyama S and Komatsu M: Relationship between
hepatocellular carcinoma and subtypes of hepatitis C virus: A
nationwide analysis. J Gastroenterol Hepatol. 11:166–169. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Dusheiko G, Schmilovitz-Weiss H, Brown D,
McOmish F, Yap PL, Sherlock S, McIntyre N and Simmonds P: Hepatitis
C virus genotypes: An investigation of type-specific differences in
geographic origin and disease. Hepatology. 19:13–18. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Yamada M, Kakumu S, Yoshioka K, Higashi Y,
Tanaka K, Ishikawa T and Takayanagi M: Hepatitis C virus genotypes
are not responsible for development of serious liver disease. Dig
Dis Sci. 39:234–239. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Dong ZX, Zhou HJ, Wang JH, Xiang XG,
Zhuang Y, Guo SM, Gui HL, Zhao GD, Tang WL, Wang H and Xie Q:
Distribution of hepatitis C virus genotypes in Chinese patients
with chronic hepatitis C: Correlation with patients'
characteristics and clinical parameters. J Dig Dis. 13:564–570.
2012. View Article : Google Scholar : PubMed/NCBI
|