Introduction
Myocardial ischemia/reperfusion (I/R) is a
pathological condition, which is characterized by an initial
restriction of the blood supply to the myocardium followed by the
subsequent restoration of perfusion (1). Although reperfusion is the most
effective treatment used to rescue ischemic myocardium, reperfusion
may paradoxically lead to severe or additional myocardial injury.
Myocardial I/R injury is a common problem in clinical practice and
may result in serious consequences. The exact pathophysiology
mechanism underlying myocardial I/R injury is complicated and has
yet to be fully understood. However, numerous studies support that
the inflammatory response is important in the pathogenesis of
myocardial I/R injury (2,3) due to the inhibition of inflammation
significantly attenuating I/R-induced myocardial injury (4,5). There
is increasing evidence to suggest that the inflammatory response
during myocardial I/R is closely associated with high mobility
group box 1 (HMGB1) (6,7).
HMGB1 is a non-chromosomal nuclear protein that may
be actively secreted from activated immune cells or passively
released from necrotic and apoptotic cells (8). Numerous studies demonstrated that
extracellular HMGB1 is a potent pro-inflammatory mediator (9,10), and
is important in triggering the inflammatory response during
myocardial I/R (11,12). Furthermore, it has been reported that
HMGB1 exerts its pro-inflammatory effects via its specific
receptors, which mainly include a receptor for advanced glycation
end products (RAGE), Toll-like receptor (TLR)-2 and TLR-4 (13). The interaction of HMGB1 and its
specific receptors activates nuclear factor-κB (NF-κB), and
ultimately leads to increased expression and release of numerous
inflammatory factors (14). Thus,
the HMGB1-RAGE/TLR-2/TLR-4-NF-κB pathway is an important
inflammatory signaling pathway (13).
Picroside II is a primary active constituent of
traditional Chinese medicine. Picrorhizae has been extensively used
in China to treat numerous diseases, including upper respiratory
tract diseases, disorder of the liver, dyspepsia and chronic
diarrhea (15). Previously, it has
been reported that picroside II has anti-inflammatory properties
and exerts beneficial effects in the nervous and urinary systems.
In the rat model of middle cerebral artery occlusion and
reperfusion, pretreatment with picroside II could significantly
improve the neurobehavioral function and inhibit neurocyte
apoptosis, which was correlated with a decrease of inflammatory
factor production by inhibition of the TLR-4/NF-κB signaling
pathway (15). In addition, the
study by Wang et al (16)
demonstrated that picroside II was able to decrease I/R-induced
renal fibrosis in rats by inhibition of long-term inflammation.
Recently, it has been reported that picroside II has a protective
effect on myocardial I/R injury in rats (17). Furthermore, our previous
investigation also demonstrated that picroside II was able to
inhibit hypoxia/reoxygenation (H/R)-induced cardiomyocyte apoptosis
(18); however, the exact mechanism
underlying the cardioprotective effects of picroside II in
myocardial I/R injury is not fully understood.
Due to inflammation being important in myocardial
I/R injury, picroside II having anti-inflammatory properties, and
the HMGB1-RAGE/TLR-2/TLR-4-NF-κB pathway being an important
inflammatory signaling pathway, the present study aimed to explore
whether the protective effect of picroside II on myocardial I/R
injury in rats is associated with suppressing the inflammatory
response by inhibition of the HMGB1-RAGE/TLR-2/TLR-4-NF-κB
signaling pathway.
Materials and methods
Animals
Male Sprague-Dawley rats (age, 8 weeks; weight,
250–300 g) were obtained from the Experimental Animal Center of
Central South University (Changsha, China). All rats were housed in
standard laboratory conditions in an air conditioned room at
temperature 24±1°C, with a 12 h light/dark cycle with a relative
humidity of 50–60%. All rats had free access to food and water. The
study was performed in accordance with the NIH Guide for the Care
and Use of Laboratory Animals and was approved by the Central South
University Veterinary Medicine Animal Care and Use Committee. The
present study was approved by the ethical committee of Ruikang
Hospital Affiliated to Guangxi University of Chinese Medicine
(Nanning, China).
Experimental protocols
The rats were randomly divided into 3 groups (n=8 in
each group): Group 1, sham-operated control (sham), in which the
rats were subjected to surgical manipulation without myocardial
ischemia; Group 2, the I/R group, in which the rats were subjected
to myocardial ischemia for 1 h followed by reperfusion for 3 h; and
Group 3, the picroside II + I/R group, in which the rats were
administered 10 mg/kg picroside II dissolved in sterile saline via
the tail vein 30 min prior to left coronary artery occlusion. The
model of myocardial I/R was established according to a previously
reported method by Li et al (19). Briefly, rats were anesthetized by an
intraperitoneal injection of 45 mg/kg sodium pentobarbital
(Shanghai West Tang Bio-Tech Co., Ltd., Shanghai, China) and then
placed in the supine position. The body temperature was maintained
at 37°C by an electric heating pad. The trachea was cannulated for
artificial ventilation at a rate of 55 breaths/min using a
volume-controlled rodent respirator, and the lead-II of the
electrocardiogram was monitored with subcutaneous stainless steel
electrodes. The chest was opened through a thoracotomy in the left
4 intercostal spaces. After the pericardium was incised, the
anterior wall of the ventricle was exposed, a 4/0 silk suture was
attached to a small curved needle and was placed around the left
coronary artery close to its origin. The complete occlusion of the
coronary artery was confirmed by an ST segment elevation in lead-II
and from the change of the ventricular color (from red to white).
After a 1-h occlusion, the rats underwent 3-h reperfusion. The
reperfusion was verified by the return of color in the ischemic
area. Sham rats underwent a similar operation with the exception of
the coronary artery ligation.
Reagents
Picroside II (purity >99%) was purchased from the
Chinese National Institute for the Control of Pharmaceutical and
Biological Products (Beijing, China). 2,3,5-triphenyltetrazolium
chloride (TTC) was obtained from Sigma-Aldrich (St. Louis, MO,
USA). The creatine kinase (CK) and lactate dehydrogenase (LDH)
assay kits were purchased from Nanjing Jiancheng Bioengineering
Institute (Nanjing, China). The terminal deoxynucleotidyl
transferase-mediated dUTP nick-end labeling (TUNEL) kit was from
Roche (Indianapolis, IN, USA). The antibodies targeting HMGB1,
RAGE, TLR-2, TLR-4 and β-actin were purchased from Abcam
(Cambridge, UK). TRIzol reagent was a product of Invitrogen (Thermo
Fisher Scientific, Inc., Waltham, MA, USA). The First Strand cDNA
Synthesis kit was obtained from MBI Fermentas, Inc. (Vilnius,
Lithuania) and the ELISA kits of TNF-α, IL-6, IL-1β, intercellular
adhesion molecule-1 (ICAM-1), HMGB1 and NF-κB p65 were purchased
from Shanghai Jiang Lai Biotechnology Co., Ltd. (Shanghai,
China).
Assessment of myocardial injury
Histological evaluation
The morphological changes of the cardiac tissue were
evaluated by hematoxylin and eosin staining. Briefly, following 3-h
reperfusion, the rats were sacrificed with an overdose of sodium
pentobarbital (220 mg/kg; i.p. injection) and their hearts were
harvested. Following the removal of the fatty tissues, the atria
and right ventricle, and the remaining left ventricle was fixed in
4% paraformaldehyde and prior to embedding in paraffin. The
paraffin-embedded tissue was sliced into 5 µm thick sections, and
the sections were then stained with hematoxylin and eosin according
to standard protocol (20). The
morphological changes to the cardiac tissue were observed under a
light microscope (Leica DM4000B; Leica Microsystems GmbH, Wetzlar,
Germany).
CK and LDH activity assay
To assess myocardial injury, the activity of CK and
LDH was determined. Briefly, after 3-h reperfusion, the blood
samples were collected from the carotid artery, and then
centrifuged at 1,000 × g for 20 min at 4°C. The activity of
CK and LDH was detected using the commercially available
colorimetric assay kits (Nanjing Jiancheng Bioengineering
Institute, Nanjing, China). in accordance with the manufacturer's
instructions.
Infarct size
The infarct size was measured by TTC staining.
Briefly, after 3-h reperfusion the left coronary artery was
occluded again at the original site, and 1 ml Evans blue dye (2%)
was injected via the femoral vein. The risk area was analyzed using
negative staining with Evans blue. Next, the rat was sacrificed
with an overdose of sodium pentobarbital (220 mg/kg; i.p.
injection) and the entire heart was rapidly excised. Following the
removal of the fatty tissues, atria and right ventricle, the
remaining left ventricle was frozen at −80°C. The frozen left
ventricle was cut transversely into 5 sections (~2 mm) and the risk
area was separated from the colored non-ischemic area (blue).
Furthermore, the sections were incubated in 1% TTC at 37°C for 15
min, and the infarcted myocardium was stained white, whereas the
viable myocardium was stained red. The infarct and the risk area
were analyzed using an image analyzer (version 6.0; Media
Cybernetics Inc., Rockville, MD, USA), and the infarct size was
expressed as a percentage of the risk area volume (%, infarct
size/risk area).
Apoptosis analysis
Cardiomyocyte apoptosis was analyzed by TUNEL
staining. Briefly, at the end of 3 h reperfusion, the heart was
removed and washed in phosphate-buffered saline. The anterior wall
tissues of the left ventricle were fixed in 4% paraformaldehyde and
embedded in paraffin. The paraffin-embedded cardiac tissues were
then cut with a thickness of 5 µm, and the sections were then
stained using a TUNEL kit following the manufacturer's
instructions. The number of TUNEL-positive cardiomyocytes was
visualized under a light microscope, and the apoptosis percentage
was expressed as a ratio of apoptotic cells to total cardiomyocytes
per field.
ELISA
Following reperfusion for 3 h, the blood samples
were collected and centrifuged 1,000 × g for 20 min at 4°C,
and the serum samples were stored at −80°C until further analysis.
The concentrations of TNF-α (cat. no JL13202), IL-6 (cat. no
JL15538), IL-1β (cat. no JL15543), ICAM-1 (cat. no JL15530) and
HMGB1 (cat. no JL13892) in the serum and NF-κB p65 (cat. no
JL10501) in nuclear lysates of the myocardium were detected using
commercially available ELISA kits (Shanghai Jiang Lai Biotechnology
Co., Ltd., Shanghai, China) in accordance with the manufacturer's
instructions.
Reverse transcription-quantitative polymerase
chain reaction (RT-qPCR) analysis
A 100 mg myocardial tissue sample was collected from
each rat for RNA isolation. A mortar and pestle, along with liquid
nitrogen, were used for disruption of samples. Homogenization of
each sample was completed by using a syringe and needle. Total RNA
was extracted from 100 mg myocardium using TRIzol reagent
(Invitrogen; Thermo Fisher Scientific, Inc.), and the concentration
was determined by a spectrophotometric absorbance measurement at
260 nm. A 1 µg RNA aliquot was converted into cDNA using the M-MLV
reverse transcriptase kit (Promega Corporation, Madison, WI, USA),
and the cDNA was then used for qPCR. Quantitative analysis of the
mRNA expression was performed using the ABI 7300 quantitative PCR
system (Thermo Fisher Scientific, Inc.) with the Power SYBR-Green
PCR Master Mix kit. PCR primers (synthesized by Sangon Biotech Co.,
Ltd., Shanghai, China). were as follows: HMGB1 (forward,
5′-ATGGGCAAAGGAGATCCTA-3′ and reverse, 5′-ATTCATCATCATCATCTTCT-3′);
RAGE (forward, 5′-TCTCAGAAGCCCAAGGAAGAGT-3′ and reverse,
5′-CCTAGGTCTGAAGGCCCTGAGT-3′); TLR-2 (forward,
5′-ACGCAGTGAGTGGTGCAAGTAT-3′ and reverse,
5′-CTTCTTCAATGGGTTCCAGCAA-3′); TLR-4 (forward,
5′-GGCATCATCTTCATTGTCCTTG and reverse, 5′-AGCATTGTCCTCCCACTCG-3′)
and β-actin (forward, 5′-AGGGAAATCGTGCGTGAC-3′ and reverse,
5′-CGCTCATTGCCGATAGTG-3′). The PCR amplification profiles consisted
of denaturation at 95°C for 10 min, followed by 40 cycles of
denaturation at 95°C for 15 sec and annealing at 60°C for 60 sec.
In addition, the relative expression values were normalized to the
expression value of β-actin using the 2−ΔΔCq method
(21). All amplification reaction
for each sample was performed four times.
Western blot analysis
Myocardial proteins were extracted using the Tissue
Total Protein Extraction kit (Beyotime Institute of Biotechnology,
Jiangsu, China). Nuclear proteins were isolated using a Nuclear
Extraction kit (Beyotime Institute of Biotechnology) in accordance
with the manufacturer's instructions. The protein concentration was
quantified by a Bradford protein assay (Beyotime Institute of
Biotechnology). Equal amounts of protein (40 µg from each group)
were loaded onto a 10% sodium dodecyl sulfate-polyacrylamide gel,
and then transferred onto a polyvinylidene fluoride membrane.
Following blocking for 1 h with 5% skimmed milk at room
temperature, the membranes were incubated with the primary antibody
for HMGB1 (rabbit polyclonal antibody; diluted 1 µg/ml (1:1,000);
cat. no. ab191583), RAGE (rabbit polyclonal antibody; 1:1,000; cat.
no. ab37647), TLR-2 (rabbit monoclonal antibody; 1:5,000; cat. no.
ab108998), TLR-4 (mouse monoclonal antibody; 1:5,000; cat. no.
ab30667) and β-actin (rabbit polyclonal antibody; 1:1,000; cat. no.
ab1801) overnight at 4°C, followed by incubation with the
corresponding horseradish-conjugated secondary antibody (goat
anti-rabbit; 1:10,000; cat. no. ab175773 or goat anti-mouse;
1:10,000; cat. no. ab97040) for 1 h at room temperature. The
protein bands were quantified using scanning densitometry, and the
results were normalized to the expression of β-actin.
Statistical analysis
SPSS statistical software was used for statistical
analysis (version 18.0; SPSS, Inc., Chicago, IL, USA) Data are
expressed as the mean ± standard error of the smean. All the values
were analyzed using analysis of variance and the Newman-Keuls
Student's t-test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Effect of picroside II on I/R-induced
myocardial injury
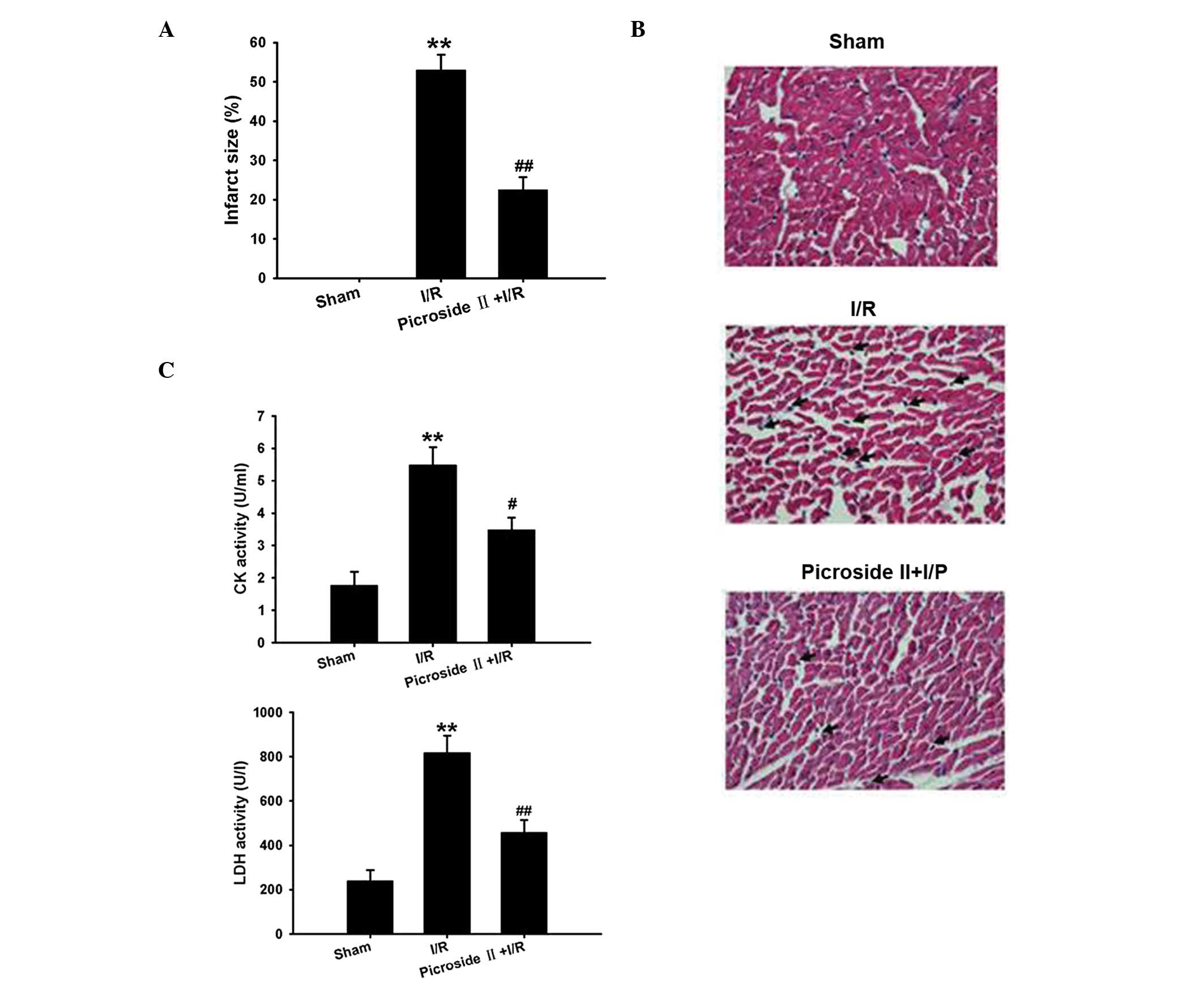
As shown in Fig. 1A,
myocardial infarction was not observed in the sham group. In the
I/R group, the infarct size was 52.95±3.98% following ischemia for
1 h and reperfusion for 3 h. Hematoxylin and eosin staining
revealed that the structure of the myocardium in the sham group was
arranged regularly, and the cell boundary was clear. Compared with
the sham group, the myocardial structure in the I/R group presented
irregularity, with some of cells shrinking and getting distorted or
ruptured. There was marked inflammatory cell infiltration in the
myocardial intercellular space (indicated by the arrows; Fig. 1B). Consistent with the above results,
the activity of CK and LDH that were used as indicators of
myocardial injury were markedly increased following ischemia for 1
h and reperfusion for 3 h (Fig. 1C).
However, pretreatment of picroside II could markedly inhibit these
effects induced by myocardial I/R (Fig.
1).
Effect of picroside II on I/R-induced
cardiomyocyte apoptosis
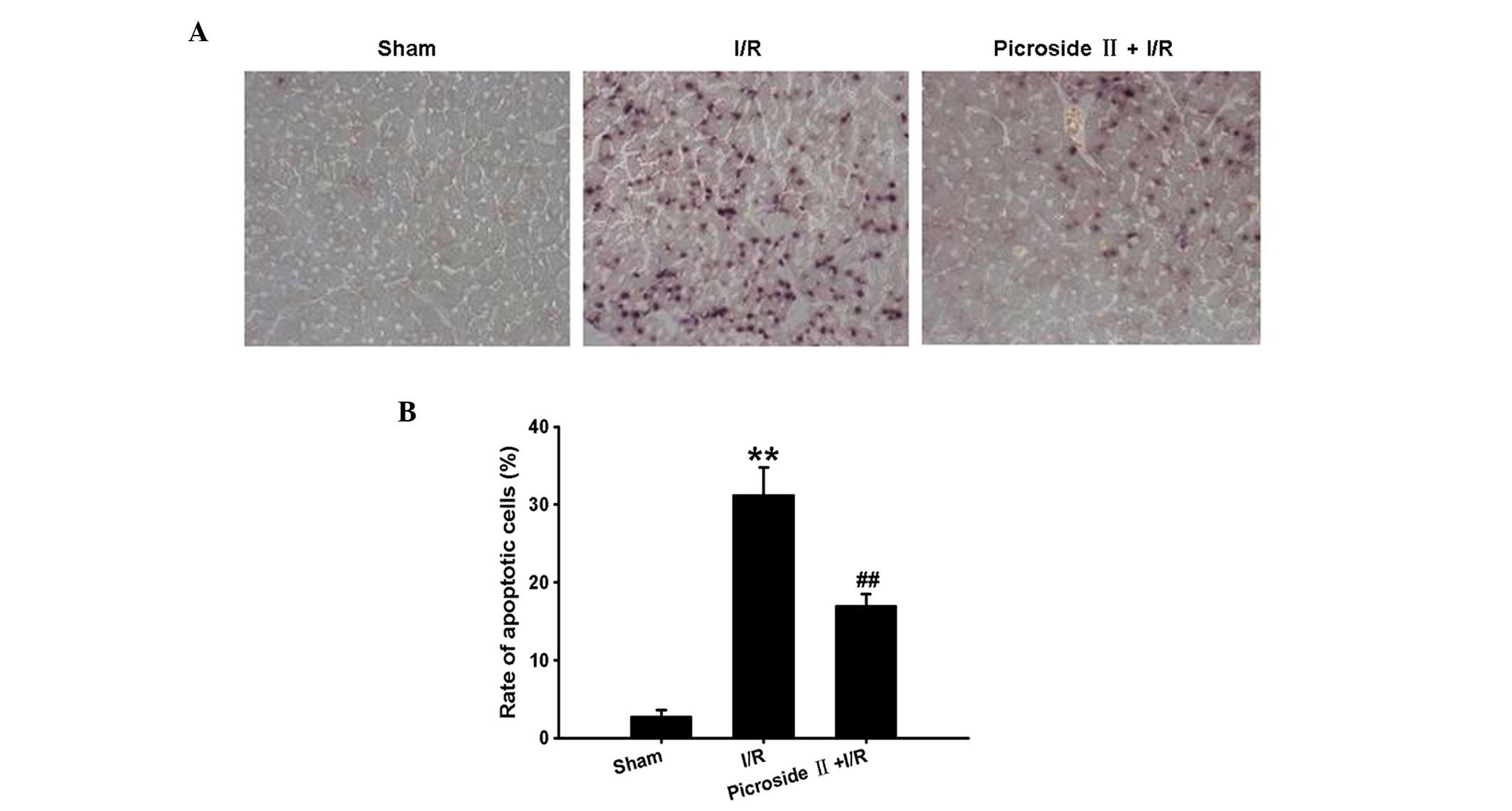
TUNEL staining revealed that the percentage of
apoptotic cells was remarkably increased following ischemia for 1 h
and reperfusion for 3 h compared with the sham group. However, this
effect induced by I/R was significantly ameliorated by
administration of picroside II (Fig.
2).
Effect of picroside II on I/R-induced
inflammatory factor production
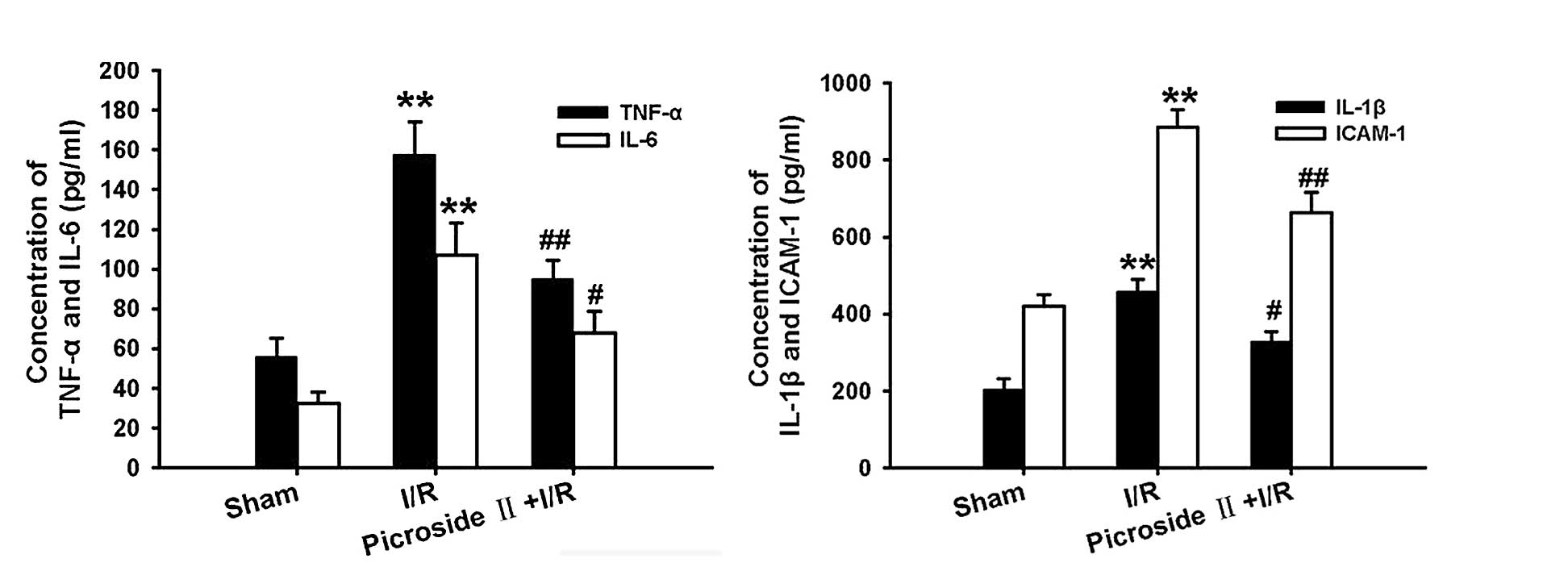
Following ischemia for 1 h and reperfusion for 3 h,
the concentration of TNF-α, IL-6, IL-1β and ICAM-1 were remarkably
increased compared with the sham group. However, this effect
induced by I/R was significantly attenuated by pretreatment of
picroside II (Fig. 3).
Effect of picroside II on I/R-induced
HMGB1 expression and release
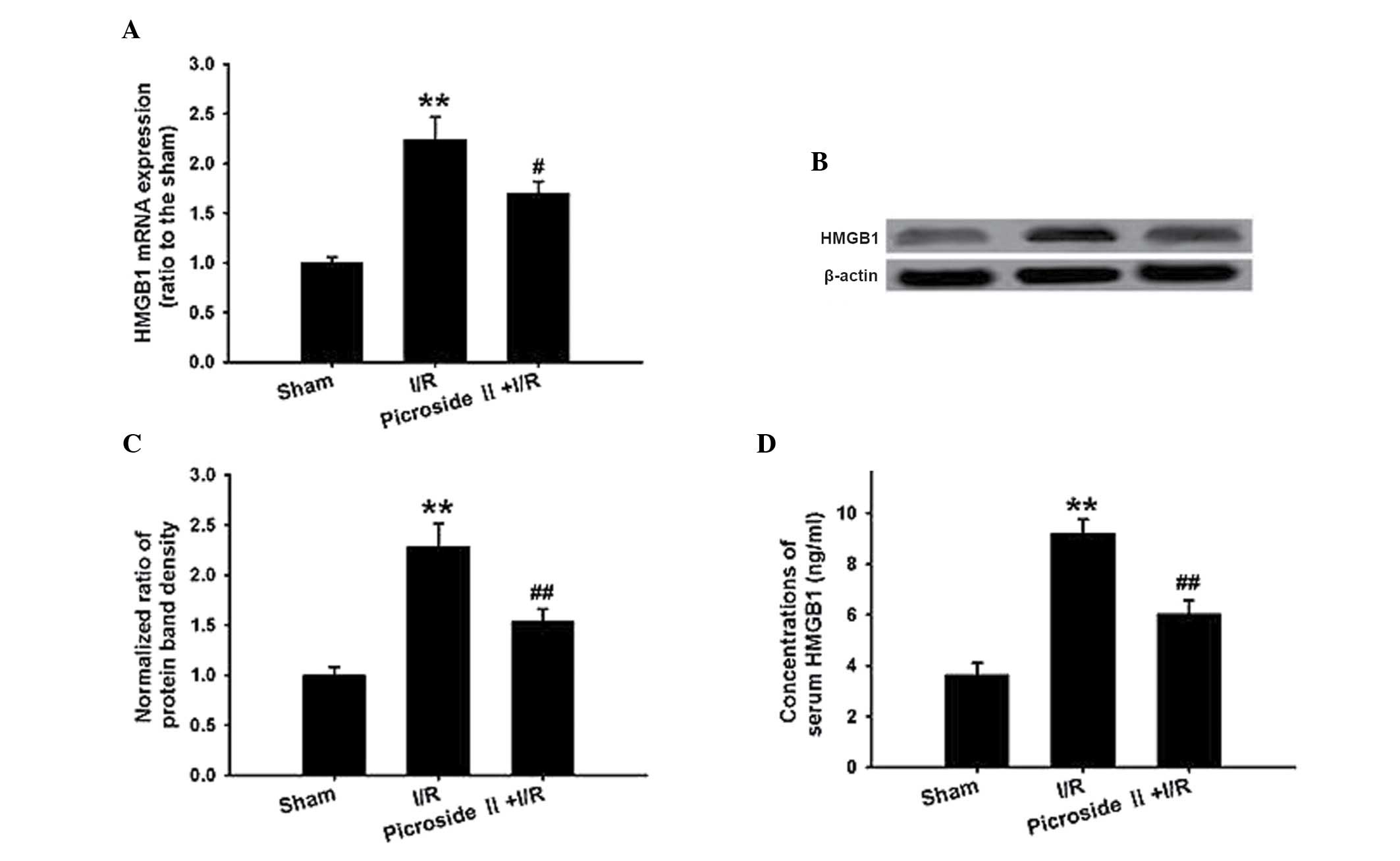
qPCR and western blotting analysis demonstrated that
the HMGB1 mRNA and protein expression were significantly
upregulated in the I/R group compared with that in the sham group.
Consistent with these results, the concentration of HMGB1 in the
serum was also significantly increased in the I/R group. However,
the increased expression and concentration of HMGB1 were
significantly attenuated by pretreatment with picroside II
(Fig. 4).
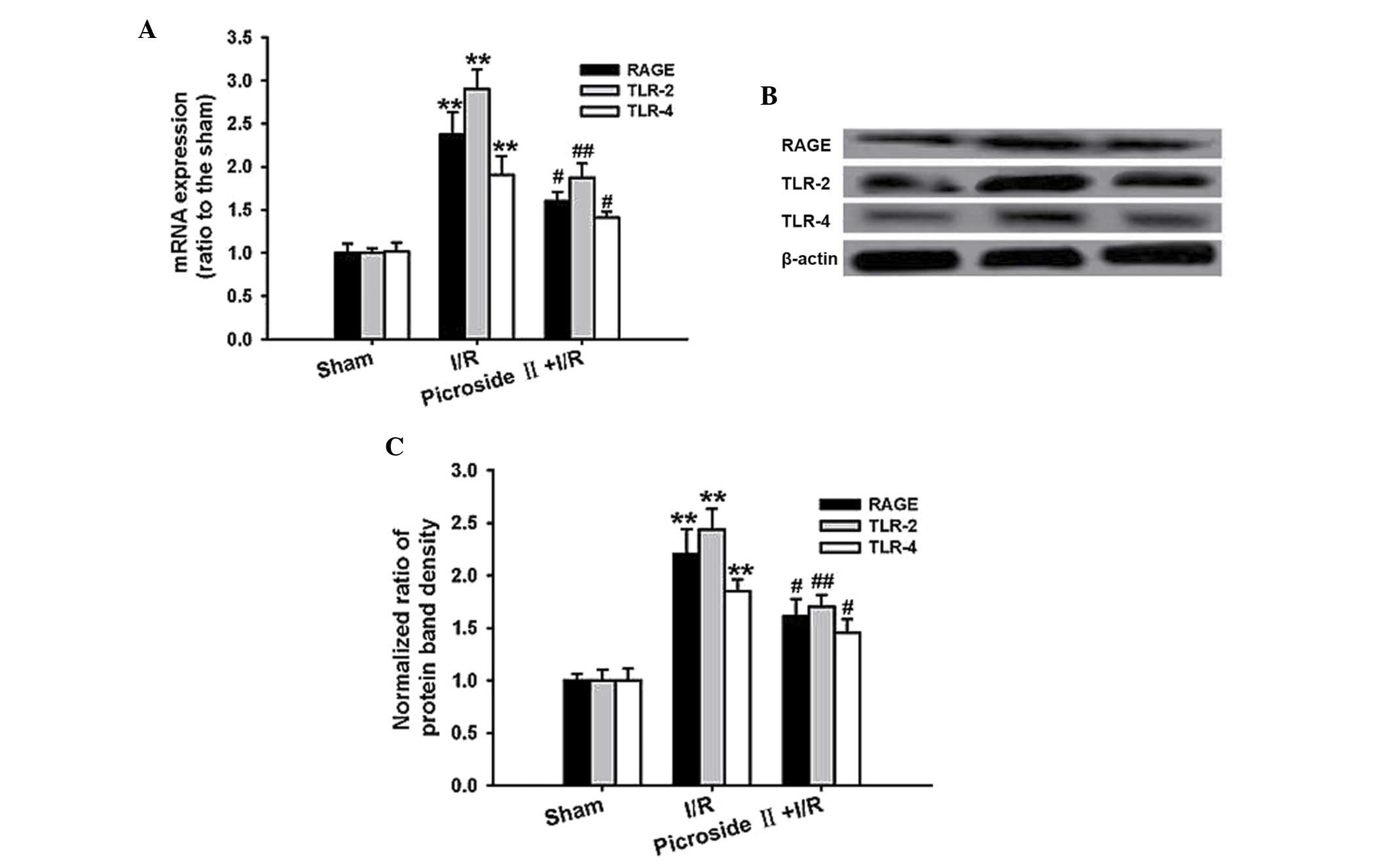
Effect of picroside II on I/R-induced
HMGB1 receptor expression
qPCR analysis demonstrated that the mRNA expression
of RAGE, TLR-2 and TLR-4 was markedly upregulated after ischemia
for 1 h and reperfusion for 3 h. Consistent with the mRNA
expression, the protein expression of RAGE, TLR-2 and TLR-4 was
also significantly increased in the I/R group. However, the
increased expression of mRNA and protein was remarkably
downregulated by pretreatment of picroside II (Fig. 5).
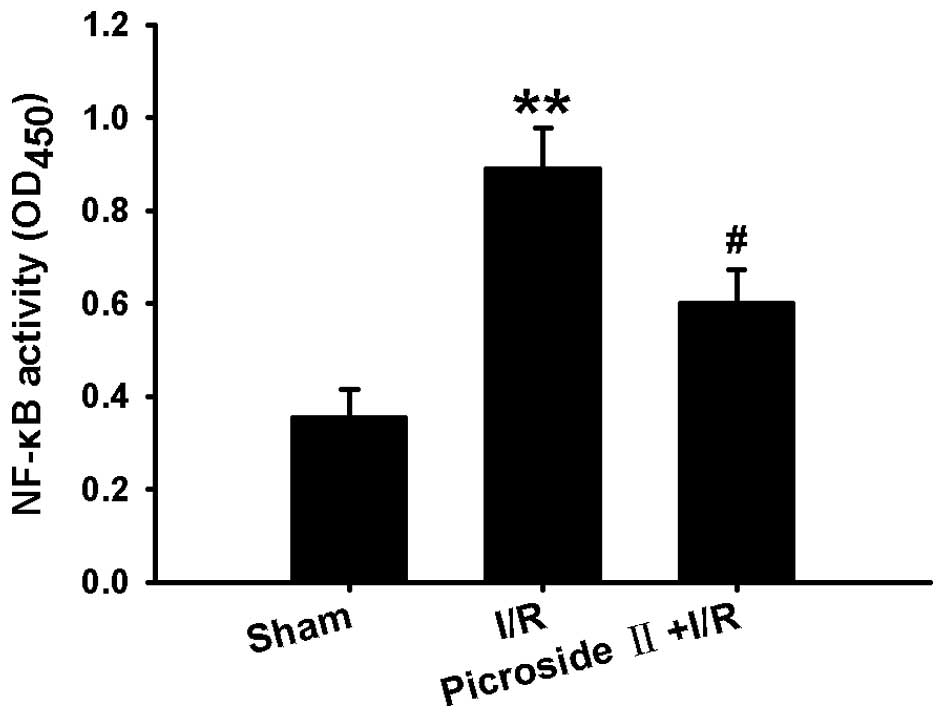
Effect of picroside II on I/R-induced
NF-κB activation
Since the activation of NF-κB is necessary to
inflammatory factor production, the NF-κB activity was investigated
in the present study. Following ischemia for 1 h and reperfusion
for 3 h, the NF-κB activity was increased, as indicated by the
increased optical density of NF-κB p65 in the nuclear lysates.
However, this effect induced by I/R was significantly inhibited by
administration of picroside II (Fig.
6).
Discussion
In the present study, a protective effect of
picroside II on I/R-induced myocardial injury was identified, which
was indicated by ameliorating myocardial morphology and decreasing
infarct size, cardiomyocyte apoptosis and the activity of CK and
LDH. The results also demonstrated that pretreatment with picroside
II was able to decrease I/R-induced inflammatory factor production,
inhibit HMGB1 expression and release, downregulate RAGE, TLR-2 and
TLR-4 expression, and decrease NF-κB activity.
Myocardial I/R injury is a common problem following
coronary artery bypass graft, heart transplantation and acute
coronary syndrome amongst other problems (22). Although ischemic heart disease is one
of the main reasons for morbidity and mortality worldwide, the
exact pathophysiological mechanism underlying myocardial I/R injury
is not fully understood. Currently, it is well-recognized that the
inflammatory response is one of the primary mechanisms underlying
myocardial I/R injury (2,3). Myocardial I/R may cause local sterile
inflammation and produce numerous inflammatory factors, including
TNF-α, IL-6, IL-1β and ICAM-1 amongst others (23). In addition, myocardial injury is
thought to be a result of an intensive inflammatory response
initiated by the infiltration of leukocytes and the production of
inflammatory factors (22).
Infiltrating cells, particularly neutrophils, are directly toxic to
the myocardium by releasing proteases and occluding the
microvasculature (24). Excessive
production of inflammatory factors damages the myocardium not only
by triggering deleterious responses but also by amplifying ongoing
responses to build a cascade of injury (25). This cascade refers to the excessive
production of inflammatory factors could cause vascular endothelial
cell damage and increase vascular permeability, and further
activate inflammatory cells that increase the inflammatory response
(26). Numerous studies have
demonstrated that inhibition of the inflammatory response could
markedly suppress myocardial injury induced by I/R in various
animal models (27–29). Therefore, decrease in inflammation
may be a potential therapeutic strategy for myocardial I/R
injury.
Recently, numerous studies suggested that the
inflammatory response during myocardial I/R injury is closely
associated with HMGB1 (6,7). HMGB1 is a single peptide chain
consisting of 215 amino acid residues with a molecular weight of
~30 kDa (30). It is an
evolutionarily conserved nuclear protein in all mammals and is
expressed in virtually all types of cells (31). Besides exerting its function in the
nucleus, extracellular HMGB1 can mediate various physical or
pathological processes, including stimulating inflammatory factor
expression and release mainly by binding to its specific receptors,
such as RAGE, TLR-2 and TLR-4 (32).
Therefore, HMGB1 has been recognized as an important
pro-inflammatory mediator and is important in initiating and
amplifying the inflammatory response (9–12). In
addition, it has been reported that the HMGB1-triggered
inflammatory response is crucial in myocardial I/R injury since a
decrease in HMGB1 expression and release could significantly
inhibit the inflammatory response and ameliorate I/R-induced
myocardial injury (11,33). Therefore, inhibition of the
HMGB1-mediated inflammatory signaling pathway may be a promising
therapeutic strategy for myocardial I/R injury.
Picroside II is an iridoid glucoside isolated from
Picrorhiza scrophulariiflora Pennell, which has been
reported to possess a wide range of pharmacological properties,
including anti-oxidative, anti-apoptotic and liver protective
effects amongst others (34–36). Furthermore, it has been reported that
picroside II is capable of protecting the heart or kidney from
I/R-induced injury by inhibition of the inflammatory response
(15,16). These studies suggested that picroside
II has an anti-inflammation property. Furthermore, studies from our
laboratory (Central South University, Changsha, China) and others
have demonstrated that picroside II has a protective effect on H/R-
or I/R-induced cardiomyocyte injury (17,18),
however, the exact mechanism remains unclear. Due to the important
role of the inflammatory response in the pathogenesis of myocardial
I/R injury and the anti-inflammatory property of picroside II, we
hypothesize that the protective mechanism of picroside II on
myocardial I/R injury may be associated with the inhibition of the
inflammatory response. In the present study, pretreatment with
picroside II was observed to inhibit I/R-induced myocardial injury
concomitantly by decreasing the inflammatory factor production. As
mentioned above, the HMGB1-RAGE/TLR-2/TLR-4-NF-κB signaling pathway
is important and the HMGB1-triggered inflammatory response is
crucial in myocardial I/R injury. The present study presumes that
the anti-inflammatory effect of picroside II may be associated with
the suppression of the HMGB1-RAGE/TLR-2/TLR-4-NF-κB signaling
pathway. A further study demonstrated that picroside II
significantly inhibited I/R-induced inflammatory factor production
concomitantly with a decreased HMGB1 expression and release,
downregulated expression of RAGE, TLR-2 and TLR-4, and decreased
NF-κB activity. These results were concordant with our previously
mentioned hypothesis.
In summary, the present study suggested that
pretreatment with picroside II was able to protect the myocardium
from I/R-induced injury. This beneficial effect was associated, at
least partly, with inhibition of the inflammatory response by
suppressing the HMGB1-RAGE/TLR-2/TLR-4-NF-κB signaling pathway. The
present study provides a theoretical basis regarding the protective
effect of picroside II on myocardial ischemia/reperfusion injury,
and also serves as a foundation for the development of novel
therapies to be used in cases of ischemic heart disease.
Acknowledgements
This study was supported by the National Nature
Science Foundation of China (grant nos. 81460613, 81101476 and
81260337), the Guangxi Nature Science Foundation of China (grant
nos. 2013GXNSFBA019126 and 2013GXNSFBA019188), the Bureau of Public
Health of Guangxi Province (grant no. Z2013206) and the Guangxi
Administration of Traditional Chinese Medicine (grant no.
GZZC14-36).
References
|
1
|
Eltzschig HK and Eckle T: Ischemia and
reperfusion-from mechanism to translation. Nat Med. 17:1391–1401.
2011. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Vilahur G and Badimon L:
Ischemia/reperfusion activates myocardial innate immune response:
The key role of the toll-like receptor. Front Physiol. 5:4962014.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kitano K, Usui S, Ootsuji H, Takashima S,
Kobayashi D, Murai H, Furusho H, Nomura A, Kaneko S and Takamura M:
Rho-kinase activation in leukocytes plays a pivotal role in
myocardial ischemia/reperfusion injury. PLoS One. 9:e922422014.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Guo J, Wang SB, Yuan TY, Wu YJ, Yan Y, Li
L, Xu XN, Gong LL, Qin HL, Fang LH and Du GH: Coptisine protects
rat heart against myocardial ischemia/reperfusion injury by
suppressing myocardial apoptosis and inflammation. Atherosclerosis.
231:384–391. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wang Y, Sun J, Liu C and Fang C:
Protective effects of crocetin pretreatment on myocardial injury in
an ischemia/reperfusion rat model. Eur J Pharmacol. 741:290–296.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Diao H, Kang Z, Han F and Jiang W:
Astilbin protects diabetic rat heart against ischemia-reperfusion
injury via blockade of HMGB1-dependent NF-κB signaling pathway.
Food Chem Toxicol. 63:104–110. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ding HS, Yang J, Chen P, Yang J, Bo SQ,
Ding JW and Yu QQ: The HMGB1-TLR4 axis contributes to myocardial
ischemia/reperfusion injury via regulation of cardiomyocyte
apoptosis. Gene. 527:389–393. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hu G, Zhang Y, Jiang H and Hu X: Exendin-4
attenuates myocardial ischemia and reperfusion injury by inhibiting
high mobility group box 1 proteinexpression. Cardiol J. 20:600–604.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tsung A, Tohme S and Billiar TR:
High-mobility group box-1 in sterile inflammation. J Intern Med.
276:425–543. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Magna M and Pisetsky DS: The role of HMGB1
in the pathogenesis of inflammatory and autoimmune diseases. Mol
Med. 20:138–146. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Herzog C, Lorenz A, Gillmann HJ, Chowdhury
A, Larmann J, Harendza T, Echtermeyer F, Müller M, Schmitz M,
Stypmann J, et al: Thrombomodulin's lectin-like domain reduces
myocardial damage by interfering with HMGB1-mediated TLR2
signalling. Cardiovasc Res. 101:400–410. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ding HS, Yang J, Gong FL, Yang J, Ding JW,
Li S and Jiang YR: High mobility group [corrected] box 1 mediates
neutrophil recruitment in myocardial ischemia-reperfusion injury
through toll like receptor 4-related pathway. Gene. 509:149–153.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li JZ, Wu JH, Yu SY, Shao QR and Dong XM:
Inhibitory effects of paeoniflorin on
lysophosphatidylcholine-induced inflammatory factor production in
human umbilical vein endothelial cells. Int J Mol Med. 31:493–497.
2013.PubMed/NCBI
|
|
14
|
Nogueira-Machado JA and de Oliveira Volpe
CM: HMGB-1 as a target for inflammation controlling. Recent Pat
Endocr Metab Immune Drug Discov. 6:201–209. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Guo Y, Xu X, Li Q, Li Z and Du F:
Anti-inflammation effects of picroside 2 in cerebral ischemic
injury rats. Behav Brain Funct. 6:432010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang L, Liu XH, Chen H, Chen ZY, Weng XD,
Qiu T and Liu L: Picroside II decreases the development of fibrosis
induced by ischemia/reperfusion injury in rats. Ren Fail.
36:1443–1448. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wu N, Li W, Shu W and Jia D: Protective
effect of picroside II on myocardial ischemia reperfusion injury in
rats. Drug Des Devel Ther. 8:545–554. 2014.PubMed/NCBI
|
|
18
|
Li JZ, Yu SY, Mo D, Tang XN and Shao QR:
Picroside II inhibits hypoxia/reoxygenation-induced cardiomyocyte
apoptosis by ameliorating mitochondrial function through a
mechanism involving a decrease in reactive oxygen species
production. Int J Mol Med. 35:446–452. 2015.PubMed/NCBI
|
|
19
|
Li TT, Zhang YS, He L, Li NS, Peng J and
Li YJ: Protective effect of phloroglucinol against myocardial
ischaemia-reperfusion injury is related to inhibition of
myeloperoxidase activity and inflammatory cell infiltration. Clin
Exp Pharmacol Physiol. 38:27–33. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhao Q, Hu X, Shao L, Wu G, Du J and Xia
J: LipoxinA4 attenuates myocardial ischemia reperfusion injury via
a mechanism related to downregulation of GRP-78 and caspase-12 in
rats. Heart Vessels. 29:667–6s78. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2-ΔΔCt method. Methods. 25:402–408. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hu X, Zhang K, Xu C, Chen Z and Jiang H:
Anti-inflammatory effect of sodium butyrate preconditioning during
myocardial ischemia/reperfusion. Exp Ther Med. 8:229–232.
2014.PubMed/NCBI
|
|
23
|
Liang Z, Liu LF, Yao TM, Huo Y and Han YL:
Cardioprotective effects of Guanxinshutong (GXST) against
myocardial ischemia/ reperfusion injury in rats. J Geriatr Cardiol.
9:130–136. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Turer AT and Hill JA: Pathogenesis of
myocardial ischemia-reperfusion injury and rationale for therapy.
Am J Cardiol. 106:360–368. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lin Y, Chen L, Li W and Fang J: Role of
high-mobility group box-1 in myocardial ischemia/reperfusion injury
and the effect of ethyl pyruvate. Exp Ther Med. 9:1537–1541.
2015.PubMed/NCBI
|
|
26
|
Yu L, Li Q, Yu B, Yang Y, Jin Z, Duan W,
Zhao G, Zhai M, Liu L, Yi D, et al: Berberine Attenuates Myocardial
Ischemia/Reperfusion Injury by Reducing Oxidative Stress and
Inflammation Response: Role of Silent Information Regulator 1. Oxid
Med Cell Longev. 2016:16896022016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hu H, Zhai C, Qian G, Gu A, Liu J, Ying F,
Xu W, Jin D, Wang H, Hu H, et al: Protective effects of tanshinone
IIA on myocardial ischemia reperfusion injury by reducing oxidative
stress, HMGB1 expression and inflammatory reaction. Pharm Biol.
53:1752–1758. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhang R, Wugeti N, Sun J, Yan H, Guo Y,
Zhang L, Ma M, Guo X, Jiao C, Xu W, et al: Effects of vagus nerve
stimulation via cholinergic anti-inflammatory pathway activation on
myocardial ischemia/reperfusion injury in canine. Int J Clin Exp
Med. 7:2615–2623. 2014.PubMed/NCBI
|
|
29
|
Zhao ZG, Tang ZZ, Zhang WK and Li JG:
Protective effects of embelin on myocardial ischemia-reperfusion
injury following cardiac arrest in a rabbit model. Inflammation.
38:527–533. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Shen X and Li WQ: High-mobility group box
1 protein and its role in severe acute pancreatitis. World J
Gastroenterol. 21:1424–1435. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wu AH, He L, Long W, Zhou Q, Zhu S, Wang
P, Fan S and Wang H: Novel mechanisms of herbal therapies for
inhibiting HMGB1 secretion or action. Evid Based Complement
Alternat Med. 2015:4563052015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yang S, Xu L, Yang T and Wang F:
High-mobility group box-1 and its role in angiogenesis. J Leukoc
Biol. 95:563–574. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kang ZC, Jiang WL, Xu Y, Zhu HB and Hou J:
Cardioprotection with 8-O-acetyl shanzhiside methylester on
experimental myocardial ischemia injury. Eur J Pharm Sci.
47:124–130. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Meng FJ, Hou ZW, Li Y, Yang Y and Yu B:
The protective effect of picroside II against hypoxia/reoxygenation
injury in neonatal rat cardiomyocytes. Pharm Biol. 50:1226–1232.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wang L, Liu X, Chen H, Chen Z, Weng X, Qiu
T and Liu L: Effect of picroside II on apoptosis induced by renal
ischemia/reperfusion injury in rats. Exp Ther Med. 9:817–822.
2015.PubMed/NCBI
|
|
36
|
Gao H and Zhou YW: Anti-lipid peroxidation
and protection of liver mitochondria against injuries by picroside
II. World J Gastroenterol. 11:3671–3674. 2005. View Article : Google Scholar : PubMed/NCBI
|