Introduction
Myocardial infarction leads to the production of a
large number of oxygen free radicals which damage myocardial
tissues and induce myocardial apoptosis and inflammation. The
resulting compensatory hypertrophy and fibrosis of the surviving
myocardium and extracellular matrix deposition leads to left
ventricular dysfunction with subsequent heart failure (1,2).
Curcumin is a small natural polyphenol compound found in tumeric,
which is a spice derived from the rhizomes of the plant Curcuma
longa Linn. Commercially available curcumin is usually a
mixture containing curcumin, hexahydrocurcumin, demethoxycurcumin
and bisdemethoxycurcumin, in which curcumin accounts for >70% of
the total mixture and serves as the primary active ingredient.
Previous studies have indicated that curcumin exerts
cardioprotective effects in patients and animal models with acute
or chronic myocardial infarction (3–6). In
particular, a previous clinical study demonstrated that curcumin
was able to decrease the incidence of in-hospital myocardial
infarction from 30% (placebo) to 13.1% in the patients following
coronary artery by decreasing C-reactive protein, malondialdehyde
and N-terminal pro-B-type natriuretic peptide levels (3). Animal studies have also demonstrated
that curcumin promotes cardiac injury, limits myocardial infarction
and improves cardiac function following acute and chronic
myocardial ischemia (4–6). It is currently thought that the
antioxidant and anti-inflammatory effects of curcumin account for
its cardioprotective effects (5,6);
however, the detailed mechanisms remain unclear.
In the current study, a myocardial infarction rat
model was established by ligation of the left anterior descending
coronary artery via thoracotomy. Curcumin was then provided by
intragastric administration to the model rats for 4 weeks. Then,
the histopathological changes to the infarction area, the apoptosis
levels in the myocardial infarction area, and the effects of
curcumin on nuclear factor-κB (NF-κB), peroxisome
proliferator-activated receptor-γ (PPAR-γ) and B-cell
leukemia/lymphoma-2 (Bcl-2) mRNA expression following myocardial
infarction were examined. The aim of the present study was to
reveal the effects of curcumin on the myocardium following
myocardial infarction, as well as the possible protective mechanism
underlying these effects. The results of this study may justify the
practice of elective opening of the occluded blood vessels to
improve cardiac function.
Materials and methods
Animals
A total of 32 healthy Sprague-Dawley rats (weight,
180±10 g), including 16 males and 16 females, were randomly
assigned to a blank control group (n=8), a sham-operation group
(n=8) and an operation group (n=16). The animals were provided by
the Experimental Animal Center of Xinxiang Medical College
(Xinxiang, China) and bred in experimental animal rooms of the
Xinxiang Medical University Forensic Laboratory with good
ventilation, natural light and a normal circadian cycle. The indoor
temperature was maintained at 21–27°C. The animals were maintained
and experiments were conducted in accordance with the Institutional
Animal Care and Use guidelines.
Preparation of the myocardial
infarction model
The rats were acclimatized for a week as previously
outlined by Lv et al (7) and
Ng and Kamm (8). A rat myocardial
infarction model was established by ligation of the left anterior
descending coronary artery via thoracotomy. Briefly, the rats were
anesthetized by intraperitoneal injection of 10% chloral hydrate
(0.3 ml/100 g; Dalian Meiun Biotech Co., Ltd., Dalian, China).
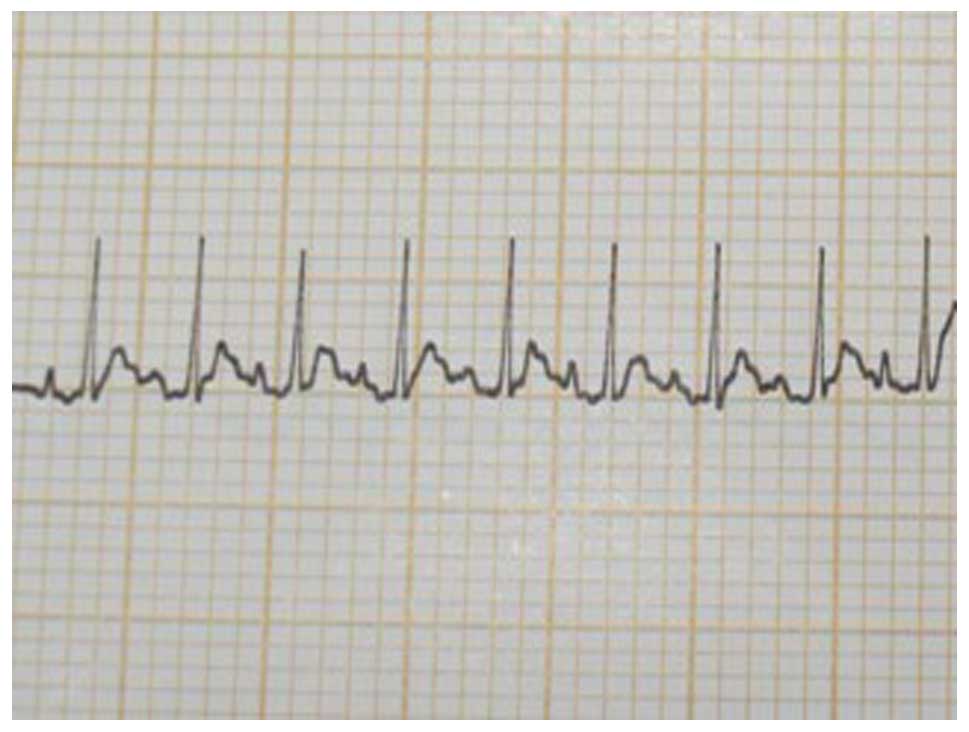
Electrocardiogram (ECG) lead II tracing was recorded when
respiration and cardiac rhythm were stabilized (Fig. 1). The skin in the middle of the neck
was incised vertically ~1 cm in length. The trachea was exposed by
separating the subcutaneous tissue from the neck muscles. A thread
was inserted below the trachea to fix the endotracheal cannula. The
endotracheal cannula was placed at the level of the third or fourth
cricoid cartilage following transverse incision of the trachea, and
connected with an animal ventilator to assist breathing. An
incision of ~5 cm in length was made to the center of chest. The
intercostal muscles were bluntly dissected at the level of the 3–4
intercostal space. The heart was visualized after the pericardium
was pulled apart. Using the great cardiac vein as a marker, a
needle was inserted from a point ~2 mm below the left atrial
appendage and ~2 mm in depth. The needle exited between the
pulmonary conus and left atrial appendage. The skin incision was
sutured and the heart immediately restored. The color change and
movement of the left ventricular myocardium were observed. The
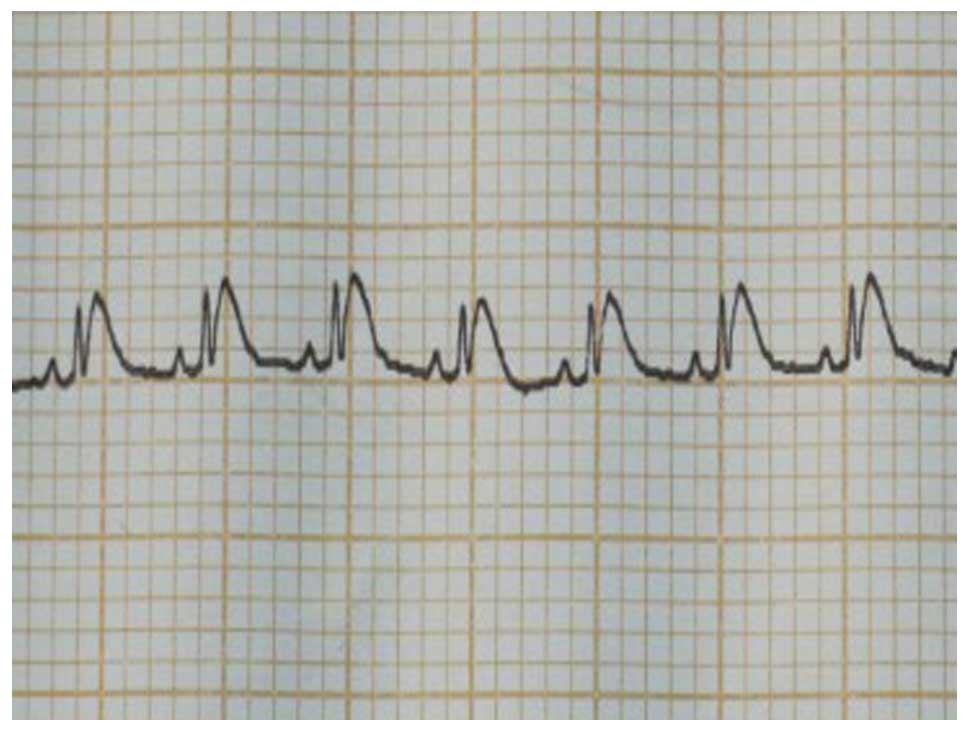
myocardial infarction model was considered successful when the left
ventricle showed signs of cyanosis and reduced wall movement, and
ST segment or T-wave elevation was observed (Fig. 2). Pleural effusion was drained and
the chest wall was closed. The ventilator was eliminated following
waking of the rats from the anesthetic. The thread was loosely tied
between the pulmonary conus and the left atrial appendage without
tight ligation for the rats in the sham-operation group. The rats
in blank control group received no treatment. The rats were
subsequently closely observed and managed if necessary following
the surgical procedure in order to improve the survival rate of the
animals.
Administration of curcumin
A total of 24 h after the surgical procedure, the 16
surviving rats in the operation group were randomized to a
myocardial infarction group (n=8) and curcumin group (n=8). The
rats in the curcumin group were treated with intragastric
administration of 150 mg/kg/body weight curcumin (Sinopharm
Chemical Reagent Co., Ltd., Shanghai, China) once a day, as
previously described (5). The rats
in the other groups were given distilled water of the same
volume.
Sample processing
A total of 4 weeks after intragastric
administration, the rats were anesthetized. Myocardial tissue
samples were obtained from each rat and divided into two sample
sets. The general appearance of the heart of the rats in the
sham-operation group was similar to that of the blank control
group. Tissue samples were obtained within 2 mm of the infarction
area prior to being rinsed with saline. A sample of tissue was
placed in 4% paraformaldehyde solution and embedded in paraffin.
Another sample of tissue was frozen at −80°C in a refrigerator for
future extraction of tissue mRNA.
Hematoxylin and eosin (H&E)
staining
Myocardial tissues were embedded in paraffin and
5-µm sections were subsequently prepared. Sections were
deparaffinized, rehydrated and stained with H&E as previously
described (9) and visualized under a
light microscope (magnification, ×400).
Terminal deoxyribonucleotidyl
transferase-mediated dUTP-biotin nick end-labeling (TUNEL)
staining
Myocardial slices were deparaffinized and rehydrated
as previously mentioned. The apoptosis of myocytes was assessed by
a TUNEL apoptosis kit (Roche Diagnostics, Basel, Switzerland)
according to the manufacturer's instructions.
Immunohistological staining.
Myocardial slices were prepared as previously mentioned
Slices were treated with citrate buffer at 98°C for
20 min. Following blocking with 10% goat serum at 37°C for 15 min,
the slices were incubated with rabbit anti-PPAR-γ polyclonal
antibody (Peprotech Inc., Rocky Hill, NJ, USA; 1:200) or rabbit
anti-NF-κBp6 polyclonal antibody (Peprotech Inc.; 1:100) at 4°C
overnight. Following washing with PBS three times, the slices were
incubated with biotin-labeled goat anti-rabbit IgG solution
(Beijing Zhongshan Golden Bridge Biotechnology Co., Ltd., Beijing,
China) at 37° for 30 min, and then washed with PBS three times.
Slices were subsequently incubated with horseradish
peroxidase-conjugated streptavidin (Beijing Zhongshan Golden Bridge
Biotechnology Co., Ltd.) and DAB solution (Beijing Zhongshan Golden
Bridge Biotechnology Co., Ltd.). Motic Images Advanced 3.2 software
was used for band detection, image capture and quantification.
Reverse transcription-polymerase chain
reaction (RT-PCR) analysis
Total RNA was extracted from myocardial tissues
using a Biozol RNA Extraction kit (Biomiga Inc., San Diego, CA,
USA), as per the manufacturer's instructions. Tissues were
harvested from the left ventricles, 2 mm in from of the border
zones. Prior to the RT and PCR transcription reactions, total RNA
was treated with DNase I (Thermo Fisher Scientific, Inc.; cat no:
AM2222) to remove the genomic DNA. A total of 1 µg RNA was used to
synthesize cDNA with a BioRT Reverse Transcription kit (Hangzhou
Bioer Technology Co., Ltd., Hangzhou, China; cat no: KIT0315), and
100 ng cDNA was subsequently used for the RT-PCR reaction with an
RT-PCR kit (Hangzhou Bioer Technology Co., Ltd.; cat no: 402876),
according to the manufacturer's instructions. PCR products were
separated by electrophoresis with 1.5% agarose gel and visualized
using ethidium bromide (Shang Hai Haoran Biological Technology Co.,
Shanghai, China; Ltd., cat no: 1203-10) on a UV transilluminator
(Shanghai Clinx Science Instruments, Shanghai, China). DNA markers
were provided by Takara Biotechnology Co., Ltd. (Beijing, China).
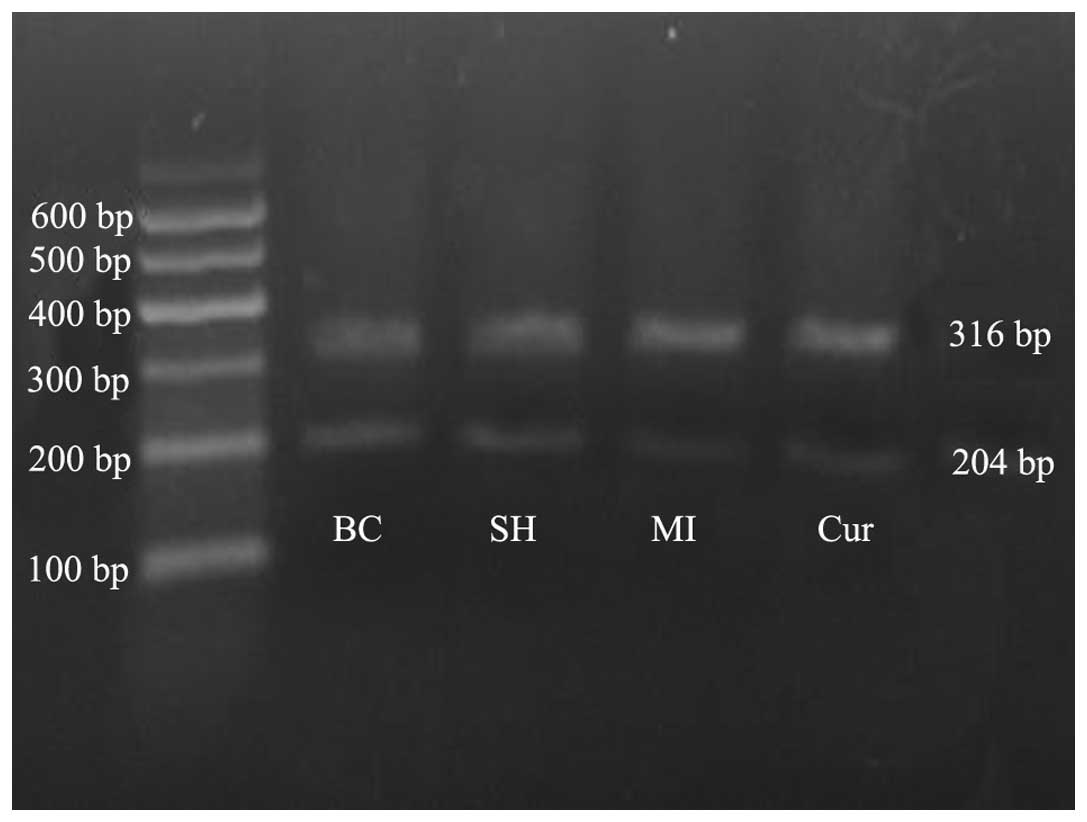
Bcl2 and β-actin primers were synthesized by Shanghai Sun Biotech
Co., Ltd. (Shanghai, China) and their sequences were as follows:
Bcl2, forward 5′-CCGCTACCGTCCGATACTTCA-3′ and reverse
5′-AAGACACAGAGGCCGCATGCTTG-3′; and β-actin, forward
5′-CAGATACAGCTCGCCTAGAAG-3′ and reverse
5′-GATTGACTGCTCTGCTCCTCA-3′. DNA marker was provided by Takara
Biotechnology Co., Ltd. (Beijing, China). Relative expression of
Bcl-2 was calculated by quantifying the gray scale values of the
target gene expression, relative to β-actin, which performed using
ImageJ software (National Institutes of Health, Bethesda, MD,
USA).
Imaging and statistical analysis
Motic Images Advanced version 3.2 software (Motic
China Group Co., Ltd., Xiamen, China), Gene Tools (Gene Tools, LLC,
Philomath, OR, USA) and SPSS 17.0 (SPSS, Inc., Chicago, IL, USA)
for Windows were used to analyze immunohistochemical images, RT-PCR
results, and data sets, respectively. The measurement data were
presented as means ± standard deviation and tested for normality
and homogeneity of variance. The data were subjected to analysis of
variance by group if applicable. A least significant difference
t-test was used for further pairwise comparison. If the data were
not applicable for analysis of variance, they were converted
accordingly and then tested by analysis of variance. If
heterogeneity of variance and/or non-normal distribution was still
present, Kruskal-Wallis rank sum test was used to compare the data.
All the hypothesis tests were two-sided. P<0.05 was considered
to indicate a statistically significant difference.
Results
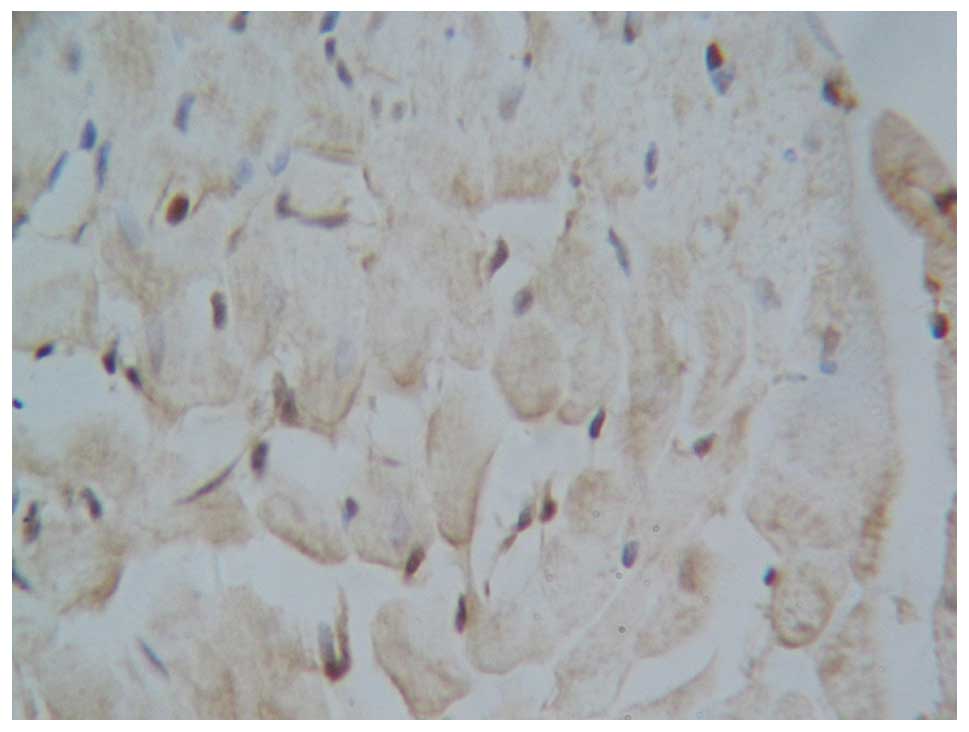
Morphological results for the left
ventricular cardiomyocytes of the rats
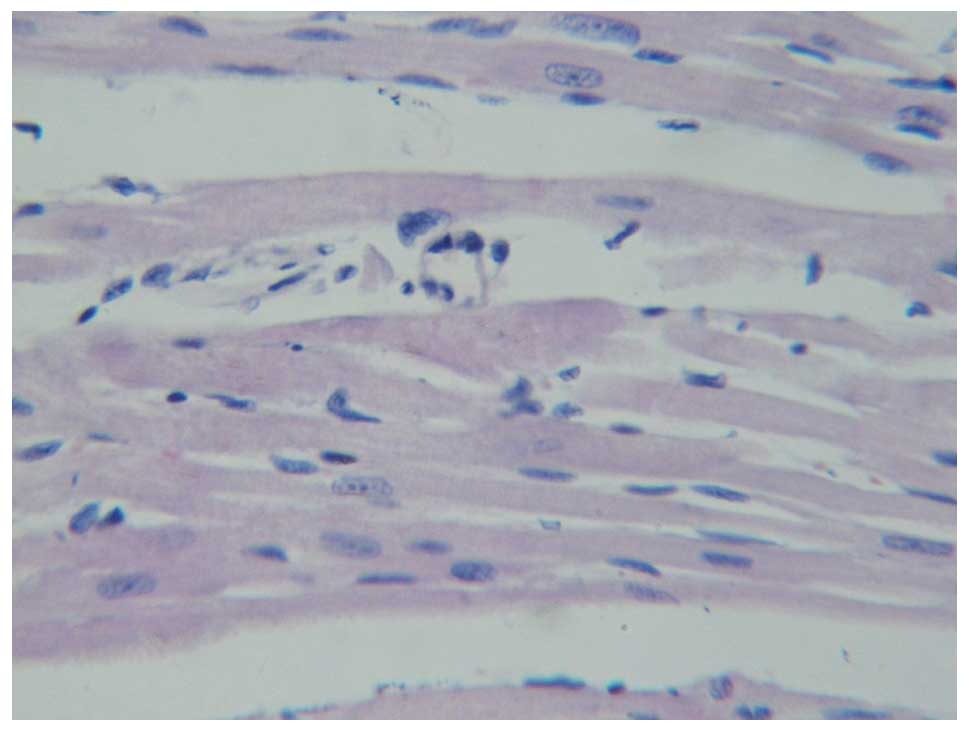
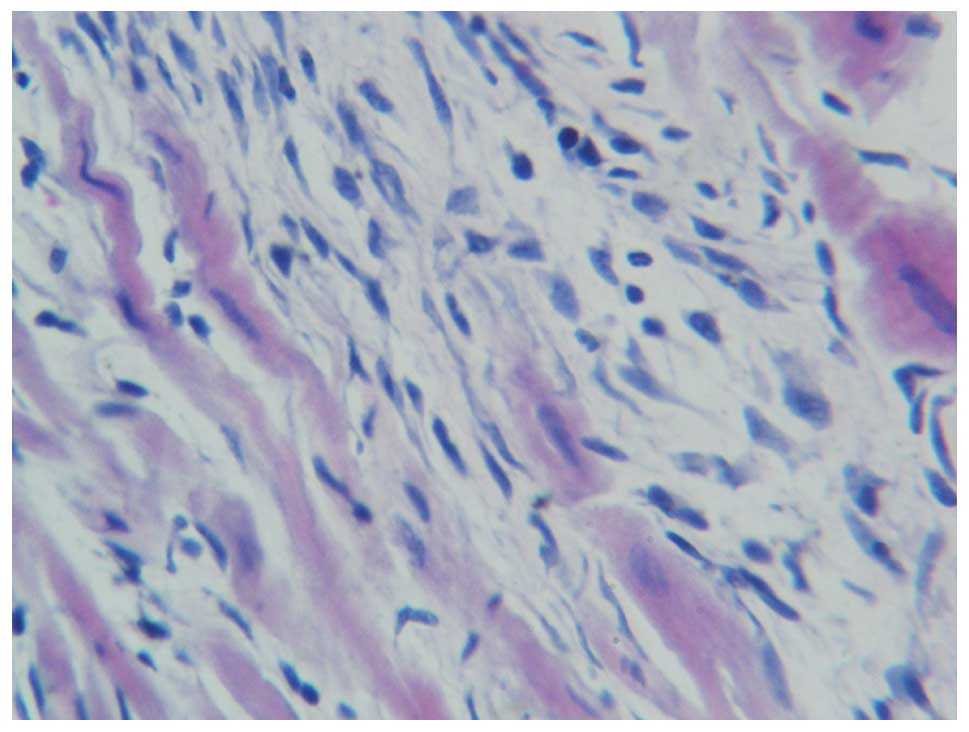
Following hematoxylin and eosin staining, the
myocardial cells of the left ventricle of the rats in the blank
control group (Fig. 3) and
sham-operation group (Fig. 4) were
cylindrical, parallel to each other and in orderly patterns under a
light microscope. The cells were not damaged in structure with oval
nuclei located in the center. The transverse striation was clearly
observable. There was no necrotic or apoptotic cells but some white
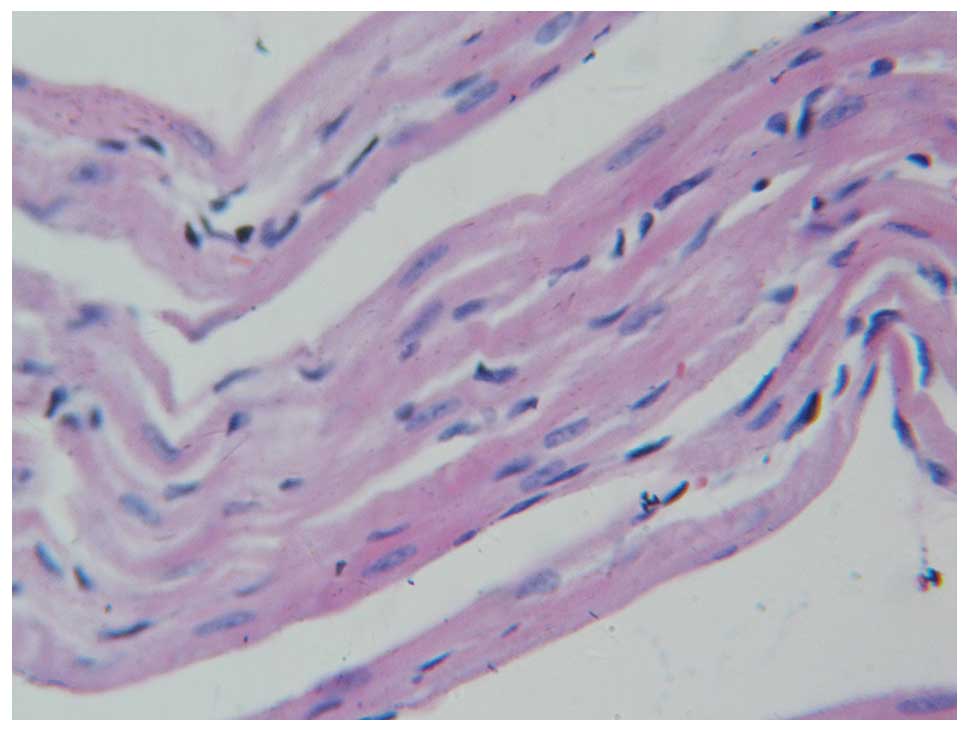
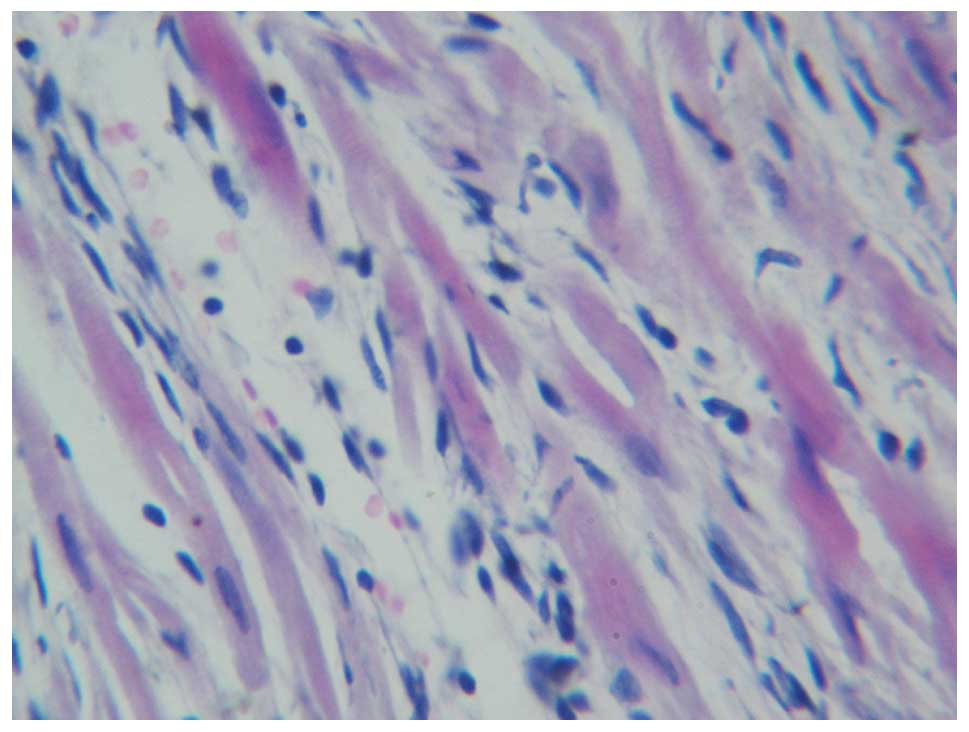
blood cells were visible. For the myocardial infarction group
(Fig. 5) and curcumin group
(Fig. 6), the left ventricular wall
was pale and thin, with left ventricular bulging. The cells at the
infarction zone of the left ventricle were shrunken with
hyperchromatic cytoplasm, lysis and breakage of myocardial fibers,
and karyopyknosis and karyorrhexis. Infiltration of inflammatory
cells and increased fibroblasts and fiber components were also
observed. Compared with the myocardial infarction group, necrotic
myocardial cells were still observed in the curcumin group
(Fig. 6), although fibroplasia at
the infarction site and inflammatory cell infiltration were
improved.
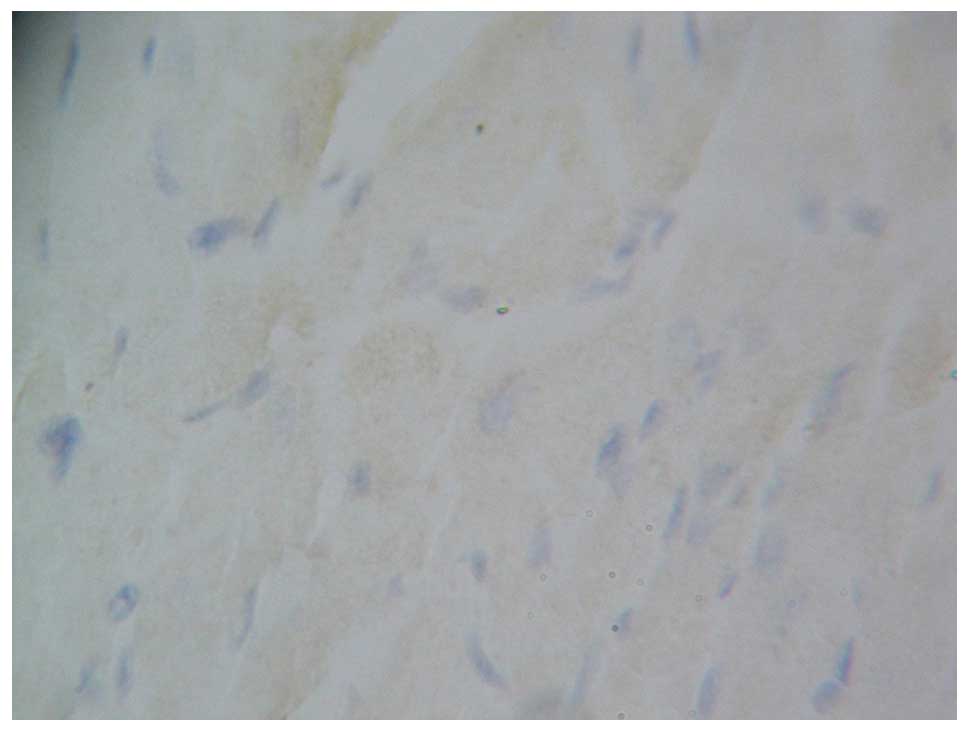
Myocardial apoptosis as determined by
TUNEL
The cells with tan myocardial nuclei were considered
apoptotic cells. The nuclei of myocardial cells from the rats in
the blank control group (Fig. 7) and
sham-operation group (Fig. 8) were
blue with almost no evidence of apoptosis. The apoptosis index did
not indicate significant difference between the two groups
(P>0.05). Four weeks after myocardial infarction, the cells
surrounding the infarction area were predominantly apoptotic cells
in the myocardial infarction group (Fig.
9). The difference was statistically significant (P<0.001)
compared with the blank control or sham-operation group. The number
of apoptotic cells in the curcumin group (Fig. 10) was lower, as compared with those
in the myocardial infarction group. The difference in the apoptosis
index was statistically significant (P<0.001), although the
number of apoptotic cells remained higher than in the blank control
or sham-operation group (P<0.001; Table I).
 | Table I.Apoptosis index of the myocardial
cells of rats in each group (mean ± standard deviation). |
Table I.
Apoptosis index of the myocardial
cells of rats in each group (mean ± standard deviation).
| Group | n | Apoptosis index
(%) |
|---|
| Blank control | 6 | 1.881±0.921 |
| Sham-operation | 6 |
0.979±0.811a |
| Myocardial
infarction | 6 |
38.383±3.880b,c |
| Curcumin (150
mg/kg·d) | 6 |
15.153±1.175b,c,d |
| F-value |
| 500.280 |
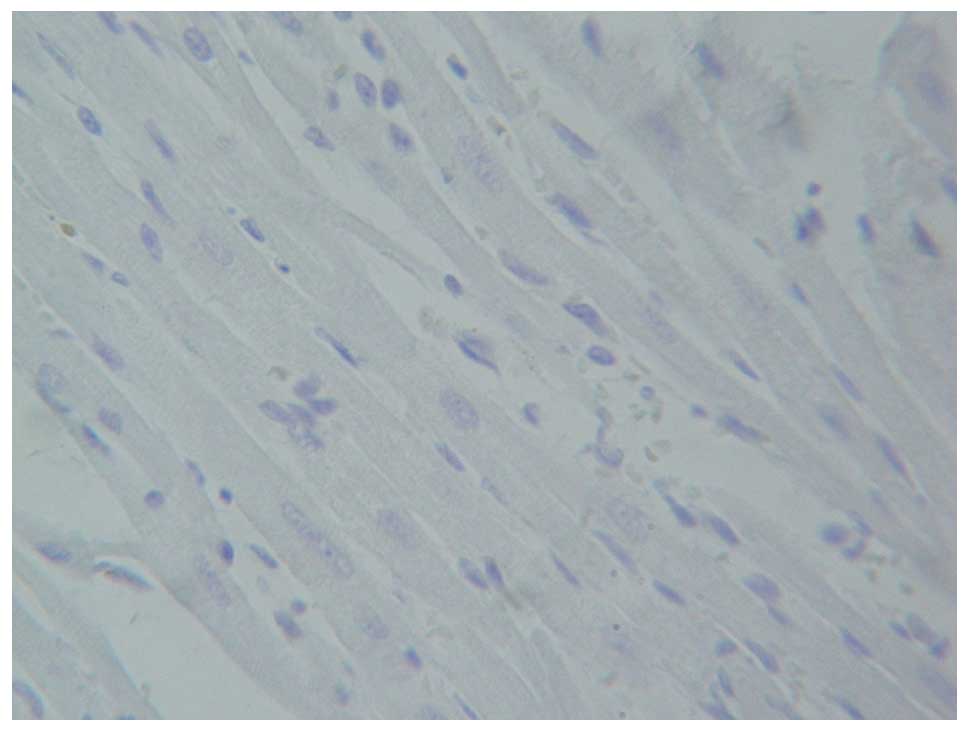
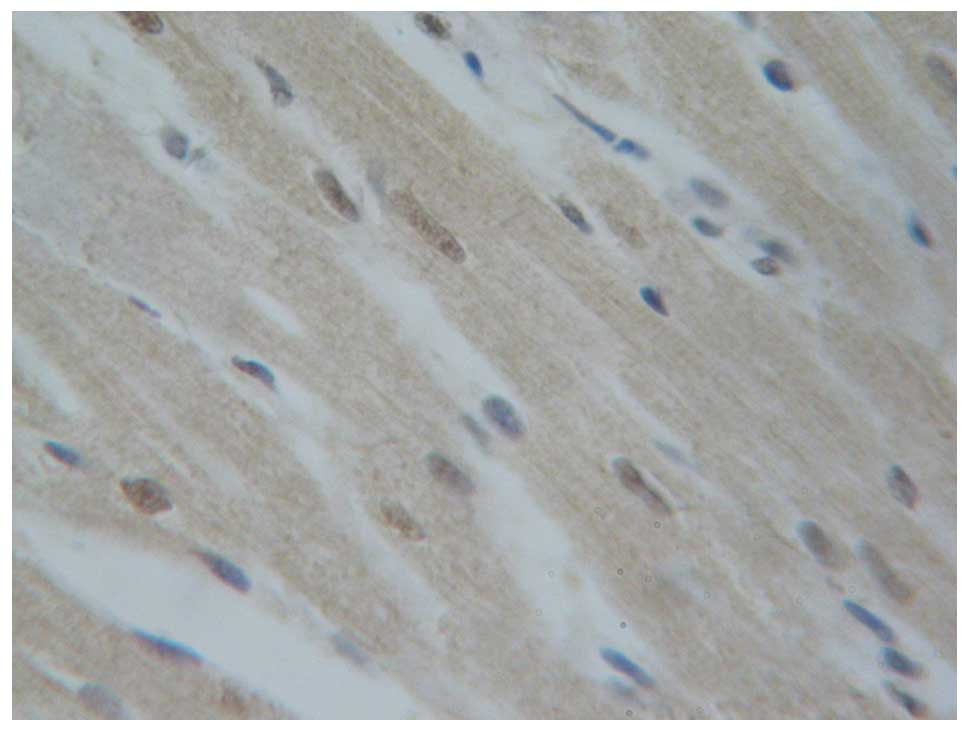
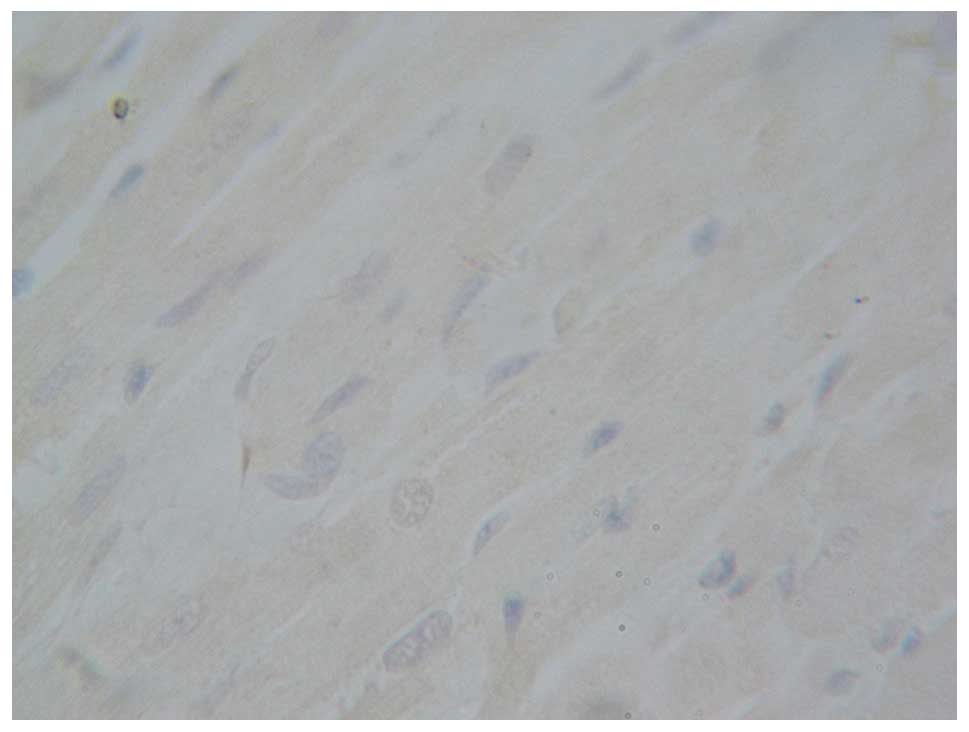
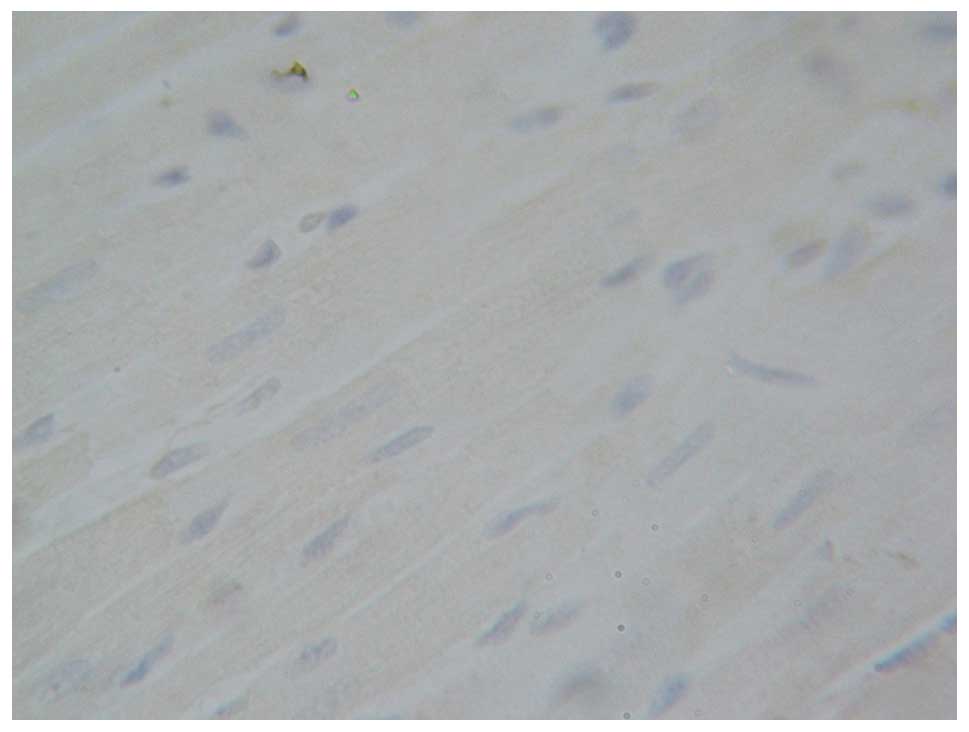
Immunohistochemical analysis of
NF-κBp65 and PPAR-γ proteins
The result was considered positive if the
immunohistochemical analysis demonstrated the presence of brown
particles under microscopic analysis. Low levels of NF-κBp65
expression were observed in the cytoplasm in both the blank control
group and the sham-operation group. No significant difference was
observed between these two groups (P>0.05). NF-κBp65 expression
in both the nucleus and cytoplasm (especially the nucleus)
increased significantly in the myocardial infarction group, as
compared with the curcumin group (P<0.01). The expression of
NF-κBp65 in the curcumin group was significantly lower than that in
the myocardial infarction group, but remained higher compared with
that of the blank control or sham-operation groups (P<0.01;
Figs. 11–14; Table
II).
 | Table II.Optical density of NF-κB and PPAR-γ
protein expression in the cardiomyocytes of rats (mean ± standard
deviation). |
Table II.
Optical density of NF-κB and PPAR-γ
protein expression in the cardiomyocytes of rats (mean ± standard
deviation).
| Group | N | NF-κBp65 | PPAR-γ |
|---|
| Blank control | 6 | 0.173±0.010 | 0.101±0.007 |
| Sham-operation | 6 |
0.166±0.008a |
0.101±0.005e |
| Myocardial
infarction | 6 |
0.325±0.004b,c |
0.107±0.006e,f |
| Curcumin (150
mg/kg) | 6 |
0.275±0.010b,c,d |
0.131±0.019g,h,i |
| F-value |
| 585.431 | 411.149 |
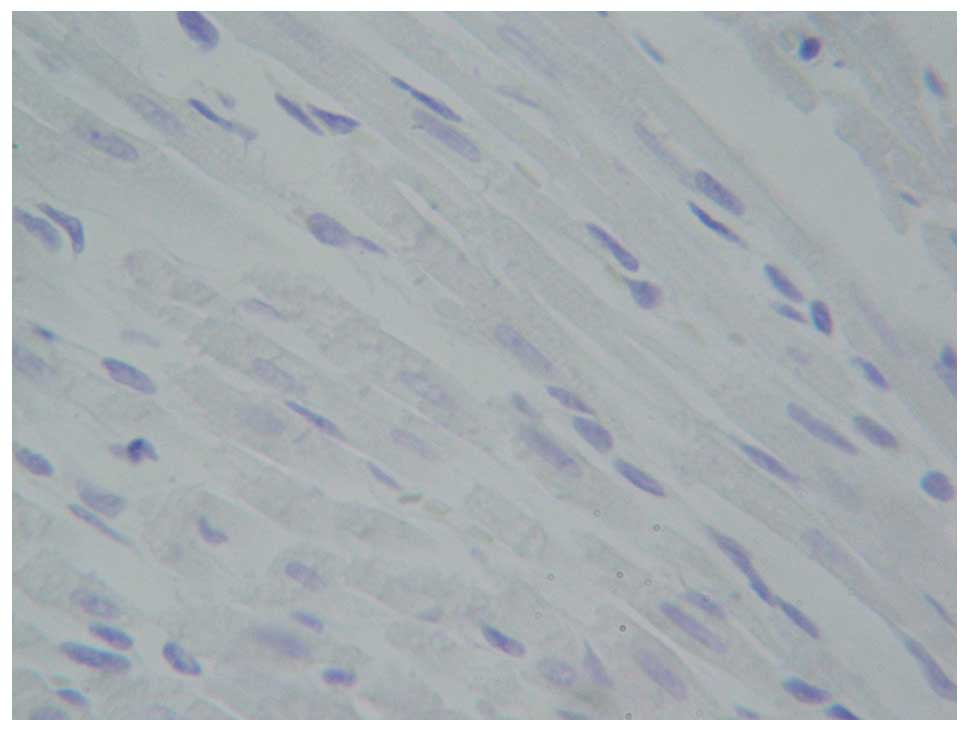
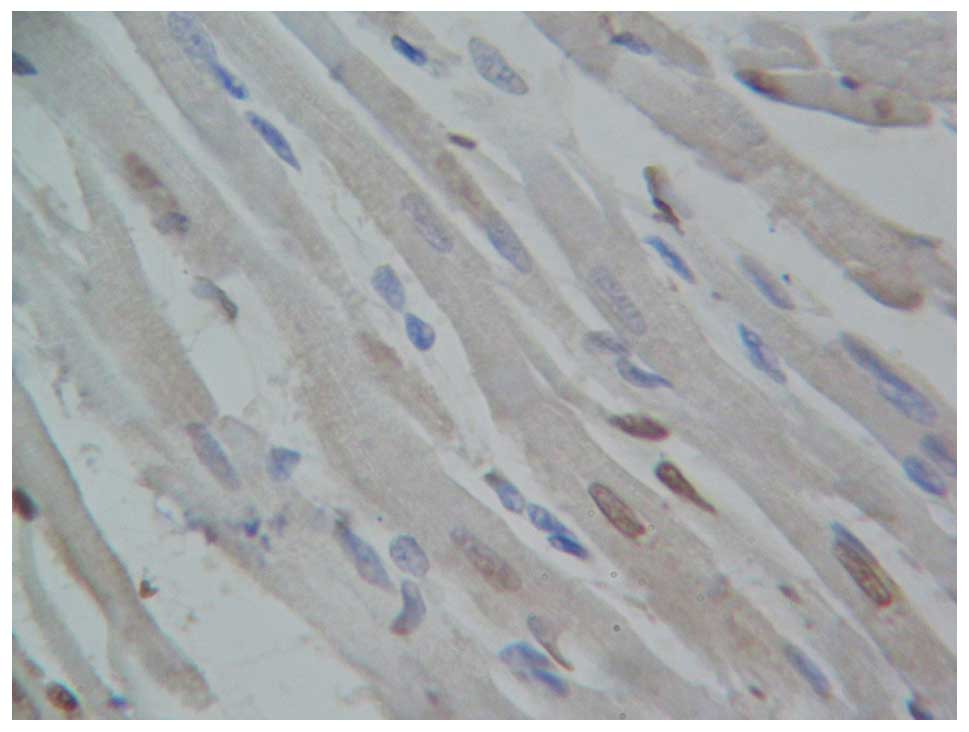
Low expression levels of PPAR-γ were observed in the
nucleus of both the blank control and sham-operation groups. There
was no significant difference between these two groups (P>0.05).
PPAR-γ expression in the myocardial infarction group was higher
compared with that in blank control or sham-operation group,
although this difference was not statistically significant
(P>0.05). Notably, the levels of PPAR-γ expression in the
nucleus were higher in the curcumin group, compared with those in
the blank control, sham-operation or myocardial infarction groups.
This difference was statistically significant (P<0.01; Figs. 15–18; Table
II).
mRNA expression levels of Bcl-2
RT-PCR results demonstrated that Bcl-2 mRNA
expression levels were not significantly different between the
blank control and sham-operation group (P>0.05). The mRNA
expression levels of Bcl-2 in the myocardial infarction group were
significantly lower compared with those of the blank control or
sham-operation group (P<0.01). Bcl-2 mRNA expression levels in
the curcumin group were significantly higher compared with those in
the myocardial infarction group (P<0.01) but remained lower
compared with those of the blank control or sham-operation group
(P<0.01; Fig. 19, Table III).
 | Table III.Bcl-2 mRNA/β-actin gray scale values
of myocardial tissues of rats (mean ± standard deviation). |
Table III.
Bcl-2 mRNA/β-actin gray scale values
of myocardial tissues of rats (mean ± standard deviation).
| Group | N | Bcl-2
mRNA/β-actin |
|---|
| Blank control | 6 | 0.519±0.024 |
| Sham-operation | 6 |
0.499±0.023a |
| Myocardial
infarction | 6 |
0.234±0.018b,c |
| Curcumin (150
mg/kg) | 6 |
0.341±0.019b,c,d |
| F value |
| 271.827 |
Discussion
Myocardial infarction is associated with necrosis
and apoptosis which induces dysfunction of tissues and organs.
Persistent apoptosis leads to the progressive loss of cells, which
is known to be an important cause for myocardial fibrosis,
ventricular dilation, cardiac remodeling, gradual decrease of
cardiac function and heart failure following myocardial infarction
(10,11). Therefore, inhibition of apoptosis
following myocardial infarction and protecting myocardial cells
against loss has become an important focus (12). A critical aspect of cardiovascular
research is the development of novel drugs and the investigation of
the effects of existing drugs on the protection of the ischemic
myocardium, the promotion of myocardial angiogenesis, and the
improvement of cardiac remodeling and function following myocardial
infarction.
Curcumin is a widely used drug that has been the
subject of investigation in the field of cardiovascular research
(13). The principal functions of
curcumin include: i) Anti-inflammation: Curcumin functions in
alleviating the pain induced by inflammation predominantly by
activating inflammatory mediators such as lipoxygenase and
cyclooxygenase, and transcription factors such as activator
protein-1 (14,15); ii) antioxidant: There are two benzene
rings in the chemical structure of curcumin, with one ring having a
phenol hydroxy and the other a phenol methoxy. These are able to
eliminate oxygen free radicals, serve as hydrogen donors or adjust
other mediators involved in the oxidation reaction against
antioxidant and anti-lipid peroxidation; iii) regulation of blood
lipids: Curcumin may lower the level of total cholesterol,
triglyceride and low density lipoprotein, increase the level of
high-density lipoprotein, inhibit the oxidative modification of low
density lipoprotein (16), and
reduce the damage to the vessel walls by oxidized low density
lipoprotein; iv) curcumin may also reduce myocardial ischemia
re-perfusion injury (17), lower
blood viscosity, inhibit the formation of atherosclerotic plaque,
stabilize plaque and inhibit adaptive hypertrophy (18,19) of
the myocardium following myocardial infarction.
The results of the present study demonstrated that
following four weeks of administration of curcumin, the left
ventricular myocardium of rats in the myocardial infarction group
and curcumin-treated group turned pale and the walls grew thin. The
ventricular wall of the rats in the myocardial infarction group
bulged. Cells at the infarction area were atrophic associated with
hyperchromatic cytoplasm, lysis and breakage of myocardial fiber,
karyopyknosis and karyorrhexis, infiltration of inflammatory cells
and increasing fibroblasts. Compared with the myocardial infarction
group, broken myocardial cells were also observed in the curcumin
group, although there were more living myocardial cells, and fiber
hyperplasia at the infarction area and inflammatory cell
infiltration decreased. An increased number of apoptotic cells was
observed surrounding the infarction area in the myocardial
infarction group, which is consistent with the results of a
previous study (19). The apoptotic
cells surrounding the infarction area of the curcumin group
decreased significantly, which indicates that curcumin is able to
inhibit the apoptosis of myocardial cells following myocardial
infarction, thereby protecting the myocardium.
The increase in cell apoptosis is an important
pathological process following myocardial infarction. Bcl-2 is an
important anti-apoptotic factor, which is able to inhibit the
apoptosis induced by various factors, including Bcl-X1 and Bax, and
enhance the resistance of cells to DNA damage, increasing cell
survival (20). The results of the
present study demonstrated that Bcl-2 mRNA expression decreased in
the myocardial infarction group, whereas Bcl-2 expression in the
curcumin group (150 mg/kg/body weight) increased significantly
compared with the myocardial infarction group due to the effect of
curcumin. These results suggest that curcumin is able to increase
Bcl-2 mRNA expression, thereby inhibiting the apoptosis of
myocardial cells.
NF-κB is a regulatory factor that controls the
transcription of DNA; it is a key nuclear transcription factor that
has important functions in signaling. NF-κB is involved in gene
expression and regulation, including the development of immunity,
inflammation, and tumor development. The activation of NF-κB leads
to the expression of various target genes, thereby promoting cell
proliferation and inhibiting apoptosis (21). Apoptosis is regulated by numerous
genes, among which Bcl-2, an anti-apoptosis gene, is a
proto-oncogene closely associated with apoptosis and a key
downstream target gene of NF-κB (20). When NF-κB associates with a
stimulating factor, it dissociates from its inhibition protein IκB
and becomes activated, following which it translocates to the
nucleus (22). Transcription and
translation of the downstream target genes in the nucleus are
subsequently activated to regulate inflammatory responses, tissue
injury, oxidative stress, cell differentiation, tissue
proliferation and apoptosis (23).
Therefore, regulating the effects of NF-κB effectively influences
the physiology and pathophysiology of the above-mentioned
processes. In the present report, increased infiltration of
inflammatory cells at the infarction area was observed in the
myocardial infarction group. NF-κB expression levels (predominantly
located in the nucleus) were significantly higher compared with
those of the blank control or sham-operation group. In other words,
NF-κB was activated due to myocardial infarction. The rats in the
curcumin group (150 mg/kg/body weight) were treated with curcumin
for four weeks. The infiltrated inflammatory cells were
significantly reduced and the protein expression levels of NF-κB
decreased significantly compared with myocardial infarction
group.
PPAR-γ inhibits inflammation via the NF-κB-mediated
signal transduction pathway, and mitigates cell damage by reducing
the levels of oxygen radicals released by neutrophils (24). Activated PPAR-γ is likely to promote
the regeneration of myocardial cells in the infarction area. PPAR-γ
is not only involved in fat formation, lipid and glucose
metabolism, but also has an important impact on vascular biology
and inflammatory response, especially in the pathogenesis of
atherosclerosis (25). PPAR-γ
protects the vascular endothelium, regulates inflammatory cytokines
and the expression of adhesion molecules on the cell surface,
inhibits macrophage activation, promotes the reverse transport of
cholesterol, inhibits proliferation of vascular smooth muscle cell
and stabilizes the atherosclerotic plaque (7,26). The
results showed the presence of low PPAR-γ expression in the
myocardial tissue samples of both the blank control group and the
sham-operation group. PPAR-γ expression in the curcumin group (150
mg/kg/body weight) increased significantly, which indicates that
curcumin is able to promote PPAR-γ expression, thereby making it
possible to protect the myocardium by stimulating the transcription
of genes downstream of PPAR-γ.
In conclusion, curcumin has a protective effect on
ischemic and hypoxic myocardium by increasing Bcl-2 and PPAR-γ
expression, inhibiting apoptosis and NF-κB expression, and reducing
inflammation. Curcumin is specifically indicated for patients whose
coronary artery cannot be opened through an emergency surgical
procedure. PCI may be provided to open the occluded blood vessels
following remission of acute disease or when appropriate to
activate the hibernating (contractile dysfunction) or stunned
(transient ischemia) myocardium, slow ventricular remodeling, and
improve and enhance heart function. Therefore, it remains important
to implement revascularization surgery on the basis of
pharmacotherapy in clinical practice. The results of the present
study provide a preliminary investigation into the inhibition of
myocardial cell necrosis and apoptosis. Further clinical research
on the protective effects of curcumin on human ischemic myocardial
cells is required.
Acknowledgements
The present study was supported by the Henan Funding
Program for College Young Backbone Teachers (grant no.
2009GGJS-085).
References
|
1
|
Qipshidze N, Metreveli N, Mishra PK,
Lominadze D and Tyagi SC: Hydrogen sulfide mitigates cardiac
remodeling during myocardial infarction via improvement of
angiogenesis. Int J Biol Sci. 8:430–441. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tuzun E, Bick R, Kadipasaoglu C, Conger
JL, Poindexter BJ, Gregoric ID, Frazier OH, Towbin JA and
Radovancevic B: Modification of a volume-overload heart failure
model to track myocardial remodeling and device-related reverse
remodeling. ISRN Cardiol. 2011:8310622011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wongcharoen W, Jai-Aue S, Phrommintikul A,
Nawarawong W, Woragidpoonpol S, Tepsuwan T, Sukonthasarn A, Apaijai
N and Chattipakorn N: Effects of curcuminoids on frequency of acute
myocardial infarction after coronary artery bypass grafting. Am J
Cardiol. 110:40–44. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jeong CW, Yoo KY, Lee SH, Jeong HJ, Lee CS
and Kim SJ: Curcumin protects against regional myocardial
ischemia/reperfusion injury through activation of RISK/GSK-3β and
inhibition of p38 MAPK and JNK. J Cardiovasc Pharmacol Ther.
17:387–394. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wang NP, Wang ZF, Tootle S, Philip T and
Zhao ZQ: Curcumin promotes cardiac repair and ameliorates cardiac
dysfunction following myocardial infarction. Br J Pharmacol.
167:1550–1562. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sunagawa Y, Sono S, Katanassaka Y,
Funamoto M, Hirano S, Miyazaki Y, Hojo Y, Suzuki H, Morimoto E,
Marui A, et al: Optimal dose-setting study of curcumnin for
improvement of left ventricular systolic function after myocardial
infarction in rats. J Pharmacol Sci. 126:329–336. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lv FH, Wang YL, Kong J, et al: Effects of
curcumin on expression of apoptosis-related genes in myocardial
cells of rats with acute myocardial infarction. J Appl Clin
Pediatr. 26:1010–1011. 2011.
|
|
8
|
Ng SC and Kamm MA: Therapeutic strategies
for the management of ulcerative colitis. Inflamm Bowel Dis.
15:935–950. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li N, Tian Y, Wang C, Zhang P and You S:
Protective effect of Lai Fu Cheng Qi decoction on severe acute
pancreatitis-induced myocardial injury in a rat model. Exp Ther
Med. 9:1133–1140. 2015.PubMed/NCBI
|
|
10
|
Konstantinova EV, Khomyakova NF,
Konstantinova NA, Podkolzina AV and Sapozhnikov AM: Relationship
between apoptosis and expression of heat shock proteins in
peripheral blood lymphocytes of patients with myocardial
infarction. Bull Exp Biol Med. 150:682–684. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Rondeau I, Picard S, Bah TM, Roy L,
Godbout R and Rousseau G: Effects of different dietary ω-6/3
polyunsaturated fatty acids ratios on infarct size and the limbic
system after myocardial infarction. Can J Physiol Pharmacol.
89:169–176. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lee Y and Gustafsson AB: Role of apoptosis
in cardiovascular disease. Apoptosis. 4:536–548. 2009. View Article : Google Scholar
|
|
13
|
Wongcharoen W and Phrommintikul A: The
protective role of curcumin in cardiovascular diseases. Int J
Cardiol. 2:145–151. 2009. View Article : Google Scholar
|
|
14
|
Basnet P and Skalko-Basnet N: Curcumin: An
anti-inflammatory molecule from a curry spice on the path to cancer
treatment. Molecules. 16:4567–4598. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Han YK, Lee SH, Jeong HJ, Kim MS, Yoon MH
and Kim WM: Analgesic effects of intrathecal curcumin in the rat
formalin test. Korean J Pain. 25:1–6. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yuan HY, Kuang SY, Zheng X, Ling HY, Yang
YB, Yan PK, Li K and Liao DF: Curcumin inhibits cellular
cholesterol accumulation by regulating SREBP-1/caveolin-1 signaling
pathway in vascular smooth muscle cells. Acta Pharmacol Sin.
29:555–563. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
González-Salazar A, Molina-Jijón E, Correa
F, Zarco-Márquez G, Calderón-Oliver M, Tapia E, Zazueta C and
Pedraza-Chaverri J: Curcumin protects from cardiac reperfusion
damage by attenuation of oxidant stress and mitochondrial
dysfunction. Cardiovasc Toxicol. 11:357–364. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Morimoto T, Sunagawa Y, Fujita M and
Hasegawa K: Novel heart failure therapy targeting transcriptional
pathway in cardiomyocytes by a natural compound, curcumin. Circ J.
74:1059–1066. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wongcharoen W and Phrommintikul A: The
protective role of curcumin in cardiovascular diseases. Int J
Cardiol. 133:145–151. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ola MS, Nawaz M and Ahsan H: Role of Bcl-2
family proteins and caspases in the regulation of apoptosis. Mol
Cell Biochem. 351:41–58. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kiefel H, Pfeifer M, Bondong S, Hazin J
and Altevogt P: Linking L1CAM-mediated signaling to NF-κB
activation. Trends Mol Med. 4:178–187. 2011. View Article : Google Scholar
|
|
22
|
Oeckinghaus A, Hayden MS and Ghosh S:
Crosstalk in NF-κB signaling pathways. Nat Immunol. 8:695–708.
2011. View
Article : Google Scholar
|
|
23
|
Sunagawa Y, Wada H, Suzuki H, Imaizumi A,
Fukuda H, Hashimoto T, Katanasaka Y, Shimatsu A, Kimura T, et al: A
novel drug delivery system of oral curcumin markedly improves
efficacy of treatment for heart failure after myocardial infarction
in rats. Biol Pharm Bull. 2:139–144. 2012. View Article : Google Scholar
|
|
24
|
Hisada S, Shimizu K, Shiratori K and
Kobayashi M: Peroxisome proliferator-activated receptor gamma
ligand prevents the development of chronic pancreatitis through
modulating NF-kappaB-dependent proinflammatory cytokine production
and pancreatic stellate cell activation. Rocz Akad Med Bialymst.
50:142–147. 2005.PubMed/NCBI
|
|
25
|
Ahmadian M, Suh J M, Hah N, Liddle C,
Atkins AR, Downes M and Evans RM: PPARγ signaling and metabolism:
The good, the bad and the future. Nat Med. 19:557–566. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lv FH, Gao JZ and Zhang JY: Role of
peroxisome proliferator-activated receptor signaling pathway in
mice with hyperlipidemia. Chin J Geriatr. 30:1047–1050. 2011.
|