Introduction
Human cervical cancer (HCC) is the fourth most
frequently diagnosed cancer and the fourth leading cause of
cancer-related mortality in women (1). An estimated 52.8 million new cases and
26.6 million cases of mortality as a result of the disease were
recorded in 2012 (2). However, the
development of a widespread program for cervical cytologic
screening (termed the Papanicolaou test) has substantially reduced
HCC mortality (3). The majority
(~84.3%) of cases occur in resource-poor countries, where the
disease accounts for ~12% of all cancers in women, and has an
overall 5-year survival rate of ~72% (2) The greatest risk factor for HCC is
infection with certain types of the human papillomavirus (HPV),
followed by smoking (4). The primary
subtypes of HCC are squamous cell carcinoma and adenocarcinoma, and
squamous cell carcinoma is the most commonly diagnosed subtype
(5). The International Federation of
Gynecology and Obstetrics (FIGO) staging system is used to
determine the prognosis for patients, and provides the basis for
therapeutic decision making (5).
However, only 1% of women who test positive for HPV develop HCC
(6). Therefore, it is important to
identify a sensitive and specific cancer-related molecule that will
serve as a predictive biomarker for clarifying the underlying
molecular mechanisms of HCC, in addition to being a valuable
diagnostic and therapeutic target.
The leukocyte-associated immunoglobulin-like
receptor-1 (LAIR-1) is a member of the immunoglobulin (Ig)
superfamily, and is a type І transmembrane glycoprotein. LAIR-1 is
composed of a C2-type Ig-like domain and two immunoreceptor
tyrosine-based inhibition motifs (ITIMs). It is broadly expressed
on nearly all immune cells, such as T-cells, B-cells, and NK cells;
however, it is not expressed on erythrocytes and platelets
(7). It is also expressed on
megakaryocytes (MKs) (8) and
osteoclasts (9). Poggi et al
(10) demonstrated that LAIR-1 was
not expressed in high-risk B-cell chronic lymphocytic leukemia
(CLL), and the intensity of expression in low- and
intermediate-risk CLL was associated with the CLL stage and
progression of the disease. However, a limited amount of attention
has focused on LAIR-1 expression in solid tumors (11). The extracellular matrix (ECM) has
been determined to promote the progression of a solid tumor
(12). Collagen, an important
component of ECM, has been confirmed to be the high-affinity ligand
for LAIR-1 (13). Accordingly,
LAIR-1 may be involved in tumor development.
The present study reports that LAIR-1 was markedly
expressed in HCC specimens, and it was identified that
overexpression of LAIR-1 was associated with clinicopathological
features.
Thus, the present study found that the
overexpression of LAIR-1 reduced tumor proliferation and increased
tumor apoptosis of cervical carcinoma cells. These data suggest
that HCC patients with a high level of LAIR-1 expression may not
experience disease progression. This suggests that LAIR-1 may be an
effective therapeutic biomarker and an alternative therapeutic
target.
Materials and methods
Patients and tissue specimens
Fresh HCC and normal adjacent tissues were collected
from 124 patients confirmed to have HCC between 2010 and 2012 at
the Department of Gynecology, Affiliated Hospital of Binzhou
Medical University (Yantai, China). All patients had squamous cell
carcinoma tumors. No patients received radiotherapy, chemotherapy
or immunotherapy prior to surgery. Patient ages ranged between 26
and 73 years (mean age, 44.8±10.4 years). Tumor differentiation was
assessed according to the FIGO staging system (5). Ethical approval was obtained from the
Ethics Committee of Binzhou Medical University. Informed consent
was obtained from all patients.
Immunohistochemical (IHC)
staining
IHC staining was conducted to examine the altered
expression of LAIR-1 in tumors and normal tissues. Formalin-fixed
and paraffin-embedded sections were deparaffinized and rehydrated.
Antigen retrieval was performed in a microwave at 98°C for 30 min.
The sections were treated with 3% hydrogen peroxide for 5 min, and
then blocked with 1% bovine serum albumin (AR1006; Boster
Biological Technology, Ltd., Wuhan, China) for 30 min. The sides
were incubated with monoclonal mouse anti-human LAIR-1 (ab14826;
Abcam, Cambridge, UK) at 1:500 dilution at 4°C overnight. Mouse IgG
(BA1046; Boster Biological Technology, Ltd.) served as the negative
control. The biotinylated anti-mouse secondary antibody (BA1001;
Boster Biological Technology, Ltd.) was incubated the following day
with the sides for 20 min at room temperature, followed by further
treatment with a streptavidin-alkaline phosphatase complex (Boster
Biological Technology, Ltd.) for 20 min. The signal was detected
using the BCIP/NBT substrate solution (Boster Biological
Technology, Ltd.).
Semiquantitative assessment of IHC
staining
The expression of LAIR-1 in the nucleus and
cytoplasm was reviewed and scored by two independent observers who
had no prior knowledge of the clinical information. The
immunoreactivity was evaluated using a semiquantitative scoring
system in which 0 = no staining, 1 = weak staining, 2 = moderate
staining, and 3 = intense staining. The percentage of LAIR-1
positive cells scored as 0 was 0% (no positive cells), 1 was ≤25%,
2 was between 25% and 50%, 3 was between 50% and 75%, and 4 was
>75%. The immunoreactive score (IRS) was calculated as follows:
Proportion of positive cells score × intensity score. Values of IRS
ranging between 0 and 12 were classified as follows: - (IRS: 0), +
(IRS: 1–4), and ++ (IRS: 6–12).
Cell culture
Cervical squamous carcinoma cell lines (Ca Ski,
ME-180, C-33 A, SiHa and MS751) were purchased from the Cell Bank
of Shanghai Institutes for Biological Sciences (Shanghai, China).
ME-180 cells were cultivated in McCoy's 5A medium (Sigma-Aldrich;
Merck Millipore, Darmstadt, Germany), and C-33 A, SiHa and MS751
cells were maintained in Minimum Essential Medium (Gibco; Thermo
Fisher Scientific, Inc., Waltham, MA, USA). Ca Ski cells were
cultured in RPMI-1640 (Hyclone; GE Healthcare Life Sciences, Logan,
UT, USA) medium. Media were supplemented with 10% fetal bovine
serum (Hyclone) and incubated at 37°C under 5% CO2 and
saturated moisture.
Stable transfection
Sequence-verified LAIR-1 cDNA was cloned into a
GV218 lentivirus vector (GeneChem Co., Ltd., Shanghai, China),
which codes the Enhanced Green Fluorescent Protein (EGFP) gene. The
accuracy of the recombinant vector was verified using automated
sequencing (Thermo Fisher Scientific Inc.). The 293T cells were
cotransfected with GV218-LAIR-1 or GV218, and other vectors
(pHelper 1.0 and 2.0; GeneChem Co., Ltd.), and the viruses were
subsequently harvested after 48 h. The lentivirus was transfected
into ME-180 cells, and cells transfected with an empty vector
served as the negative control. At 48 h after transfection, the
stable transfected cells, which were expressed as the vector
sequences coding the puromycin resistance gene, were selected using
puromycin (Sigma-Aldrich). The transfected cell lines were
confirmed using western blotting and immunofluorescence.
Western blotting
Radioimmunoprecipitation assay lysis buffer
(Beyotime Institute of Biotechnology, Nantong, China) was used to
lyse the cells. Subsequent to centrifugation at 10,000 × g
for 15 min at 4°C, the protein solution was collected and the
concentration was quantitated using a BCA protein assay (Thermo
Fisher Scientific Inc.). The protein (50 µg per lane) was separated
using 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis
and transferred to a polyvinylidene fluoride membrane. The membrane
was probed with anti-LAIR-1 (ab14826; Abcam) at dilution of 1:500
and anti-GAPDH (AB-P-R 001; Goodhere Biotechnology Co., Ltd.,
Hangzhou, China) at dilution of 1:10,000 at 4°C overnight. After
washing, the membrane was incubated with horseradish
peroxidase-conjugated anti-rabbit/mouse secondary antibodies
(1:2,500 dilution; BA1055/BA1051; Boster Biological Technology,
Ltd.), at room temperature for 1.5 h and visualized using enhanced
chemiluminescence plus (Millipore, Billerica, MA, USA).
Immunofluorescence staining
LAIR-1 transfected cells (L-ME-180) and cells
transfected with empty vector (V-ME-180) were seeded into a cover
glass at a density of 4×104/piece/500 µl overnight. The
following day, the cells were fixed with 4% paraformaldehyde for 1
h at 4°C. After blocking, the cells were incubated with anti-LAIR-1
at 4°C overnight prior to incubation with Cy3-conjugated anti-mouse
IgG (ZDR-5210; ZSGB-BIO, Beijing, China) at a dilution of 1:80 for
1 h at room temperature. The cells were analyzed using laser
confocal scanning microscopy (LEICA Microsystems GmbH, Wetzlar,
Germany).
Cell proliferation assay
Cells were seeded into a 96-well plate at a
concentration of 4,000/well/100 µl. The cells were quantified at 0,
24, 48, and 72 h using a Cell Counting Kit-8 (CCK-8) (Dojindo Co.,
Ltd., Kumamoto, Japan), and the absorbance was detected at 450 nm.
Cell proliferation viability was recorded according to the
following equation: Cell proliferation viability = absorbance value
(AV) / 0 h AV. The experiment was performed in triplicate and
repeated three times.
Cell apoptosis assay
Monodispersed cells (2.5×105 cells/well)
were seeded into a 6-well plate in the maintenance medium.
Subsequent to incubation for 24 h, cells were treated with 0.5
µg/ml cisplatin (Hansoh Pharmaceutical Co., Ltd., Lianyungang,
China) for 18 h, and then digested with 0.25% trypsin
(ethylenediaminetetraacetic acid-free). The Annexin V-PE Apoptosis
Detection kit (Keygen Biotechnology Co., Ltd., Nanjing, China) was
used for staining. The cells were analyzed using a flow cytometer
(BD Biosciences, Franklin Lakes, NJ, USA).
Statistical analysis
Data was analyzed using SPSS software (version 15.0;
SPSS, Inc., Chicago, IL, USA). The Wilcoxon exact test was used to
determine the LAIR-1 expression in HCC and adjacent nontumor
cervical tissues. The association between LAIR-1 and various
clinicopathological features was analyzed using the χ2
test. Continuous variables were analyzed using two-way analysis of
variance. The correlation between the pathological stage and LAIR-1
expression levels were assessed using Spearman's Rank correlation
coefficient analysis. R<0 was considered to indicate negative
correlation, and P<0.05 was considered to indicate a
statistically significant difference.
Results
LAIR-1 is upregulated in HCC clinical
samples
To investigate the association between LAIR-1
expression and cervical lesions, HCC and adjacent normal cervical
tissues were examined by IHC staining. Representative results are
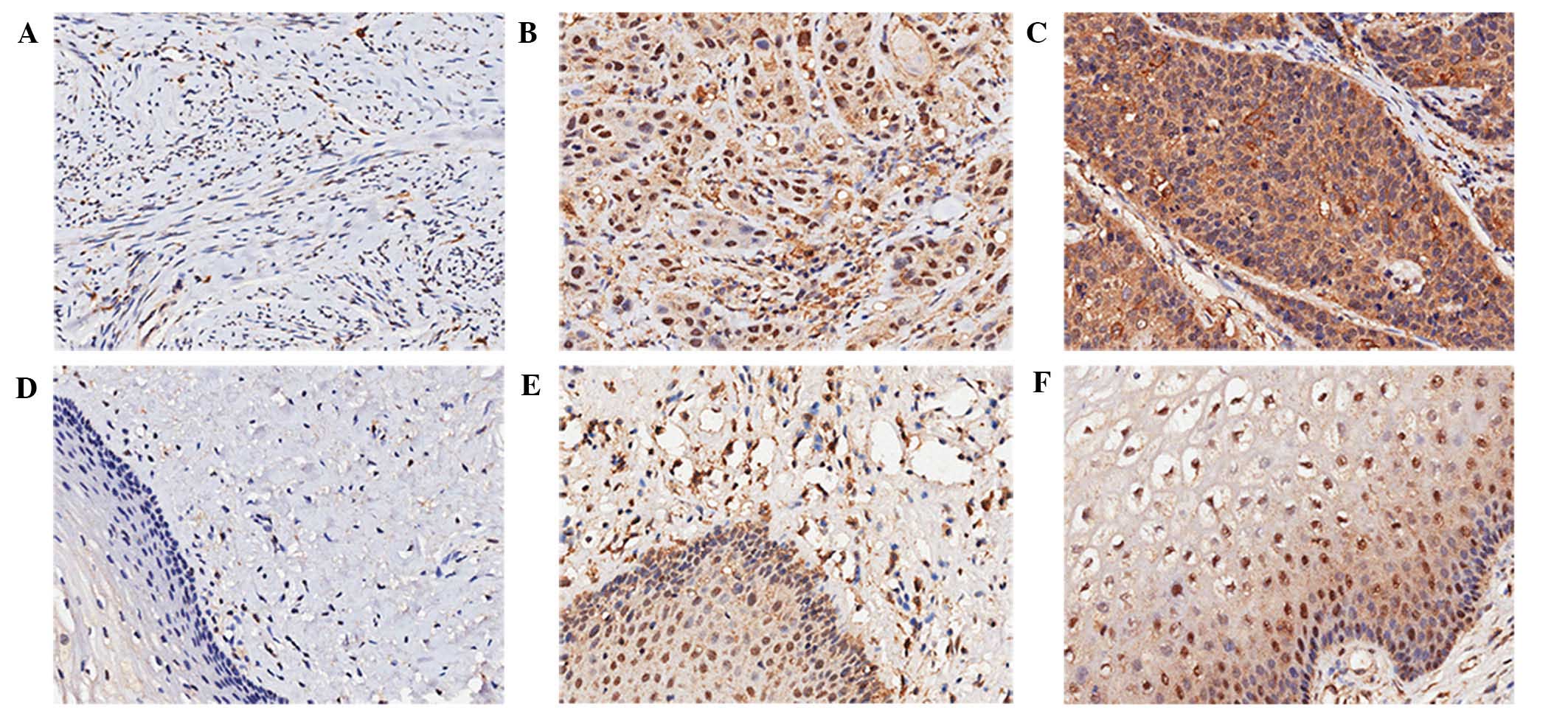
shown in Fig. 1. LAIR-1 was
localized in the cytoplasm and nucleus of the cells, and was
primarily expressed in the cytoplasm. The percentage of cells that
were >90% positive was 48.4% in the cytoplasm of HCC specimens
(data not shown). As summarized in Table
I, compared with normal adjacent tissues, LAIR-1 overexpression
in HCC tissues was statistically significant both in the cytoplasm
and nucleus (P<0.05). Of the 124 HCC patient samples analyzed,
positive staining of LAIR-1 in the cytoplasm was detected in 109
tumors (88%); 54 cases exhibited weak LAIR-1 staining, and 55 cases
showed strong LAIR-1 staining. By contrast, only 11 cases (8.9%)
exhibited weak staining in the cytoplasm of the paired
tumor-adjacent normal tissues, and no cases showed strong staining.
Although the negative rate of LAIR-1 expression in the nucleus of
HCC tissue was 49.2%, the negative rate in normal tissues was
50.8%, and the difference was statistically significant
(P=0.026).
 | Table I.Differential LAIR-1 expression in hcc
and matched normal tissues. |
Table I.
Differential LAIR-1 expression in hcc
and matched normal tissues.
|
| Cytoplasmic
LAIR-1 | Nuclear LAIR-1 |
|---|
|
|
|
|
|---|
| Tissue type | − | + | ++ | P-value | − | + | ++ | P-value |
|---|
| HCC tissues | 15
(12.1) | 54
(43.5) | 55 (44.4) | <0.001 | 61 (49.2) | 49 (39.5) | 14 (11.3) | 0.026 |
| Normal tissues | 113 (91.1) | 11 (8.9) | 0 (0.0) |
| 63 (50.8) | 56 (45.2) | 5
(4.0) |
|
LAIR-1 expression is correlated to
clinicopathological variables
The correlation between LAIR-1 expression and
clinicopathological parameters was analyzed using the χ2
test. Table II indicates that
LAIR-1 expression in the cytoplasm was significantly associated
with the number of positive lymph nodes (P<0.001); however, no
statistically significant associations between LAIR-1 expression
and age, tumor size, pathologic stage, T classification, N
classification and clinical stage (P>0.05) were detected. In the
nucleus, LAIR-1 expression was evidently associated with tumor size
(P=0.047), pathological stage (P=0.014), T classification
(P<0.001) and clinical stage (P=0.027). A negative correlation
existed between LAIR-1 expression and the pathological stage
(R=−0.185, P=0.043).
 | Table II.Correlation between human cervical
cancer clinicopathological features with LAIR-1 expression in
subcellular localization. |
Table II.
Correlation between human cervical
cancer clinicopathological features with LAIR-1 expression in
subcellular localization.
|
| Cytoplasmic
LAIR-1 | Nuclear LAIR-1 |
|---|
|
|
|
|
|---|
| Variable | − (n=15) | + (n=54) | ++ (n=55) | P-value | − (n=61) | + (n=49) | ++ (n=14) | P-value |
|---|
| Age |
|
|
| 0.756 |
|
|
| 0.780 |
| ≤45 | 9
(12.9) | 32 (45.7) | 29 (41.4) |
| 33 (47.1) | 28 (40.0) | 9
(12.9) |
|
|
>45 | 6
(11.1) | 22 (40.7) | 26 (48.1) |
| 28 (51.9) | 21 (38.9) | 5
(9.3) |
|
| Tumor size, cm |
|
|
| 0.751 |
|
|
| 0.047 |
| ≤4 | 12 (13.5) | 38 (42.7) | 39 (43.8) |
| 38 (42.7) | 41 (46.1) | 10 (11.2) |
|
|
>4 | 3
(8.6) | 16 (45.7) | 16 (45.7) |
| 23 (65.7) | 8
(22.9) | 4 (11.4) |
|
| Pathological
stage |
|
|
| 0.390 |
|
|
| 0.014 |
| G1 | 0
(0.0) | 3
(42.9) | 4
(57.1) |
| 1 (14.3) | 3
(42.9) | 3 (42.9) |
|
| G2 | 9
(9.4) | 43 (44.8) | 44 (45.8) |
| 45 (46.9) | 43 (44.8) | 8
(8.3) |
|
| G3 | 4
(23.5) | 7
(41.2) | 6
(35.3) |
| 11 (64.7) | 3
(17.6) | 3
(17.6) |
|
| Number of positive
lymph nodes |
|
|
| <0.001 |
|
|
| 0.492 |
| 0 | 9
(10.7) | 37 (44.0) | 38 (45.2) |
| 45 (53.6) | 32 (38.1) | 7
(8.3) |
|
| ≤3 | 0
(0.0) | 7
(36.8) | 12 (63.2) |
| 9
(47.4) | 7
(36.8) | 3
(15.8) |
|
|
>3 | 5
(41.7) | 3
(25.0) | 4
(33.3) |
| 5
(41.7) | 4
(33.3) | 3
(25.0) |
|
| T
classification |
|
|
| 0.090 |
|
|
| <0.001 |
| T1 | 12 (15.0) | 37 (46.3) | 31 (38.8) |
| 39 (48.8) | 37 (46.3) | 4
(5.0) |
|
| T2 | 3
(8.8) | 14 (41.2) | 17 (50.0) |
| 21 (61.8) | 9 (26.5) | 4
(11.8) |
|
| T3 | 0
(0.0) | 0 (0.0) | 5 (100.0) |
| 0 (0.0) | 2 (40.0) | 3
(60.0) |
|
| N
classification |
|
|
| 0.746 |
|
|
| 0.251 |
| N0 | 9
(10.8) | 37 (44.6) | 37 (44.6) |
| 45 (54.2) | 32 (38.6) | 6
(7.2) |
|
| N1 | 6
(15.8) | 16 (42.1) | 16 (42.1) |
| 16 (42.1) | 16 (42.1) | 6
(15.8) |
|
| Clinical stage |
|
|
| 0.914 |
|
|
| 0.027 |
| 1 | 6
(12.8) | 22 (46.8) | 19 (40.4) |
| 24 (51.1) | 22 (46.8) | 1
(2.1) |
|
| 2 | 3
(9.7) | 13 (41.9) | 15 (48.4) |
| 20 (64.5) | 8
(25.8) | 3
(9.7) |
|
| 3 | 6 (14.6) | 16 (39.0) | 19 (46.3) |
| 16 (39.0) | 17 (41.5) | 8
(19.5) |
|
LAIR-1 was overexpressed in ME-180
cell line
To research the functional significance of LAIR-1 in
HCC, its expression was detected in HCC cell lines (Ca Ski, ME-180,
C-33 A, SiHa, and MS751); however, no expression was detected (data
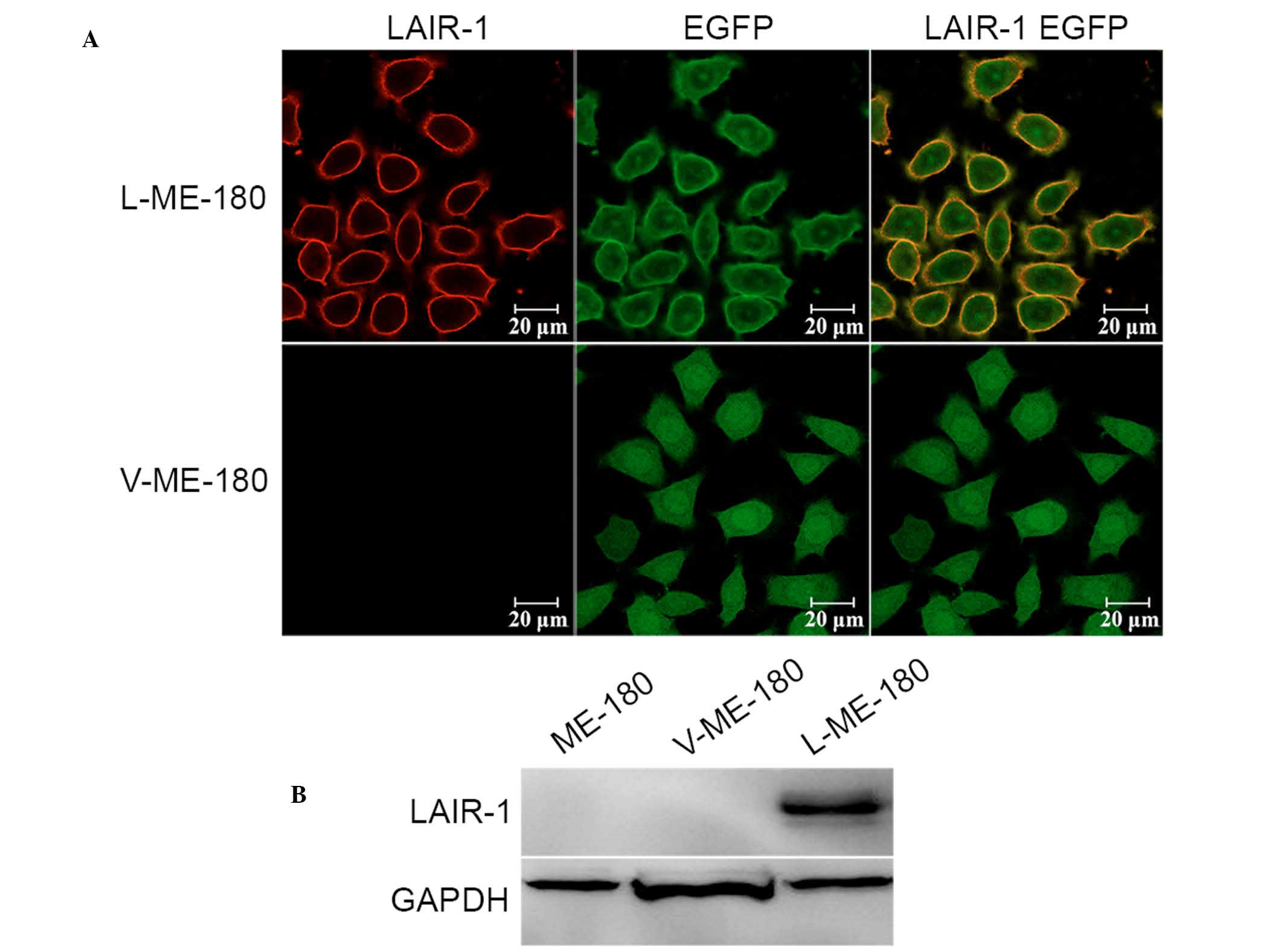
not shown). The LAIR-1 expression levels were modulated in ME-180
cells by using stable transfection with the LAIR-1 expression
plasmid. Western blotting and immunofluorescence analysis confirmed
an increase of LAIR-1 expression in ME-180 cells after
transfection. As hypothesized, we observed enhanced expression of
LAIR-1 in the ME-180 cells (L-ME-180; Fig. 2A), and the molecular weight was 70
kDa as a result of the fusion of LAIR-1 and EGFP proteins (Fig. 2B).
LAIR-1 expressed in HCC cell lines is
tumor suppressive
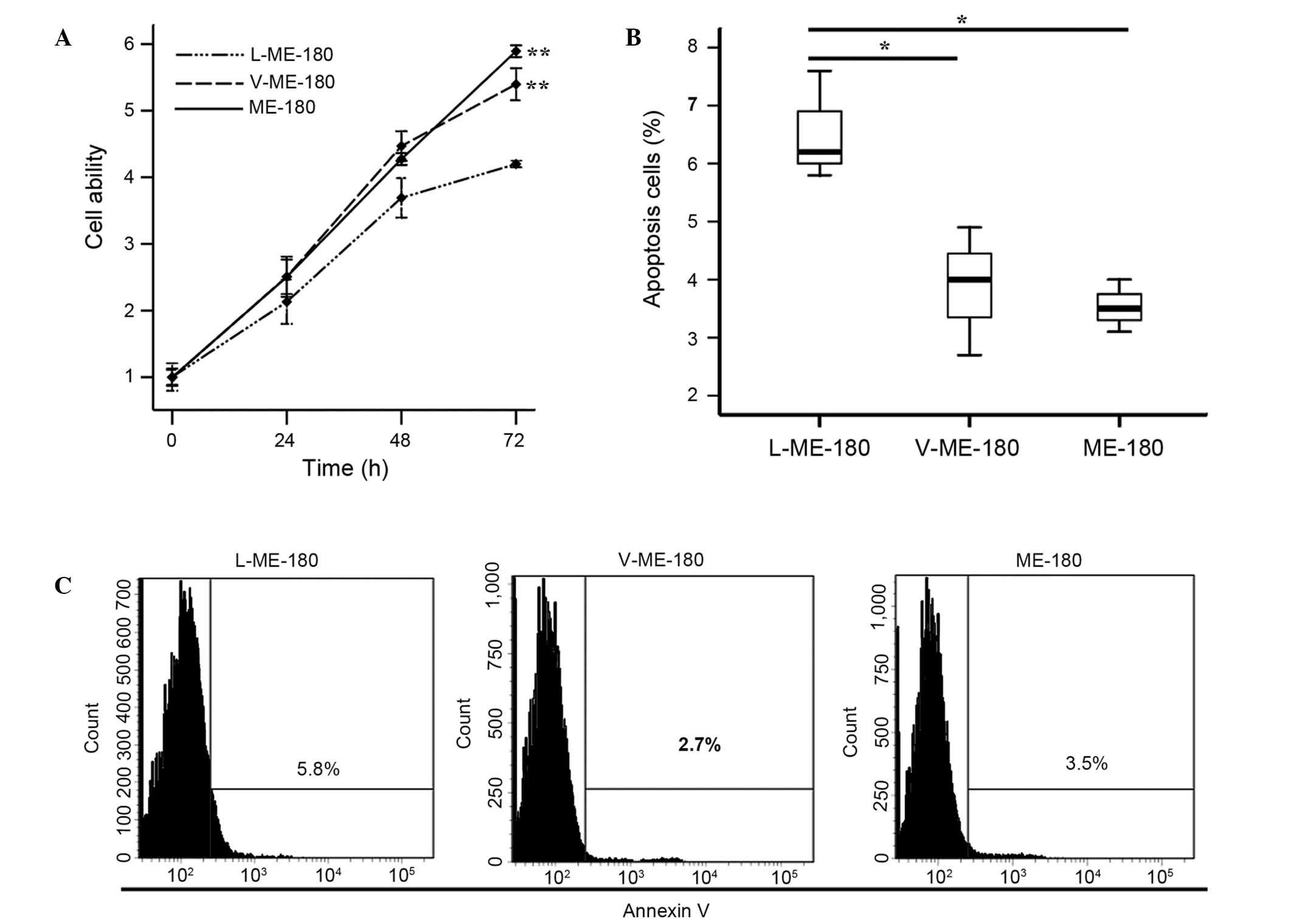
To investigate the function of LAIR-1 in the growth
of HCC cells, CCK-8 and annexin V assays were used to detect the
proliferation and apoptosis of ME-180 cells. Cell proliferation
ability was significantly reduced in the L-ME-180 cells
(P<0.001; Fig. 3A). As shown in
Fig. 3B, compared with V-ME-180 and
ME-180 cells, respectively, the mean apoptotic rate of L-ME-180
cells increased (P=0.021 or P=0.020). In summary, the expression of
LAIR-1 inhibits the proliferation and promotes the apoptosis of
ME-180 cells.
Discussion
In industrialized countries, HCC incidence has been
significantly reduced as a result of organized screening programs
(14). However, new cases of HCC in
less developed regions without access to health care account for
84.3% of the worldwide incidence (1). High-risk HPV infection is an essential
and causal factor for HCC. However, establishing a cytology-based
program in low-resource countries has several limitations. Since
2000, a range of novel biomarkers has been identified to advance
the current understanding of HCC molecular mechanisms, including
viral biomarkers (HPV DNA detection, HPV integration, HPV oncogene
mRNA and host and viral methylation) and cellular biomarkers
(p16ink4a, MCM2, Top2a, ki-67, 3q, and 5p chromosomal
instability) (15). Numerous
researchers are currently investigating various biomarker
candidates.
LAIR-1 is primarily expressed on immune cells, and
is also presented on CD34+ hematopoietic progenitor
cells (7), MKs (8) and osteoclasts (9). By using an IHC staining approach for
analyzing LAIR-1 expression in primary untreated HCC and normal
cervical epithelia, the current study observed LAIR-1 to be
localized in the cytoplasm and nucleus of HCC cells. The strong
expression of LAIR-1 in HCC tissues indicated that LAIR-1 may serve
a role in HCC progression. According to the statistical analysis to
elucidate the correlation between LAIR-1 expression and
clinicopathological variables, it was confirmed that the level of
LAIR-1 expression in the cytoplasm was associated with the number
of positive lymph nodes in patients with HCC, and that LAIR-1
expression in the cell nucleus was associated with pathological
stage, T classification and clinical stage. In conclusion, the
expression levels of LAIR-1 were associated HCC progression.
The present study used the ME-180 HCC cell line to
further investigate the function of elevated LAIR-1 expression in
HCC tissues. LAIR-1 expression is not detected on common HCC cell
lines. The current study established that LAIR-1 may be stably
transfected into ME-180 cells. Previous studies have demonstrated
the inhibitory function of LAIR-1 (16–18).
Poggi et al (19) stated that
LAIR-1 is able to inhibit proliferation and induce apoptosis of the
myelomonocytic THP1, MM6 and U937 cell lines through a
caspase-independent pathway. LAIR-1 engagement may also
downregulate granulocyte-macrophage colony-stimulating
factor-mediated survival and proliferation of acute myeloid
leukemia blasts (20). The present
study demonstrated that LAIR-1 expression suppresses the ability of
ME-180 cells to proliferate and induces their apoptosis. The two
ITIMs of LAIR-1 are commonly considered as the basis for inhibitory
function. Phosphatizing the tyrosine residues of ITIMs can recruit
the effectors of Src homology phosphotyrosine phosphatase-1 (SHP-1)
and SHP-2, and send an intracellular signal. SHP-1 has been
reported to be a negative regulator of angiogenesis (21), and is overexpressed in breast cancer
(22). In addition, upregulation of
SHP-2 has been found in gastric cancer (23) and HCC (24,25).
Cells or tumor stroma can produce collagen (12), and cross-linking LAIR-1 with collagen
can phosphatize ITIMs, recruit SHP-1 and SHP-2 (26), and weaken activation signals. Future
studies will focus on the role of LAIR-1 in the growth of HCC
cells, and the underlying mechanisms.
In conclusion, high expression of LAIR-1 in HCC is
associated with clinicopathological variables and inhibits the
growth ability of HCC cell lines. The present results suggests that
LAIR-1 may be a novel biomarker for diagnosis and a treatment
target for patients with HCC.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant no. 81001300), the
Shandong Provincial Natural Science Foundation of China (grant no.
ZR2010HM072) and the Scientific Research Development Program of
Shandong Provincial Education Department of China (grant no.
J08LG04).
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global Cancer Statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Dhillon PK, Yeole BB, Dikshit R, Kurkure
AP and Bray F: Trends in breast, ovarian and cervical cancer
incidence in Mumbai, India over a 30-year period, 1976–2005: An
age-period-cohort analysis. Br J Cancer. 105:723–730. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gadducci A, Barsotti C, Cosio S, Domenici
L and Genazzani A Riccardo: Smoking habit, immune suppression, oral
contraceptive use, and hormone replacement therapy use and cervical
carcinogenesis: A review of the literature. Gynecol Endocrinol.
27:597–604. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Benedet JL, Bender H, Jones H III, Ngan HY
and Pecorelli S: FIGO staging classifications and clinical practice
guidelines in the management of gynecologic cancers. FIGO Committee
on Gynecologic Oncology. Int J Gynaecol Obstet. 70:209–262. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cain JM and Howett MK: Preventing cervical
cancer. Science. 288:1753–1755. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Meyaard L: LAIR and collagens in immune
regulation. Immunol Lett. 128:26–28. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Xue J, Zhang X, Zhao H, Fu Q, Cao Y, Wang
Y, Feng X and Fu A: Leukocyte-associated immunoglobulin-like
receptor-1 is expressed on human megakaryocytes and negatively
regulates the maturation of primary megakaryocytic progenitors and
cell line. Biochem Biophys Res Commun. 405:128–133. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang Y, Ding Y, Huang Y, Zhang C, Boquan
J and Ran Z: Expression of leukocyte-associated immunoglobulin-like
receptor-1 (LAIR-1) on osteoclasts and its potential role in
rheumatoid arthritis. Clinics (Sao Paulo). 68:475–481. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Poggi A, Catellani S, Bruzzone A,
Caligaris-Cappio F, Gobbi M and Zocchi MR: Lack of the
leukocyte-associated Ig-like receptor-1 expression in high-risk
chronic lymphocytic leukaemia results in the absence of a negative
signal regulating kinase activation and cell division. Leukemia.
22:980–988. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cao Q, Fu A, Yang S, He X, Wang Y, Zhang
X, Zhou J, Luan X, Yu W and Xue J: Leukocyte-associated
immunoglobulin-like receptor-1 expressed in epithelial ovarian
cancer cells and involved in cell proliferation and invasion.
Biochem Biophys Res Commun. 458:399–404. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lu P, Weaver VM and Werb Z: The
extracellular matrix: A dynamic niche in cancer progression. J Cell
Biol. 196:395–406. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lebbink RJ, de Ruiter T, Adelmeijer J,
Brenkman AB, van Helvoort JM, Koch M, Farndale RW, Lisman T,
Sonnenberg A, Lenting PJ and Meyaard L: Collagens are functional,
high affinity ligands for the inhibitory immune receptor LAIR-1. J
Exp Med. 203:1419–1425. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tewari KS, Sill MW, Long HJ, Penson RT,
Huang H, Ramondetta LM, Landrum LM, Oaknin A, Reid TJ, Leitao MM,
Michael HE and Monk BJ: Improved survival with Bevacizumab in
advanced cervical cancer. N Engl J Med. 370:734–743. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sahasrabuddhe VV, Luhn P and Wentzensen N:
Human papillomavirus and cervical cancer: Biomarkers for improved
prevention efforts. Future Microbiol. 6:1083–1098. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Meyaard L, Adema GJ, Chang C, Woollatt E,
Sutherland GR, Lanier LL and Phillips JH: LAIR-1, a novel
inhibitory receptor expressed on human mononuclear leukocytes.
Immunity. 7:283–290. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ouyang W, Ma D, Lin D, Sun Y, Liu X, Li Q,
Jia W, Cao Y, Zhu Y and Jin B: 9.1C3 is identical to LAIR-1, which
is expressed on hematopoietic progenitors. Biochem Biophys Res
Commun. 310:1236–1240. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Maasho K, Masilamani M, Valas R, Basu S,
Coligan JE and Borrego F: The inhibitory leukocyte-associated
Ig-like receptor-1 (LAIR-1) is expressed at high levels by human
naive T cells and inhibits TCR mediated activation. Mol Immunol.
42:1521–1530. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Poggi A, Pellegatta F, Leone BE, Moretta L
and Zocchi MR: Engagement of the leukocyte-associated Ig-like
receptor-1 induces programmed cell death and prevents NF-kappaB
nuclear translocation in human myeloid leukemias. Eur J Immunol.
30:2751–2758. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zocchi MR, Pellegatta F, Pierri I, Gobbi M
and Poggi A: Leukocyte-associated Ig-like receptor-1 prevents
granulocyte-monocyte colony stimulating factor-dependent
proliferation and Akt1/PKB alpha activation in primary acute
myeloid leukemia cells. Eur J Immunol. 31:3667–3675. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Seo DW, Li H, Qu CK, Oh J, Kim YS, Diaz T,
Wei B, Han JW and Stetler-Stevenson WG: Shp-1 mediates the
antiproliferative activity of tissue inhibitor of
metalloproteinase-2 in human microvascular endothelial cells. J
Biol Chem. 281:3711–3721. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yip SS, Crew AJ, Gee JM, Hui R, Blamey RW,
Robertson JF, Nicholson RI, Sutherland RL and Daly RJ:
Up-regulation of the protein tyrosine phosphatase SHP-1 in human
breast cancer and correlation with GRB2 expression. Int J Cancer.
88:363–368. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jiang J, Jin MS, Kong F, Wang YP, Jia ZF,
Cao DH, Ma HX, Suo J and Cao XY: Increased expression of tyrosine
phosphatase SHP-2 in Helicobacter pylori-infected gastric cancer.
World J Gastroenterol. 19:575–580. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Meng F, Zhao X and Zhang S: Expression and
significance of SHP-2 in human papillomavirus infected cervical
cancer. J Huazhong Univ Sci Technolog Med Sci. 32:247–251. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tao XH, Shen JG, Pan WL, Dong YE, Meng Q,
Honn KV and Jin R: Significance of SHP-1 and SHP-2 expression in
human papillomavirus infected Condyloma acuminatum and cervical
cancer. Pathol Oncol Res. 14:365–371. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Verbrugge A, Rijkers ES, de Ruiter T and
Meyaard L: Leukocyte-associated Ig-like receptor-1 has SH2
domain-containing phosphatase-independent function and recruits
C-terminal Src kinase. Eur J Immunol. 36:190–198. 2006. View Article : Google Scholar : PubMed/NCBI
|