Introduction
Cerebral hemorrhage remains a worldwide health
burden, causing high morbidity, mortality, and costs to health care
systems, and is the primary cause of serious long-term disabilities
in the developed and developing countries (1). Recent advances in stem cell research
have led to the development of cell-based therapies for tissue
damage caused by injury or disease (2). This growing field of medicine brings
the promise of stem cell therapies that may restore the original
tissues. In this pursuit, advancements in neurogenesis through the
use of mesenchymal stem cells (MSCs) may provide the fundamental
components that are missing from classical single molecule-based
pharmaceutical interventions to enhance functional recovery after a
cerebral hemorrhagic episode.
MSCs are a heterogeneous population of stem cells
that are able to differentiate into various cell types, including
osteoblasts, chondrocytes, adipocytes and muscle cells (3). The use of human MSCs has shown enormous
therapeutic potential in neurological regenerative medicine
(4–7). MSCs can be isolated from various
tissues, including bone marrow, adipose tissue, synovium,
periosteum, skeletal muscles, and umbilical cord tissues (8). In previous studies, we demonstrated the
immunologic compatibility and osteogenic properties of human
umbilical cord-derived mesenchymal stem cells (Hu-MSCs)in tibia
non-union in rats (9,10) and patients (11). The primary aim of the present study
was to examine in cerebral hemorrhage both with bone marrow
mononuclear cells/Hu-MSCs and with conventional surgical
approaches, which enhanced regenerative support from transplanted
cells to effectively treat acute and chronic disorders of the
nervous system. The secondary objective was to determine the safety
of cell therapy in patients with cerebral hemorrhage.
Materials and methods
Study population
A retrospective analysis was performed on a cohort
of 24 patients treated for cerebral hemorrhage. The patients were
admitted to the Department of Neurosurgery, Siping Hospital of
China Medical University (Siping, China) from October 1, 2007 to
October 1, 2009.
The present study was approved by the ethics
committee of Siping Hospital of China Medical University. All the
patients in the cohort were ≥18 years of age. Cerebral hemorrhage
patients were treated within the first 6 h of the onset of the
hemorrhagic episode. Patients with life-threatening conditions,
limited follow-up, missing stratification information, or without
standard indications for rehabilitation were excluded from the
study. The criteria for exclusion were chosen to ensure that all
the patients were treated according to the best medical practices.
Written informed consent was obtained from the patients prior to
any and all procedures.
For data analysis, 24 patients were classified into
one of three groups, with all groups receiving conventional
surgical treatment: group 1, the control group, had 8 patients who
received hematoma removal surgery alone; group 2 had 7 patients who
received additional autologous bone marrow mononuclear cell
transplant; and group 3 had 9 patients who received an additional
umbilical cord MSCs allograft instead. The general description of
the patients is shown in Table I.
Blood was taken from the internal jugular veins.
 | Table I.Inclusion and exclusion criteria. |
Table I.
Inclusion and exclusion criteria.
| No. of criterion
type |
|---|
| Inclusion |
| 1. Men or
women (women of non-child-bearing age only), aged 30–75 years |
| 2.
Cerebral hemorrhage observed within 6 h of the onset of
symptoms |
| 3.
Radiological: |
| Maximum
diameter of the stroke region in any dimension ≤10 mm |
| Damage
not involving more than 50% of the ipsilateral subventricular
zonea |
| 4.
Moderate to severe persistent neurologic deficit (National
Institutes of Health Stroke Scale of 6–21 inclusive) |
| Exclusion |
| 1.
Hematologic disorders or bone marrow suppression |
| 2. Severe
medical illness defined as: |
|
Severe heart
failure |
|
Severe febrile
illness |
|
Hepatic or renal
dysfunction |
|
Active cancer |
|
Any evidence of
chronic co-morbid condition or unstable acute systemic illness
which, in the investigator's opinion, could shorten survival or
limit the ability to complete the study |
| 3.
Lactating women or pregnant women as determined by positive urine
hCG test |
| 4.
Considered unwilling or unable to comply with the procedures and
study visit schedule outlined in the protocol |
| 5.
Unwilling to undergo bone marrow aspiration |
Definition of cerebral hemorrhage
The diagnosis of cerebral hemorrhage was confirmed
on radiographs at the discretion of the admitting doctor (12).
Bone marrow mononuclear cell
isolation
Bone marrow mononuclear cells were isolated from 40
ml of bone marrow harvested from the posterior iliac crest of each
patient undergoing an autologous transplant. The procedure for
harvesting human MSCs followed the method described in our previous
study (13). Briefly, the bone
marrow samples were washed with Dulbecco's modified Eagle's
medium-low glucose (DMEM-LG; Invitrogen Life Technologies,
Carlsbad, CA, USA) supplemented with 10% of an aliquot of the
patient's own blood plasma. To isolate the mononuclear cells, the
samples were subjected to a preformed Percoll™ (Sigma, St. Louis,
MO, USA) density gradient (1.073 g/ml) centrifugation step. The
total number of MSCs was ~1.8×108 cells for each
patient.
Umbilical cord harvesting
Five human umbilical cord (UC), samples were
collected after informed written consent was obtained from the
mothers. The study protocol was approved by the Institutional
Ethics Committee of Siping Hospital of China Medical University and
all the experimental animal procedures were in accordance with the
national/international guidelines for ethical conduct in the care
and use of animals. Regarding each sample, UC sections of 8–10 cm
were internally washed with phosphate-buffered saline (PBS)
containing 300 U/ml penicillin and 300 g/ml streptomycin (Gibco,
Grand Island, NY, USA) and immediately immersed in DMEM-LG (Gibco)
supplemented with 10% fetal bovine serum, 300 U/ml penicillin, and
0.3 mg/ml streptomycin. The samples were processed within 12–15 h
after collection.
Isolation and culture of adherent
cells from the UC
The UCs were filled with 0.1% collagenase
(Sigma-Aldrich, St. Louis, USA) in PBS and incubated at 37°C for 20
min as previously described (9,10). Each
UC was washed with proliferation medium and the detached cells were
harvested after gentle massage of the UC. The cells were
centrifuged at 300 × g for 10 min, resuspended in proliferation
medium, and seeded in 75-cm2 flasks at a density of
5×107 cells/ml. After incubation for 24 h, non-adherent
cells were removed and the culture medium was replaced every 3
days. Adherent cells were cultured until they reached 80–90%
confluence.
Laboratory measurements
Patient's serum levels of T-cell subtypes were
analysed using flow cytometry 5 years after the
transplantation.
Flow cytometry
To analyze the cell surface expression of the
typical protein markers, adherent cells were incubated with the
following anti-human conjugated primary antibodies (BD Biosciences,
Franklin Lakes, NJ, USA): CD4-phycoerythrin (PE) (monoclonal,
dilution: 0.2 µl/1×106 cells, catalog no.: 565999);
CD8-fluorescein isothiocyanate (monoclonal, dilution: 0.2
µl/1×106 cells, catalog no.: 557696); CD56 (monoclonal,
dilution: 0.4 µl/1×106 cells, catalog no.: 556647); and
HLA-DR-R-PE (monoclonal, dilution: 0.8 µl/1×106 cells,
catalog no.: 560943). Unconjugated markers were added to react with
anti-mouse PE-conjugated secondary antibody (eBioscience, San
Diego, CA, USA; catalog no.: 11-5921; dilution: 1/200). A total of
10,000 labeled cells were analyzed using a BD LSRFortessa™ Cell
Analyzer (BD Biosciences, San Jose, CA, USA) and the data obtained
were analyzed using BD FACSDiva software 6.0 (BD Biosciences).
Cell transplantation
The patients were treated with conventional hematoma
removal surgery within 6 h after hemorrhage. However, in the case
of the transplant patients, one end of a 10-cm silicone tube was
inserted into the hematoma cavity, the other side was placed
subcutaneously, and then sutured. After 2 weeks, and under
anesthesia with 5 ml of 2% lidocaine, the scalp was cut and the
extracranial end of the silicone tube was found. BMSCs or Hu-MSCs
were grafted through the lumen of the tube. The catheter was then
returned as before. A second injection of BMSCs or Hu-MSCs was
instilled 1 week later, and this time the indwelling tube was
pulled out carefully during the course of 10 min, and finally the
scalp was sutured. The patients were sent to the hospital ward for
recovery. Antibiotics were given to prevent infection.
Treatment protocol
The patients received a standard treatment
consisting of: i) Anti-hypertensive medication such as sodium
nitrate tome (Hongyuan Group, Quanzhou, Fujian, China); ii)
neurotrophic drugs: N-α-acetylglycinamide (Pfizer Pharmaceutical
Co., Ltd., Shanghai, China), and/or sodium
monosialotetrahexosylganglioside injection (Qilu Pharmaceutical
Co., Ltd., Jinan, China); iii) intracranial pressure control with
20% mannitol (Kelun Pharmaceutical Co., Ltd., Chengdu, China); and
iv) neurological rehabilitation sessions.
Radiological evaluation
Radiographs were obtained for the patients following
admission to the hospital before and 2 days, 4 weeks after
operation, and then 3, 6 and 7 days, and 12 months after
transplantation. Three independent observers blinded to the
assigned treatment assessed the radiographs of each patient for
cerebral hemorrhage.
Outcomes
Primary clinical evaluations included assessments of
the length of stay in the intensive care unit (ICU), readmission to
the ICU, length of stay in the hospital, infection, neurological
dysfunction [including National Institutes of Health Stroke Scale
(NIHSS), modified Rankin scale (mRS), modified Barthel index
(mBI)], time of healing, pain at the iliac crest and infection. For
analysis of the outcomes of the primary clinical evaluations, the
categorical shift in mRS at 90 days after treatment was used.
Scores from 0 to 5 were used for the mRS, by merging the last scale
of 6 (death) with that of 5 (severe disability). Clinical outcomes
were followed-up by phone interview at 4 weeks, and in person at 3,
6, 12, 36 and 60 months.
The secondary evaluations involved checking the
percentage of CD4, CD8, CD56 and HLA-DR in peripheral blood 3 years
after the transplantation to determine whether the patients had
suffered any immune rejections. Secondary and exploratory
evaluation outcomes are shown in Table
II.
 | Table II.Hematoma reabsorption time evaluated
by computed tomography scan. |
Table II.
Hematoma reabsorption time evaluated
by computed tomography scan.
| Characteristics | Control group | Auto-graft group | Allograft group |
|---|
| Hematoma reabsorbed
partly, months |
|
|
|
| Mean | 1.9 | 1.3 | 1.3 |
|
Range | 1.3–2.7 | 0.9–2.1 | 0.9–2.2 |
| Hematoma reabsorbed
completely, months |
|
|
|
| Mean | 4.5 | 3.2 | 3.3 |
|
Range | 3.5–6.1 | 2.8–4.3 | 2.8–4.1 |
Data collection
Data were collected through person-to-person
interactions after training all the interviewers to use
standardized scripts. Each interview started with the respondent
first reading a short non-technical description of the limbs'
function scale explaining the possible limb health states.
Investigators blinded to treatment allocations measured the
neurological disability of each case using the NIHSS and functional
scores for mRS and mBI.
Statistical analysis
Data obtained were presented as means ± standard
deviation values and compared between groups using one-way ANOVA.
P<0.05 was considered to indicate a statistically significant
difference.
Results
General
The patients were older in the Hu-MSC treatment
group (Table I). The patients were
followed-up for 5 years through the recheck outpatient department
and with radiological examinations to confirm the treatment
effectiveness.
Hematoma reabsorption analysis
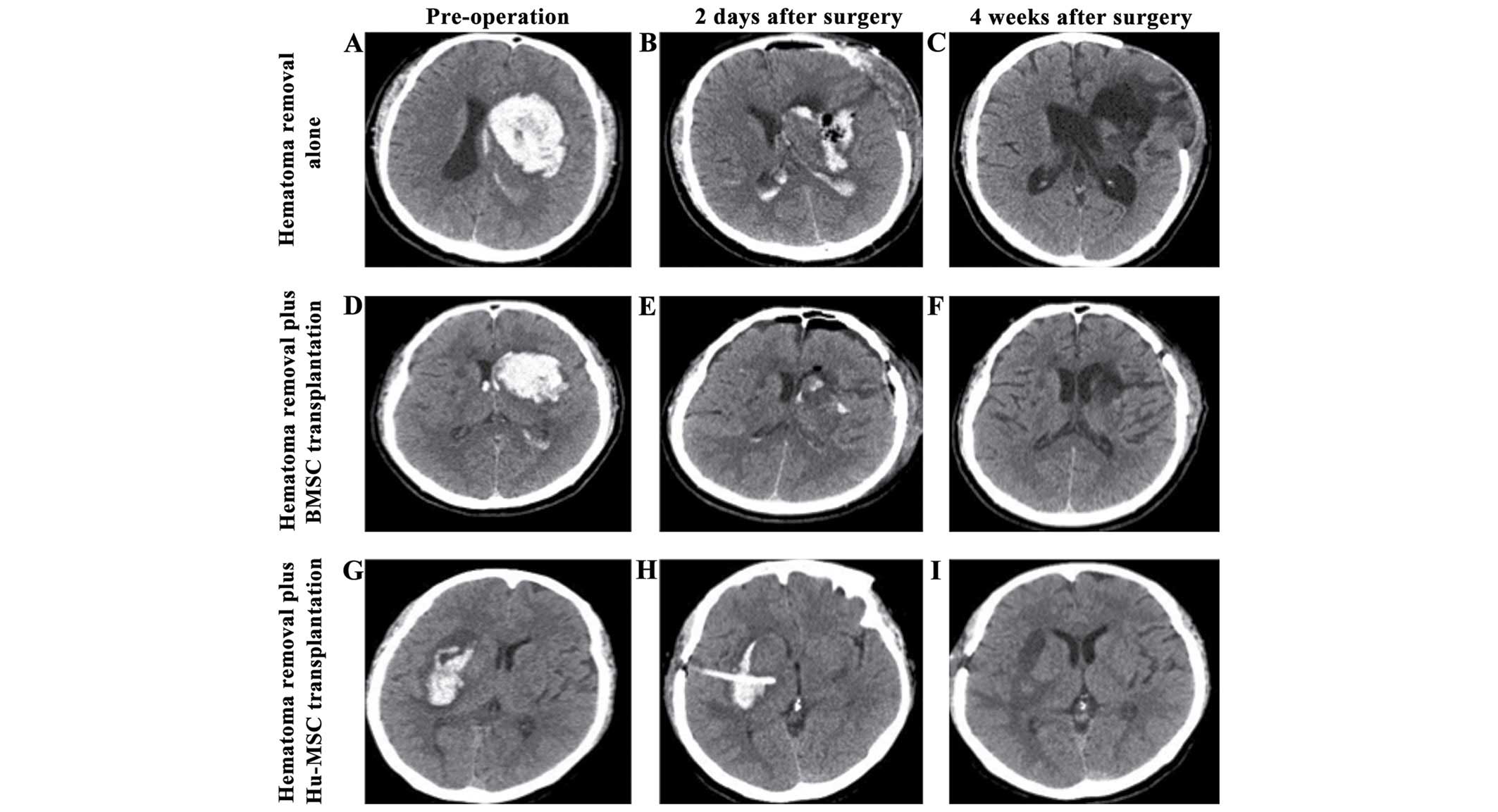
As shown in Fig. 1,
the initial hematoma volume was similar in all the cases. Two days
after hematoma removal surgery, a small amount of hematoma remained
in the cavity. At 2 weeks after Hu-MSCs (Fig. 1F) or BMSCs (Fig. 1I) transplantation, the bleeding site
was evident as low-density small narrow strips surrounded by
edematous brain tissue, while the bleeding site in the control
group consisted of a larger squared region.
Neurological disability and function
evaluation
As shown in Table
III, the mean time for hematoma reabsorption was similar in all
the groups. The computed tomography (CT) scans for brain tissue
healing showed better outcomes in the two cell transplanted groups
than in the control group (Fig.
1A-C). Approximately 3.5 months after the graft, CT scans
showed the hematoma was completely reabsorbed. NIHSS, mRS and mBI
scores were similar in the cell transplantation groups (Table IV). Nevertheless, the outcomes in
the cell-transplanted groups were better than those in the control
group at 5 years. Of note, the patients who underwent Hu-MSC
allograft had better outcomes than those who underwent autologous
BMSC transplantation starting at 3 months and during the rest of
the follow-up period, as evaluated by the NIHSS score (Table IV). After 6 months the mRS and mBI
score tendencies in each group did not vary significantly.
 | Table III.Baseline data in patients. |
Table III.
Baseline data in patients.
| Characteristics | Control group | Auto-graft group | Allograft group |
|---|
| Gender, no. |
|
|
|
| Male | 5 | 5 | 6 |
|
Female | 3 | 2 | 3 |
| Age, years |
|
|
|
| Mean | 51.32 | 42.43 | 52.36 |
|
Range | 40–55 | 38–44 | 44–58 |
| BMI,
m/kg2 |
|
|
|
| Mean | 25.73 | 24.62 | 25.13 |
|
Range | 23.6–26.2 | 22.7–25.6 | 22.9–27.3 |
| Smoking status,
package-year |
|
|
|
| Mean | 8.41 | 9.15 | 12.33 |
|
Range | 0–23 | 0–22 | 0–23 |
| Location of
hemorrhage, no. |
|
|
|
| Basal
ganglia | 5 | 4 | 5 |
|
Subcortical | 3 | 3 | 4 |
| Hypertension,
years |
|
|
|
| Mean | 14.83 | 11.47 | 16.16 |
|
Range | 11–23 | 6–22 | 9–23 |
|
Hypertension, no. | 8 | 7 | 9 |
| Diabetes mellitus,
years |
|
|
|
| Mean | 3.75 | 3.66 | 5.44 |
|
Range |
6–24 |
9–18 | 11–21 |
| Diabetes mellitus,
no. | 2 | 3 | 3 |
| Infection, no. | 4 | 3 | 4 |
| Total cholesterol,
mg/dl | 5.71±0.43 | 5.69±0.55 | 5.75±0.64 |
| Triglycerides,
mg/dl | 2.32±0.28 | 2.46±0.69 | 2.49±0.71 |
 | Table IV.Outcome data of total population. |
Table IV.
Outcome data of total population.
| Variables | Control group | Auto-graft
group | Allograft
group |
|---|
| Readmission rate to
ICUa (%) | 1/8 | 0/7 | 0/9 |
| ICU LOS (days) |
4.3±0.8 | 4.6±0.3 | 4.2±0.9 |
| Hospital LOS
(days) | 29.8±3.4 | 28.6±2.8 | 29.4±2.1 |
| NIHSS score |
|
|
|
| Values
during 24 h treatment, % readings | 19.1±0.3 | 20.4±0.7 | 21.1±0.6 |
|
NIHSS |
|
|
|
| Values
before left ICU treatment, % readings | 16.5±1.5 | 15.9±1.1 | 16.3±0.7 |
|
NIHSS |
|
|
|
| Values
during 4 weeks treatment, % readings | 14.9±0.8 | 13.3±0.7 | 13.6±0.2 |
|
NIHSS |
|
|
|
| Values
during 3 months treatment, % readings | 14.6±0.9 |
12.9±0.3b |
10.5±0.6b,c |
|
NIHSS |
|
|
|
| Values
during 6 months treatment, % readings | 13.9±0.4 |
10.6±0.6b |
9.6±0.5b,c |
|
NIHSS |
|
|
|
| Values
during 12 months treatment, % readings | 13.6±0.6 |
9.3±0.4b |
8.9±0.8b,c |
|
NIHSS |
|
|
|
| Values
during 24 months treatment, % readings | 13.1±0.3 |
9.6±0.3b |
8.1±0.3b,c |
|
NIHSS |
|
|
|
| Values
during 36 months treatment, % readings | 13.3±0.6 |
9.1±0.5b |
7.8±0.6b,c |
|
NIHSS |
|
|
|
| Values
during 60 months treatment, % readings mRS score | 13.3±0.4 |
9.3±0.3b |
7.6±0.5b,c |
Donor site complications
In total, 3 patients complained of moderate pain at
the iliac crest, and therefore the donor site-related morbidity
rate was 42.9%. However, none of the patients suffered permanent
pain at the iliac crest, harvesting-related swelling, redness,
drainage, infection or neurological deficits.
Intensity of pain and level of
treatment-dissatisfaction
During follow-up, patients of all the groups stated
to have approximately equal intensity of index operation-related
pain. Additionally, 2 patients in the autologous-transplant group
(28.5%) compared to all the patients of the allograft treatment
group (100%) were satisfied or only minimally dissatisfied with the
hematoma removal treatment. Thus, patients of the allograft group
were significantly less dissatisfied with the treatment compared to
those of the autologous group (data not shown).
Serum levels of T-cell subtypes
The serum levels of CD4, CD56 and HLA-DR in the
allograft group were negative, while the serum levels of CD8 were
4.6% positive. There were no evident differences in T-cell subtypes
among the 3 groups (data not shown).
Discussion
In the current study, we reported data of a
retrospective comparative study using autologous bone marrow
mononuclear cells, Hu-MSCs or surgery alone to treat cerebral
hemorrhage. All the patients agreed to the offered treatment
options. Success of the treatment was observed in 100% of the
Hu-MSCs grafted and the autologous bone marrow mononuclear cells
grafted groups. This is the first time, to the best of our
knowledge, that the safety of an Hu-MSCs allograft in vivo
in humans has been assessed over a period of 5 years.
Different tissue-derived adult stem cells can be
employed as donor cells for transplantation to treat neurogenetic
disorders. An important factor in considering stem cell grafting
for therapy is the possibility of an immunogenic complication such
as a host graft rejection. Taking this into consideration, levels
of CD8, CD56 and HLA-DR, which are known immunological rejection
markers, were measured in all the graft patients. There were no
elevations in the levels of CD8, CD56 or HLA-DR in either group,
while the level of CD4+ T-cells was similar in the two
groups after 5 years of the grafting procedure. Some investigators
have reported elevated endogenous neurogenesis via a
CD4+ T-cell-dependent mechanism (14). Given these results and the fact that
no adjunctive immunosuppressants were administered, the data
suggest no immunological rejection or disorder in the Hu-MSCs graft
group. A limitation of the present study is that the level of
HLA-G, which is reported as a contributing factor to inducing
stronger immunosuppression (15) and
a prognostic indicator of graft tolerance (16,17) was
not observed in the allograft group when compared to the autograft
group.
MSCs can be harvested from a variety of mesenchymal
tissues, and have different characteristics in an immune response
depending on their origin. Placenta-derived MSCs show less
inhibition to CD4+ T-cell stimulation than bone
marrow-derived stem cells (18). The
current research for favorable outcomes suggests an optimal
intra-brain administration of cells 2 weeks post-hemorrhage, and a
therapeutic dose of 1 million cells. This period of opportunity
poses a challenge in generating enough stem cells from freshly
harvested autologous tissue sources.
An aim of the present study was to evaluate the
effectiveness and tolerance of Hu-MSCs compared to the autologous
bone-marrow-mononuclear-cell-grafting in the treatment of cerebral
hemorrhage. Taking all results into account, the present findings
show that the Hu-MSCs group performed better overall than the
control or even the autologous graft group, demonstrating the
effectiveness of the Hu-MSC graft treatment for cerebral
hemorrhage. Of note, 3 patients in the autologous group suffered
moderate donor site complications at the iliac crest region. By
contrast, patients treated with Hu-MSCs claimed minimal
dissatisfaction concerning the surgery during follow-up.
In summary, the present study has shown that
patients treated with Hu-MSCs had good functional recovery, no
complications, reduced treatment dissatisfaction, and a lack of
donor site complications at the iliac crest region. Therefore we
recommend that the use of Hu-MSCs offered to suitable patients as a
valuable alternative for autologous grafting. Harvesting Hu-MSCs is
easy, and is perceived as ethically correct. In conclusion, our
findings suggest that Hu-MSCs grafting is an effective and safe
method for the treatment of cerebral hemorrhage and should be
seriously considered in future studies.
Acknowledgements
The present study was supported by the National High
Technology Research and Development Program of (863 Program), nos.
2011AA020101 and 2012A020905.
References
|
1
|
Qureshi AI, Mendelow AD and Hanley DF:
Intracerebral haemorrhage. Lancet. 373:1632–1644. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
D'souza N, Rossignoli F, Golinelli G,
Grisendi G, Spano C, Candini O, Osturu S, Catani F, Paolucci P,
Horwitz EM, et al: Mesenchymal stem/stromal cells as a delivery
platform in cell and gene therapies. BMC Med. 13:1862015.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Dominici M, Le Blanc K, Mueller I,
Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A,
Prockop DJ and Horwitz E: Minimal criteria for defining multipotent
mesenchymal stromal cells. The International Society for Cellular
Therapy position statement. Cytotherapy. 8:315–317. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Shinozuka K, Dailey T, Tajiri N, Ishikawa
H, Kaneko Y and Borlongan CV: Stem cell transplantation for
neuroprotection in stroke. Brain Sci. 3:239–261. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Borlongan CV, Glover LE, Tajiri N, Kaneko
Y and Freeman TB: The great migration of bone marrow-derived stem
cells toward the ischemic brain: Therapeutic implications for
stroke and other neurological disorders. Prog Neurobiol.
95:213–228. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Liu X, Ye R, Yan T, Yu SP, Wei L, Xu G,
Fan X, Jiang Y, Stetler RA, Liu G, et al: Cell based therapies for
ischemic stroke: From basic science to bedside. Prog Neurobiol.
115:92–115. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Dailey T, Metcalf C, Mosley YI, Sullivan
R, Shinozuka K, Tajiri N, Pabon M, Acosta S, Kaneko Y, van Loveren
H, et al: An update on translating stem cell therapy for stroke
from bench to bedside. J Clin Med. 2:220–241. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zou Z, Zhang Y, Hao L, Wang F, Liu D, Su Y
and Sun H: More insight into mesenchymal stem cells and their
effects inside the body. Expert Opin Biol Ther. 10:215–230. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Qu Z, Guo L, Fang G, Cui Z, Guo S and Liu
Y: Biological characteristics and effect of human umbilical cord
mesenchymal stem cells (hUC-MSCs) grafting with blood plasma on
bone regeneration in rats. Cell Biochem Biophys. 63:171–181. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Qu Z, Guo S, Fang G, Cui Z and Liu Y: AKT
pathway affect bone regeneration in nonunion treated with umbilical
cord-derived mesenchymal stem. Cell Biochem Biophys. 71:1543–1551.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Qu Z, Fang G, Cui Z and Liu Y: Cell
therapy for bone nonunion: A retrospective study. Minerva Med.
106:315–321. 2015.PubMed/NCBI
|
|
12
|
Kim SJ, Moon GJ, Chang WH, Kim YH and Bang
OY: STARTING-2 (STem cell Application Researches and Trials In
NeuroloGy-2) collaborators: Intravenous transplantation of
mesenchymal stem cells preconditioned with early phase stroke
serum: Current evidence and study protocol for a randomized trial.
Trials. 14:3172013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Qu ZG, Liu Y, Guo LB and Bi WW:
Percutaneous radiological autologous bone-marrow mesenchymal stem
cells grafting integrating blood plasma by injection in the part of
thigh fracture: Seven-month follow-up effect evaluation in one
case. J Clin Reh Tis Eng Res. 13:7393–7396. 2009.
|
|
14
|
Saino O, Taguchi A, Nakagomi T, Nakano-Doi
A, Kashiwamura S, Doe N, Nakagomi N, Soma T, Yoshikawa H, Stern DM,
et al: Immunodeficiency reduces neural stem/progenitor cell
apoptosis and enhances neurogenesis in the cerebral cortex after
stroke. J Neurosci Res. 88:2385–2397. 2010.PubMed/NCBI
|
|
15
|
Hunt JS, Petroff MG, McIntire RH and Ober
C: HLA-G and immune tolerance in pregnancy. FASEB J. 19:681–693.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Menier C, Rouas-Freiss N, Favier B,
LeMaoult J, Moreau P and Carosella ED: Recent advances on the
non-classical major histocompatibility complex class I HLA-G
molecule. Tissue Antigens. 75:201–206. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lee JM, Jung J, Lee HJ, Jeong SJ, Cho KJ,
Hwang SG and Kim GJ: Comparison of immunomodulatory effects of
placenta mesenchymal stem cells with bone marrow and adipose
mesenchymal stem cells. Int Immunopharmacol. 13:219–224. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Fazekasova H, Lechler R, Langford K and
Lombardi G: Placenta-derived MSCs are partially immunogenic and
less immunomodulatory than bone marrow-derived MSCs. J Tissue Eng
Regen Med. 5:684–694. 2011. View
Article : Google Scholar : PubMed/NCBI
|