Introduction
Rheumatoid arthritis is a type of systemic
autoimmune disease, which involves symmetrical chronic inflammation
of the synovial membranes, and has the following pathological
characteristics: Chronic inflammation of synovial membranes, the
gradual formation of pannus, cartilage and joint destruction,
ankylosis and joint deformity in the advanced stage and possible
muscle atrophy (1). As a result,
rheumatoid arthritis, the morbidity rate of which is 0.2–0.4%, is a
major cause of productivity loss and disability, and can cause
great suffering and a heavy financial burden (2). Furthermore, rheumatoid arthritis is a
systematic disease whose pathogenesis is unclear. However,
rheumatoid arthritis is commonly considered to be induced by
exogenous factors in addition to genetic susceptibility (3). Therefore, as no specific medicine for
this disease has yet been developed, immunosuppressive and
biological agents are widely used because of their curative
abilities, despite their side-effects (4).
Dandelion is the dry grass of the Asteraceae
(Compositae) family, which is used in the treatment of malignant
boils, skin ulcers, acute mastitis, acute conjunctivitis, pulmonary
abscess, damp-heat jaundice, heat stranguria and other inflammatory
conditions, with various effects, including detoxification,
detumescence, fluid removal and the induction of diuresis (5). One of the effective components of
dandelion is taraxasterol, which has a molecular structure
resembling that of a steroid hormone, and has been shown to have
anti-inflammatory effects in vivo and in vitro
(6). Therefore, the present study
examined whether taraxasterol exhibits a protective effect against
rheumatoid arthritis through the modulation of inflammatory
responses in mice.
Materials and methods
Animals and rheumatoid arthritis
induction
Eight-week-old chemokine (C-C motif) receptor 9
(CCR9)-deficient male mice (n=32; weight, 20–23 g) were obtained
from the Animal Resource Center of the Hebei Cangzhou Hospital of
Integrated Traditional Chinese and Western Medicine (Cangzhou,
China). The mice were maintained in individual cages at 21–24°C and
55–65% humidity with a 12-h light-dark cycle (8:00–20:00). Food and
water were provided ad libitum. The present study was
conducted according to the National Institutes of Health Guide for
the Care and Use of Laboratory Animals (2012). Mice were injected
with a collagen II monoclonal antibody cocktail (Sigma-Aldrich, St.
Louis, MO, USA) intraperitoneally (i.p.) at 5 mg/kg per mouse, as
indicated (7) for 10 days. The mice
were also injected with lipopolysaccharide (LPS; i.p., 100 µg at
day 1 or day 4; Sigma-Aldrich). Following treatment with
taraxasterol, blood samples were collected from retro-orbital
bleeds using heparin-coated glass capillaries and stored at −80°C
prior to analysis.
Animal grouping
The mice used in the experiment were randomly
distributed into four groups: Control (n=6), taraxasterol only
(n=6), rheumatoid arthritis model (n=10) and rheumatoid arthritis +
taraxasterol-treated (n=10) groups. Mice of the control or
rheumatoid arthritis model groups were treated with 0.5% sodium
tvlose (Nanjing Chemical Reagent Co., Ltd., Nanjing, China; i.p.)
once per day for 5 consecutive days following the establishment of
the collagen-induced rheumatoid arthritis model. The mice of the
taraxasterol only and rheumatoid arthritis + taraxasterol-treated
groups were treated with 10 mg/kg taraxasterol (i.p.) once per day
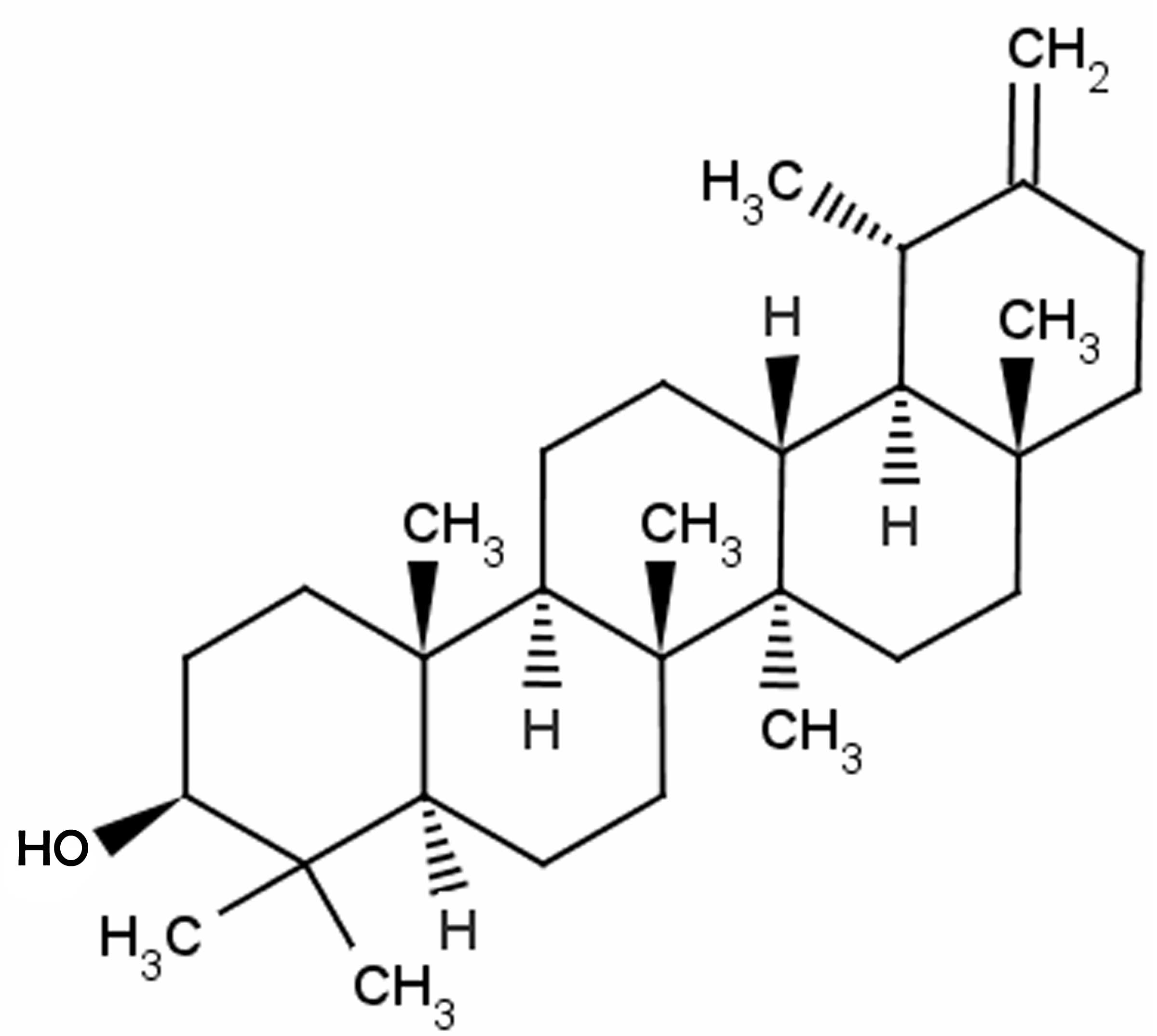
for 5 consecutive days. The chemical structure of taraxasterol
(≥98.5% purity; Sigma-Aldrich) is shown in Fig. 1.
Measurement of pain thresholds in the
rheumatoid arthritis mouse model
The mean value of the pressure pain threshold was
determined before and after modeling, and after the 5-day
treatment. This was measured using an electronic pressure pain
detector (Somedic, Hörby, Sweden).
Measurement of clinical arthritic
score in the rheumatoid arthritis mouse model
On each day of the 5-day treatment, the mice were
evaluated for arthritis using a macroscopic scoring system as
follows: 11–15, severe arthritis of the entire paw and digits;
6–10, more than two joints involved; 1–5, two joints involved and
0, no signs of arthritis.
Measurement of tumor necrosis factor
(TNF)-α, interleukin (IL)-1β and IL-6
Following the 5-day treatment, blood was collected
to evaluate the TNF-α, IL-1β and IL-6 levels with the corresponding
enzyme-linked immunosorbent assay kits according to the
manufacturer's instructions (R&D Systems, Minneapolis, MN,
USA).
Measurement of nitric oxide (NO) and
prostaglandin E2 (PGE2)
Following treatment with taraxasterol, mice were
sacrificed using cervical vertebra luxation under anesthesia. For
the measurement of NO, tissue samples were mixed with Griess
reagent and 100 µl bronchoalveolar lavage fluid, and then were
incubated for 10 min at room temperature. The optical density at
540 nm was then measured using a Synergy2 microplate reader
(BioTek, Winooski, VT, USA), and the NO level determined by
comparison with a standard curve. The PGE2 level was measured using
a commercial enzyme immunoassay, with a prostaglandin E metabolite
kit (Cayman Chemical, Ann Arbor, MI, USA).
Western blot analysis of
cyclooxygenase-2 (COX-2) and nuclear factor (NF)-κB
Following the 5-day treatment, paw tissue samples
were harvested and frozen in liquid nitrogen immediately prior to
homogenization. Protein concentrations were determined using a
bicinchoninic acid assay kit (BestBio, Shanghai, China). Equal
amounts of protein (50 mg) were separated by 10% sodium dodecyl
sulfate-polyacrylamide gel electrophoresis and subsequently
transferred onto a polyvinylidene difluoride (PVDF) membrane
(Bio-Rad Laboratories, Inc., Munich, Germany). The PVDF membrane
was blocked with 5% skimmed milk in Tris-buffered saline and Tween
20 (TBST) on a shaker for 2 h at room temperature. The membrane was
then incubated with anti-COX-2 (cat. no. sc-514489, 1:1,000; Santa
Cruz Biotechnology, Inc., Dallas, TX, USA), anti-NF-κB (cat. no.
sc-8008; 1:1,000; American Diagnostica Inc., Stamford, CT, USA) and
anti-β-actin (cat. no. sc-130300; 1:1,000; Santa Cruz
Biotechnology, Inc.) primary antibodies. After washing the membrane
three times with TBST, the membrane was incubated with secondary
antibody (cat. no. sc-2005; 1:5,000; Santa Cruz Biotechnology,
Inc.) for 2 h on the shaker at room temperature. The membrane was
then incubated with chemiluminescence reagent (ECL Plus Western
Blotting Detection system; GE Healthcare Life Sciences, Chalfont,
UK). The relative quantity of the protein was measured using
AlphaEase FC (FluorChem FC2) software (Cell Biosciences Inc., Santa
Clara, CA, USA).
Statistical analysis
Data are expressed as the mean ± standard error of
the mean. Differences between the mean values of normally
distributed data were analyzed using one-way analysis of variance
(Dunnett's t-test) and two-tailed Student's t-test. P<0.05 was
used to indicate a statistically significant difference.
Results
Protective effect of taraxasterol
against pain in the rheumatoid arthritis mouse model
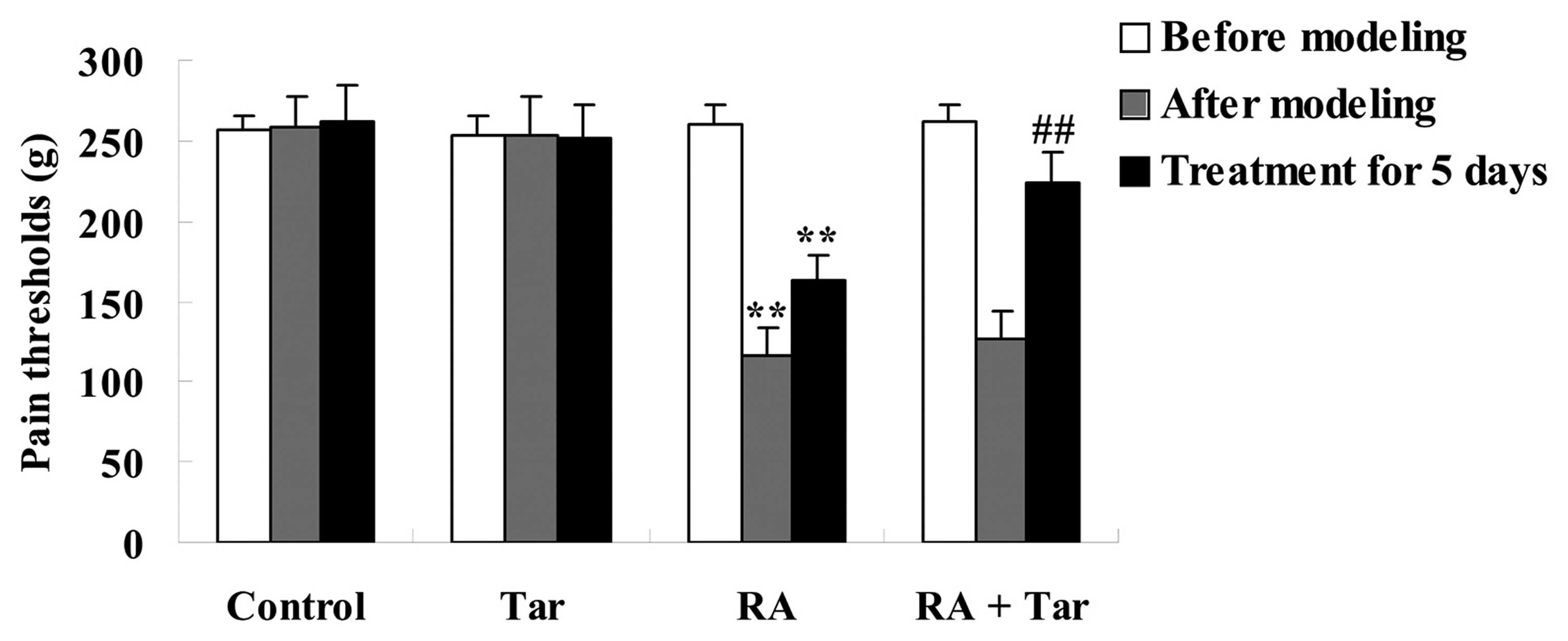
The protective effect of taraxasterol against pain
thresholds in the rheumatoid arthritis mouse model was assessed
using an electronic pressure pain detector. As shown in Fig. 2, the pain thresholds in the control
group were very similar to those of the taraxasterol only group
(P>0.05) during the entire experiment. By contrast, pain
thresholds of the animals in the rheumatoid arthritis group were
lower compared with those of the control and taraxasterol only
groups after modeling and following treatment for 5 days (Fig. 2). Furthermore, pain thresholds were
noticeably increased by treatment with taraxasterol for 5 days in
comparison with the rheumatoid arthritis group (Fig. 2).
Protective effect of taraxasterol
against clinical arthritis in the rheumatoid arthritis mouse
model
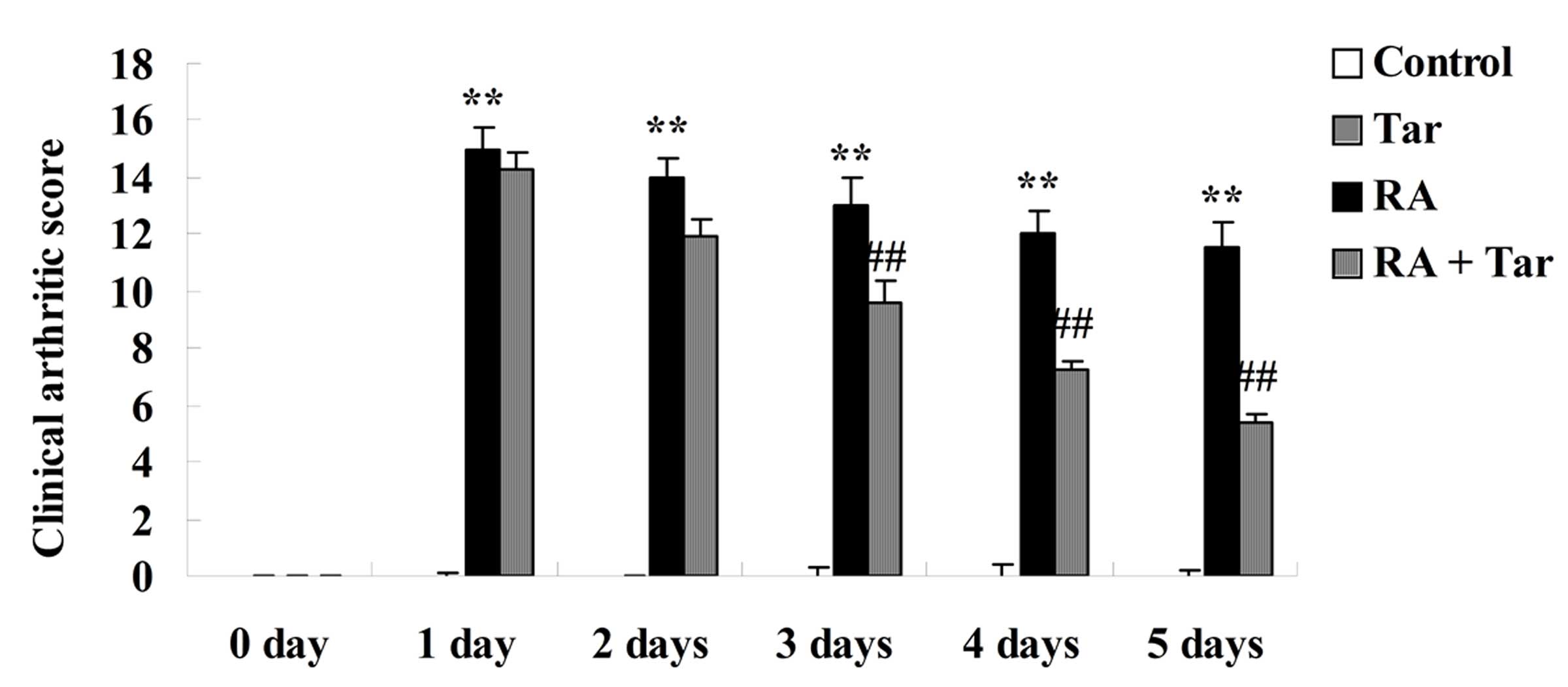
The protective effect of taraxasterol in the
rheumatoid arthritis mouse model was evaluated using the clinical
arthritic score. As shown in Fig. 3,
there was no significant difference in the clinical arthritic score
between the control and the taraxasterol only groups (P>0.05).
By contrast, rheumatoid arthritis markedly increased the clinical
arthritic scores compared with those in the control and
taraxasterol only groups (Fig. 3).
However, the clinical arthritic score of mice in the rheumatoid
arthritis + taraxasterol group was markedly suppressed in
comparison with that of the rheumatoid arthritis group (Fig. 3).
Protective effect of taraxasterol
against TNF-α, IL-1β and IL-6 in the rheumatoid arthritis mouse
model
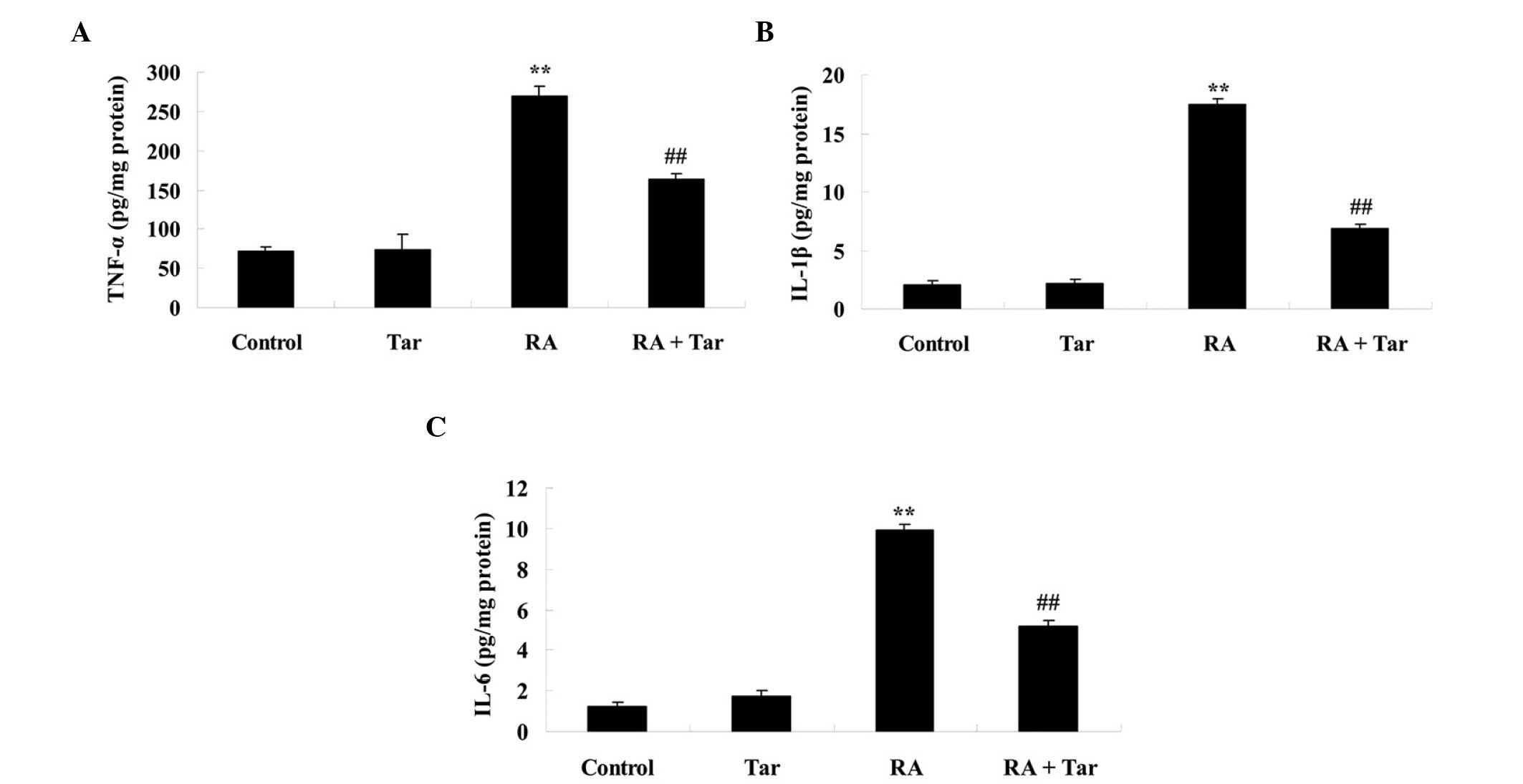
To investigate the protective effect of taraxasterol
against inflammation in mice with rheumatoid arthritis, TNF-α,
IL-1β and IL-6 levels were measured. As shown in Fig. 4, there was no significant difference
in the levels of TNF-α, IL-1β and IL-6 between the control and
taraxasterol only group (P>0.05). However, the levels of these
inflammatory factors were effectively increased in mice with
rheumatoid arthritis compared with the control and taraxasterol
only groups (Fig. 4). Furthermore,
treatment with taraxasterol decreased the levels of TNF-α, IL-1β
and IL-6 in mice with rheumatoid arthritis, when compared with the
rheumatoid arthritis group (Fig.
4).
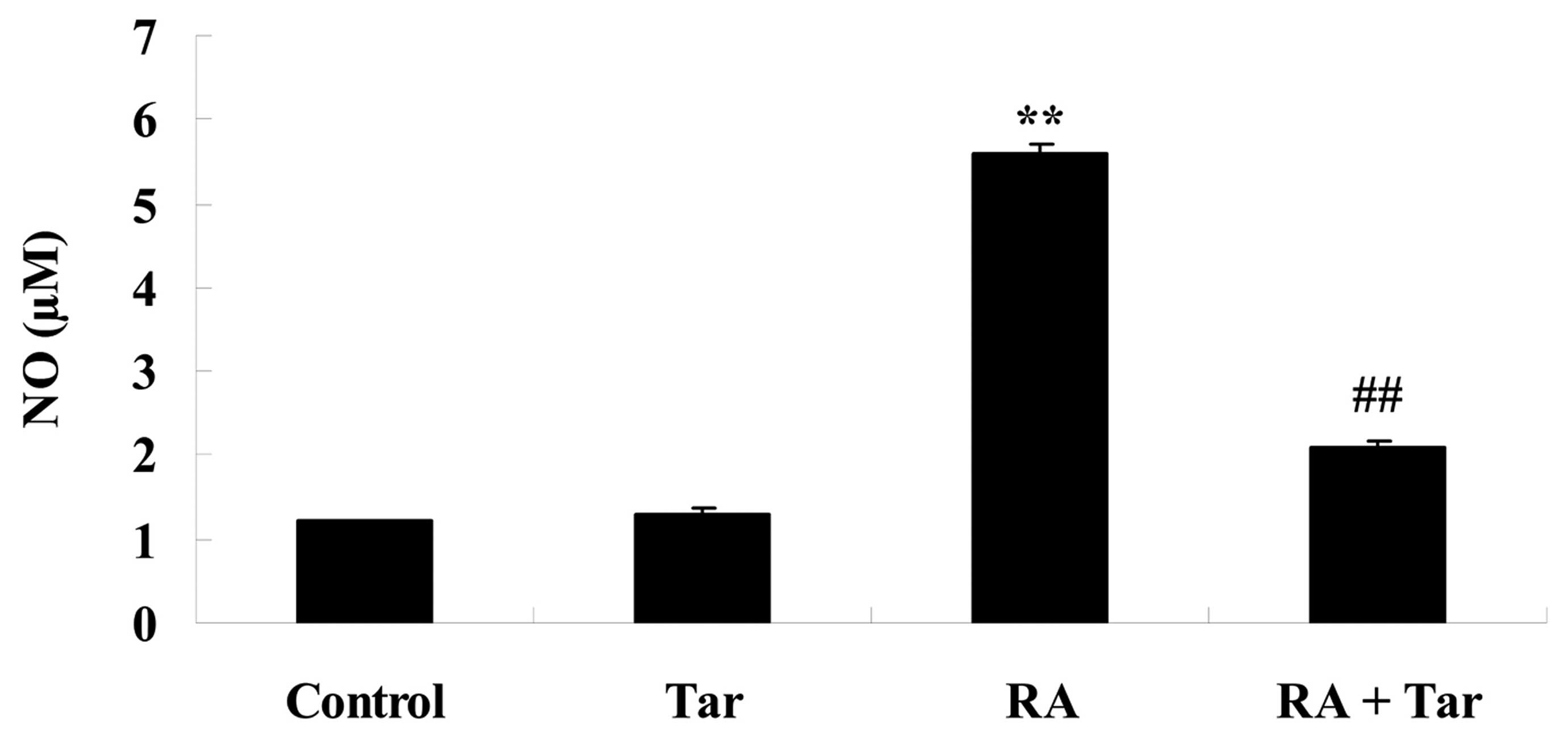
Protective effect of taraxasterol
against NO generation in the rheumatoid arthritis mouse model
NO generation was measured to investigate the
protective effect of taraxasterol against NO damage in rheumatoid
arthritis model mice. As shown in Fig.
5, no significant inter-group difference was identified between
the control and taraxasterol only group for NO generation
(P>0.05). However, there was a significant increase in NO
generation in the mice with rheumatoid arthritis compared with the
control and taraxasterol only groups (Fig. 5). However, treatment with
taraxasterol attenuated the increase in NO generation in mice with
rheumatoid arthritis compared with that in the rheumatoid arthritis
group (Fig. 5).
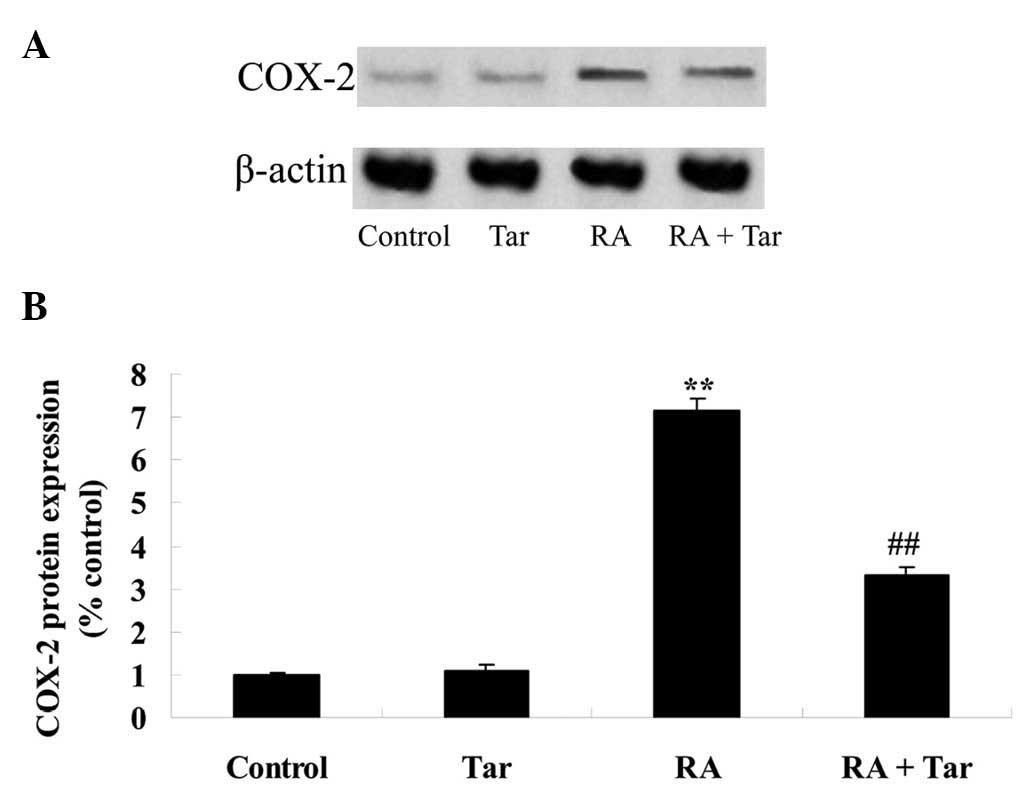
Protective effect of taraxasterol
against COX-2 in the rheumatoid arthritis mouse model
In order to define the protective effect of
taraxasterol against COX-2 in a rheumatoid arthritis mouse model,
COX-2 protein expression levels were examined using western blot
analysis. The expression level of COX-2 demonstrated no significant
difference between the control and taraxasterol only groups
(P>0.05; Fig. 6). However,
rheumatoid arthritis noticeably induced the expression of COX-2
protein compared with COX-2 expression levels in the control and
taraxasterol only groups (Fig. 6).
However, taraxasterol significantly attenuated the change in COX-2
level induced by rheumatoid arthritis (Fig. 6).
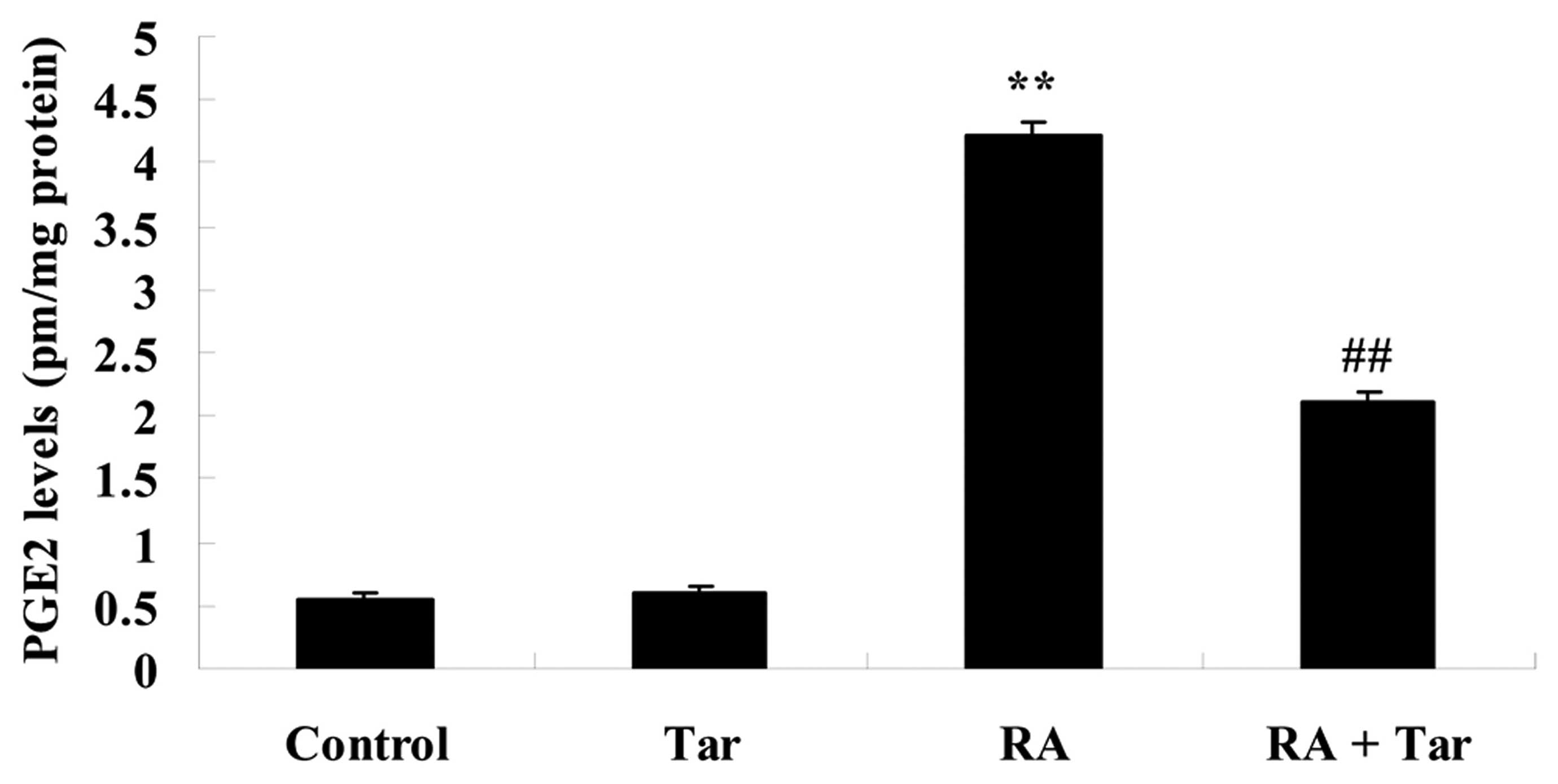
Protective effect of taraxasterol
against PGE2 in the rheumatoid arthritis mouse model
In order to explore the inhibitory effect of
taraxasterol against PGE2 in the rheumatoid arthritis mouse model,
the PGE2 level was estimated in the present study. No significant
difference was observed in the PGE2 level between the control and
taraxasterol only groups (P>0.05; Fig. 7). The PGE2 level of the rheumatoid
arthritis group was higher compared with those of the control or
taraxasterol only groups (Fig. 7).
However, administration of taraxasterol significantly reduced the
PGE2 level in the rheumatoid arthritis mouse model compared with
that in the rheumatoid arthritis group (Fig. 7).
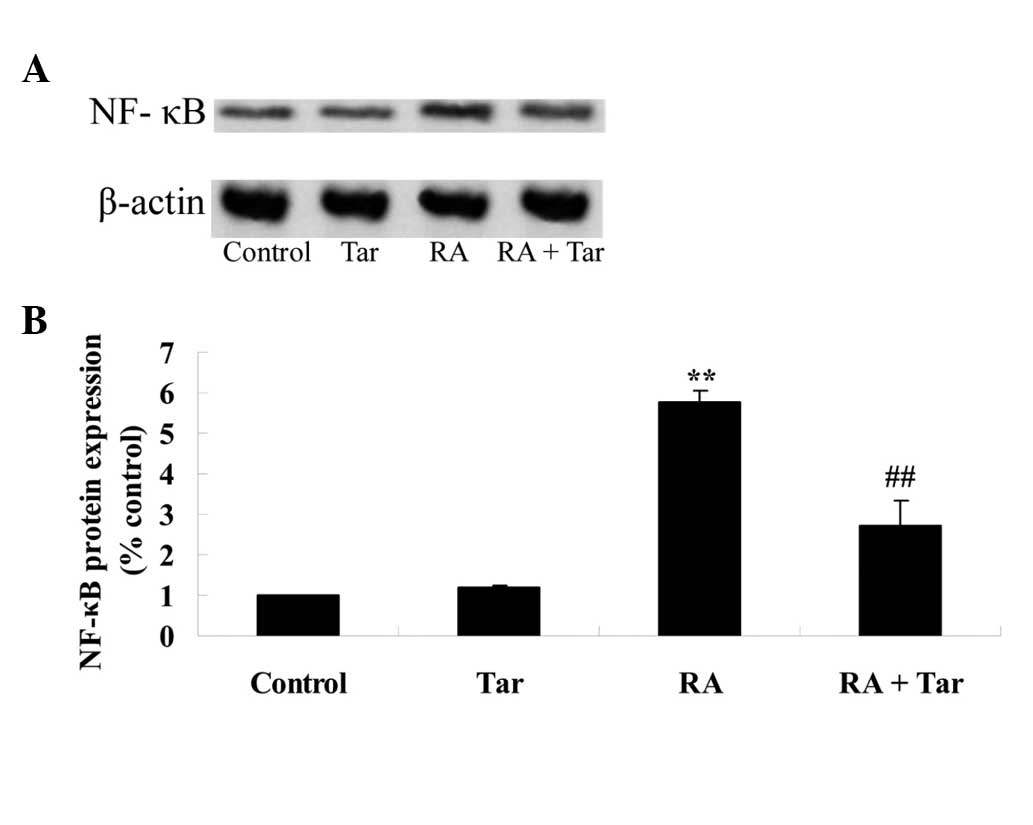
Protective effect of taraxasterol
against NF-κB in the rheumatoid arthritis mouse model
In order to explore the protective effect of
taraxasterol against NF-κB in the rheumatoid arthritis mouse model,
the protein expression level of NF-κB was measured using western
blot analysis. The results of the western blot assay revealed that
the protein expression level of NF-κB in the control group was very
similar to that in the taraxasterol only group (P>0.05; Fig. 8). As shown in Fig. 8, the protein expression of NF-κB was
significantly increased in the rheumatoid arthritis group compared
with the control and taraxasterol only groups (Fig. 8). Furthermore, treatment with
taraxasterol significantly decreased the protein expression level
of NF-κB in rheumatoid arthritis model mice compared with that in
the rheumatoid arthritis group (Fig.
8).
Discussion
Rheumatoid arthritis is an inflammatory autoimmune
disease that involves joints throughout the body. Approximately 1%
of the world's population suffers from this disease, and the
morbidity for females is 2-fold higher than that of males (8). Rheumatoid arthritis is distinguished by
chronic synovitis of joints and is known to have the following
pathological characteristics: Auto-antibody production, synovial
cell hyperplasia, inflammatory cell infiltration (such as
neutrophile granulocytes and macrophages), pannus production and
destruction of bones and cartilage, which finally lead to the
destruction and lack of function of a whole joint (9). According to official statistics,
rheumatoid arthritis is an important cause of productivity loss and
extremity disability in China, with a morbidity rate of 0.32–0.36%
(10). The pathogenesis of
rheumatoid arthritis has not been fully elucidated by previous
researchers; however, it may be associated with factors including
heredity, immunoregulation and the environment (11–13). The
present study demonstrated that treatment with taraxasterol
significantly increased pain thresholds and inhibited the clinical
arthritic scores of mice with rheumatoid arthritis. In a previous
study, Zhang et al reported that treatment with taraxasterol
protected against LPS-induced endotoxic shock in mice (14). In addition, it appears that
taraxasterol may be a useful agent for treating rheumatoid
arthritis.
Inflammatory cell infiltration in the joint synovium
is one of the key characteristics of rheumatoid arthritis. The
infiltrated cells include macrophages, T cells, B cells, dendritic
cells and neutrophile granulocytes (15). At the same time, hyperplastic
synovial cells invade the cartilago articularis. The aforementioned
cells secrete proinflammatory factors and matrix metalloproteinases
(MMPs), which can induce aggravation of inflammation and result in
the destruction of synovia, cartilage and bones (16). Macrophages around the joint synovium
are transformed from mononuclear cells in blood, the process of
which is guided by chemotactic factors. Once macrophages become
activated, they secrete a mass of inflammatory mediators (including
IL-1α, IL-1β, IL-6, IL-10, IL-15, IL-17, TNF-α and
granulocyte-macrophage colony-stimulating factor), proinflammatory
factors, growth factors, chemotactic factors and MMPs (17). All these factors can cause increased
inflammation and play a main role in injury of the synovium and
joints (18). In the present study,
it was identified that treatment with taraxasterol significantly
suppressed TNF-α, IL-1β, and IL-6 levels and the protein expression
of NF-κB in mice with rheumatoid arthritis. Zhang et al
reported that the effects of taraxasterol protect LPS-treated RAW
264.7 macrophages through suppression of the inflammatory response
(19). Piao et al
demonstrated that taraxasterol protects human osteoarthritic
chondrocytes by inhibition of IL-1β-induced inflammatory response
(6). Therefore, the
anti-inflammatory effect of taraxasterol may have potential in
preventing rheumatoid arthritis.
Activated inducible nitric oxide synthase (iNOS), an
important inflammatory mediator, can produce a large quantity of NO
that inhibits DNA synthesis, induces cell apoptosis and causes
cytotoxic effects by restraining the Kreb's cycle (20). PGE2 is another inflammatory mediator,
trace amounts of which can lead to intense inflammation; therefore,
PGE2 is important in physiological and pathological processes
(21). PGE2 is generated by
continuous enzymatic reactions as follows: Arachidonic acid is
released from the membrane phospholipid by catalysis of
phospholipase A2, prostaglandin H2 (PGH2) is generated from
arachidonic acid by catalysis with COX and finally PGE2 is created
from PGH2 by catalysis with prostaglandin E synthase (PGES)
(22). The expression of
membrane-bound PGES-1 and COX-2 and the production of PGE2 are
increased by inflammatory factors. COX-2 is an important enzyme in
the development of inflammation; its expression levels are low
under normal conditions, but are increased strongly in the presence
of LPS (23). According to a
previous study, the severity of rheumatoid arthritis can be reduced
in a dose-dependent manner by suppressing the expression of NOS,
COX-2 and PGE2 (24). In the present
study, NO, PGE2 and COX-2 protein expression levels were
significantly reduced by treatment with taraxasterol in a mouse
model of rheumatoid arthritis. Furthermore, Xiong et al
indicated that taraxasterol treatment weakens LPS-induced effects
on RAW 264.7 macrophages and reduces iNOS and COX-2 expression
(25). In addition, Piao et
al revealed that taraxasterol suppresses PGE2 and NO production
in human osteoarthritic chondrocytes (6). The effect of taraxasterol against iNOS,
COX-2 and PGE2 pathways may support its consideration as a
potential agent for the treatment of rheumatoid arthritis.
In conclusion, the present study revealed a
protective effect of taraxasterol against rheumatoid arthritis,
which is mediated by the modulation of inflammatory responses and
the iNOS, COX-2 and PGE2 pathways in mice. These results suggest
that taraxasterol may be a potential protective agent against
rheumatoid arthritis.
References
|
1
|
Vogel WV, van Riel PL and Oyen WJ:
FDG-PET/CT can visualise the extent of inflammation in rheumatoid
arthritis of the tarsus. Eur J Nucl Med Mol Imaging. 34:4392007.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bijlsma JW, Welsing PM, Woodworth TG,
Middelink LM, Pethö-Schramm A, Bernasconi C, Borm ME, Wortel CH,
ter Borg EJ, Jahangier ZN, et al: Early rheumatoid arthritis
treated with tocilizumab, methotrexate, or their combination
(U-Act-Early): A multicentre, randomised, double-blind,
double-dummy, strategy trial. Lancet. 388:343–355. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hao GF, Li YS, Liu JL and Wo MY:
Association of HLA-DQA1 (rs9272219) with susceptibility to
rheumatoid arthritis in a Han Chinese population. Int J Clin Exp
Pathol. 7:8155–8158. 2014.PubMed/NCBI
|
|
4
|
Majumdar KN, Banerjee A, Ratha J, Mandal
M, Sarkar RN and Saha KD: Leishmanial lipid suppresses tumor
necrosis factor alpha, interleukin-1beta, and nitric oxide
production by adherent synovial fluid mononuclear cells in
rheumatoid arthritis patients and induces apoptosis through the
mitochondrial-mediated pathway. Arthritis Rheum. 58:696–706. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Herz W and Mirrington RN: Identification
of pyrethrol with taraxasterol. J Pharm Sci. 55:1041966. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Piao T, Ma Z, Li X and Liu J: Taraxasterol
inhibits IL-1β-induced inflammatory response in human
osteoarthritic chondrocytes. Eur J Pharmacol. 756:38–42. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Luo L, Hu L, He L, Tang ZL, Song XG,
Dirckinck-Holmfeld L and Cai RL: Effect of moxibustion on
ultrastructure of synovial cells in rheumatoid arthritis rats. Zhen
Ci Yan Jiu. 36:105–109. 2011.(In Chinese). PubMed/NCBI
|
|
8
|
Ahmed S, Anuntiyo J, Malemud CJ and Haqqi
TM: Biological basis for the use of botanicals in osteoarthritis
and rheumatoid arthritis: A review. Evid Based Complement Alternat
Med. 2:301–308. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li J, Zhou T and Zhao F: Inhibitory effect
of sodium houttuyfonate on synovial proliferation in vitro in cells
from a patient with rheumatoid arthritis. Exp Ther Med.
7:1639–1642. 2014.PubMed/NCBI
|
|
10
|
Jiang P, Li H and Li X: Diabetes mellitus
risk factors in rheumatoid arthritis: A systematic review and
meta-analysis. Clin Exp Rheumatol. 33:115–121. 2015.PubMed/NCBI
|
|
11
|
Sparks JA and Costenbader KH: Genetics,
environment, and gene-environment interactions in the development
of systemic rheumatic diseases. Rheum Dis Clin North Am.
40:637–657. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wen H and Baker JF: Vitamin D,
immunoregulation, and rheumatoid arthritis. J Clin Rheumatol.
17:102–107. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Souliotis K, Papageorgiou M, Politi A,
Ioakeimidis D and Sidiropoulos P: Barriers to accessing biologic
treatment for rheumatoid arthritis in Greece: The unseen impact of
the fiscal crisis - The Health Outcomes Patient Environment (HOPE)
study. Rheumatol Int. 34:25–33. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang X, Xiong H, Li H and Cheng Y:
Protective effect of taraxasterol against LPS-induced endotoxic
shock by modulating inflammatory responses in mice. Immunopharmacol
Immunotoxicol. 36:11–16. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kikuchi J, Hashizume M, Kaneko Y,
Yoshimoto K, Nishina N and Takeuchi T: Peripheral blood CD4 (+)
CD25 (+) CD127 low regulatory T cells are significantly increased
by tocilizumab treatment in patients with rheumatoid arthritis:
Increase in regulatory T cells correlates with clinical response.
Arthritis Res Ther. 17:102015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tetlow LC and Woolley DE: The effects of 1
alpha, 25-dihydroxyvitamin D(3) on matrix metalloproteinase and
prostaglandin E(2) production by cells of the rheumatoid lesion.
Arthritis Res. 1:63–70. 1999. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hein GE, Köhler M, Oelzner P, Stein G and
Franke S: The advanced glycation end product pentosidine correlates
to IL-6 and other relevant inflammatory markers in rheumatoid
arthritis. Rheumatol Int. 26:137–141. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ma X and Xu S: TNF inhibitor therapy for
rheumatoid arthritis. Biomed Rep. 1:177–184. 2013.PubMed/NCBI
|
|
19
|
Zhang X, Xiong H and Liu L: Effects of
taraxasterol on inflammatory responses in
lipopolysaccharide-induced RAW 264.7 macrophages. J Ethnopharmacol.
141:206–211. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chenevier-Gobeaux C, Simonneau C,
Lemarechal H, Bonnefont-Rousselot D, Poiraudeau S, Rannou F, Anract
P and Borderie D: Hypoxia induces nitric oxide synthase in
rheumatoid synoviocytes: Consequences on NADPH oxidase regulation.
Free Radic Res. 46:628–636. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hishinuma T, Nakamura H, Sawai T, Uzuki M,
Itabash Y and Mizugaki M: Microdetermination of prostaglandin E2 in
joint fluid in rheumatoid arthritis patients using gas
chromatography/selected ion monitoring. Prostaglandins Other Lipid
Mediat. 58:179–186. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lee WS, Lim JH, Sung MS, Lee EG, Oh YJ and
Yoo WH: Ethyl acetate fraction from Angelica sinensis inhibits
IL-1β-induced rheumatoid synovial fibroblast proliferation and
COX-2, PGE2, and MMPs production. Biol Res. 47:412014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sung MS, Lee EG, Jeon HS, Chae HJ, Park
SJ, Lee YC and Yoo WH: Quercetin inhibits IL-1β-induced
proliferation and production of MMPs, COX-2, and PGE2 by rheumatoid
synovial fibroblast. Inflammation. 35:1585–1594. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lee EG, Lee SL, Chae HJ, Park SJ, Lee YC
and Yoo WH: Ethyl acetate fraction from Cudrania tricuspidata
inhibits IL-1β-induced rheumatoid synovial fibroblast proliferation
and MMPs, COX-2, and PGE2 production. Biol Res. 43:225–231. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Xiong H, Cheng Y and Zhang X and Zhang X:
Effects of taraxasterol on iNOS and COX-2 expression in LPS-induced
RAW 264.7 macrophages. J Ethnopharmacol. 155:753–757. 2014.
View Article : Google Scholar : PubMed/NCBI
|