Introduction
Oral squamous cell carcinoma (OSCC) is the most
frequent malignant tumor of oral cancer, accounting for at least
90% of all oral malignancies (1).
OSCC is a complex disease associated with numerous changes in
genetic and epigenetic interactions; however, the mechanisms remain
poorly understood (2). Alterations
in the histone acetylation/deacetylation balance, have been
suggested to underpin the altered gene expression patterns detected
in various pathological conditions, including cancer (3) and cardiac hypertrophy (4,5). Histone
deacetylases (HDACs) are enzymes that remove acetyl moieties from
ε-N-acetylated lysine residues in histones, resulting in chromatin
condensation and epigenetic repression of gene transcription. HDACs
also catalyse deacetylation of various non-histone proteins, such
as the tumor suppressor p53, resulting in the inhibition of its
proapoptotic transcriptional activity (6,7). In line
with their profound antiapoptotic activity, HDACs are upregulated
in various types of cancer and thus are efficiently inhibited by
chelating agents. Notably, these HDAC inhibitors (HDACi) have been
reported to be successful anticancer agents. Due to their antitumor
potential, HDACi have a long history of use in antitumor therapy
(8). Previous studies have reported
additive effects when HDACi were used in vitro to treat
prostate cancer cells (9,10). Valproic acid (VPA) is a HDACi with a
safety profile that permits its long-term use in patients (11). However few studies have assessed its
use in oral cancer. Therefore, knowledge of the effects of VPA on
OSCC and its underlying mechanisms may improve understanding of
OSCC processes, and provide future therapeutic targets for OSCC
patients.
Post-translational modification of proteins can be
modulated through acetylation, controlled by HDACs and augmented by
small ubiquitin-related modifier (SUMO)-mediated gene regulation
(12). Post-translational
modification of proteins via conjugation to SUMO-SUMOylation, has
been shown to influence protein functions associated with numerous
cellular processes, including transcription, signal transduction,
subcellular localization and gene expression (13).
SUMOylation is a reversible modification of a
dynamic process and SUMO-specific proteases (SENPs) are able to
remove SUMO from modified proteins (14). Few studies have examined SUMO
modification in oral cancer. Katayama et al (15) reported that expression levels of
SUMO1 were significantly increased in OSCC tissues and cell lines
compared with normal oral mucosa, and SUMO1 expression was also
demonstrated to be correlated with a poor patient prognosis. Ding
et al (16) reported that
SENP5 was increased and associated with tumor differentiation in 48
cases of OSCC, and Sun et al (17) found that SENP3 was overexpressed and
positively correlated with OSCC tumor differentiation.
In the present study, the role of VPA as a HDACi on
the oral tongue cancer cell line, CAL27, was investigated, and its
interactions with SENPs were characterized. Furthermore, the
therapeutic potential of VPA in treating OSCC was examined using a
xenograft model of the disease.
Materials and methods
Cell culture
Tongue cancer cell line, CAL27, was purchased from
Shanghai Key Laboratory of Stomatology, Ninth People's Hospital,
Shanghai Jiao Tong University School of Medicine, (Shanghai, China)
and cultured in Dulbecco's Modified Eagle Medium (DMEM)
supplemented with 10% fetal bovine serum (FBS; both Gibco; Thermo
Fisher Scientific, Inc., Waltham, MA, USA), 100 IU/ml penicillin
and 100 mg/ml streptomycin (both Invitrogen; Thermo Fisher
Scientific, Inc.) at 37°C in an atmosphere containing 5%
CO2. Cells were harvested at 80–90% confluency by
trypsinization with 0.25 mg/ml trypsin/EDTA (Gibco; Thermo Fisher
Scientific, Inc.), nad subsequently suspended in DMEM prior to use.
Cells were incubated in DMEM for 24–48 h before treatment with VPA
dissolved in DMSO (Gibco; Thermo Fisher Scientific, Inc.). Control
cells were treated with DMSO only.
Cell growth assay
Viability of CAL27 cells treated with VPA was
determined by standard MTT assays. Cells were seeded in 96-well
plates at a density of 1×103 cells per well and grown
overnight in DMEM supplemented with 10% FBS, 1% glutamine and 1%
penicillin-streptomycin at 37°C and 5% CO2.
FBS-supplemented medium was removed and cells were cultured in
serum-free DMEM for 2 h. VPA (99-66-1; Sigma-Aldrich; Merck
Millipore, Darmstadt, Germany) was dissolved in DMSO and used at
final concentrations of 0.5, 1.0, 1.5, 2.0, 2.5, and 3.0 mmol/l,
respectively. Following exposure to VPA for 24, 48, 72, 96, and 120
h, respectively, supernatants were removed and 20 µl MTT solution
(5 µg/ml; Sigma-Aldrich; Merck Millipore) was added to each well
for an additional 4 h at 37°C. Supernatants were subsequently
discarded and 100 µl DMSO was added to each well. Absorbance was
read at 540 nm using a microplate reader (Model 550; Bio-Rad
Laboratories, Inc., Hercules, CA, USA). Proliferation rates were
calculated by comparing the cell density of the VPA-treated cells
with that of DMSO-treated cells.
Flow cytometric analysis of apoptotic
cells
Apoptosis was measured by flow cytometry using a
Annexin V-fluorescein isothiocyanate/PI propidium iodide (PI)
apoptosis detection kit (Nanjing Keygen Biotech, Nanjing, China),
according to the manufacturer's instructions. CAL27 cells
(1×106) were seeded into 6-well plates and treated with
VPA at final concentrations of 0.5, 1.0, 1.5, 2.0 and 3.0 mmol/l,
respectively. Following treatment for 48 h, the cells were
trypsinized, washed with PBS, and resuspended in 500 µl binding
buffer containing Annexin V-Fluos labeling reagent and PI at
2×104 cells/ml. Cells were then incubated in the dark
for 15 min at room temperature and analyzed using a FACS Aria flow
cytometer (BD Biosciences, Franklin, NJ, USA). For each sample,
20,000 cells were analyzed. Apoptotic rates were calculated using
FlowJo 7.6.3 software (Tree Star, Inc., Ashland, OR, USA).
Flow cytometric analysis of the cell
cycle
Cells (1×106) were seeded into 6-well
plates and treated with VPA at final concentrations of 0.5, 1.0,
1.5, 2.0 and 3.0 mmol/l for 24 h, respectively. Cells were
harvested from each well, washed in cold phosphate-buffered saline
(PBS), and fixed overnight with cold 70% ethanol. Cells were then
incubated with PBS buffer containing 50 µg/ml PI and 50 µg/ml RNase
(both Sigma-Aldrich; Merck Millipore) for 30 min at room
temperature. Cellular PI fluorescence intensity was determined
using a FACS Aria flow cytometer. Data were analyzed using FlowJo
software to model various cell populations. Apoptosis ratios in the
VPA-treated cells were compared with those of DMSO-treated
cells.
RNA isolation and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
analysis
Total RNA was isolated from CAL27 cells treated with
VPA at final concentrations of 0.5, 1.0, 1.5, 2.0 and 3.0 mmol/l
for 12, 24, and 48 h, respectively, using TRIzol reagent
(Invitrogen; Thermo Fisher Scientific, Inc.) and DNase treatment to
remove genomic DNA. First strand cDNA was synthesized with 1 µg
total RNA using a cDNA synthesis kit (Takara Bio, Inc., Otsu,
Japan) and an RNase-free DNase kit (Qiagen, Inc., Valencia, CA,
USA). Total reaction volume was 20 µl consisting of 4 µl 5X RT
Buffer, 2 µl dNTP mixture, 1 µl RNase inhibitor, 1 µl Oligo
(dT)20 (Thermo Fisher Scientific, Inc.), 1 µl ReverTra
Ace (Toyoba Co., Ltd., Osaka, Japan), 1 µg total RNA and RNase-free
H2O. cDNA was stored at −80°C for the following
experiments. SYBR Green (Qiagen, Inc.) was used to detect PCR
products using the Bio-Rad Mini Opticon real-time PCR system in a
total volume of 20 µl, consisting of 2 µl cDNA, 10 µl
SYBR® Green Real-Time PCR MasterMix, 0.8 µl forward
primer (10 µmol/l), 0.8 µl reverse primer (10 µmol/l), and 6.4 µl
RNase-free H2O. Reaction conditions were 95°C for 1 min,
followed by 40 cycles of 95°C for 5 sec, 60°C for 30 sec, and 72°C
for 45 sec, with final extension at 72°C for 10 min. GAPDH
transcripts were also analyzed for quality assurance.
Dissociation curves were calculated and gene
expression levels were analyzed using the standard curve method. To
normalize samples, GAPDH was used. All experiments were repeated a
minimum of three times. qPCR assays were performed in 96-well
optical plates, Cq values were normalized to GAPDH Cq values, and
relative expression was calculated using the 2−ΔΔCq
method (18). Primer pairs were
synthesized by Sangon Biotech Co., Ltd., (Shanghai, China) and are
presented in Table I.
 | Table I.Primer sequences. |
Table I.
Primer sequences.
| Primer | Direction | Sequence |
|---|
| GAPDH | Forward |
5′-CTGCACCACCAACTGCTTAG-3′ |
|
| Reverse |
5′-GTCTTCTGGGTGGCAGTGAT-3′ |
| SENP1 | Forward |
5′-ACTGATAGTGAAGATGAATTTCCTGA-3′ |
|
| Reverse |
5′-CATCCTGATTCCCATTACGAA-3′ |
| SENP3 | Forward |
5′-ACTGGCTCAATGACCAGGTGATGAACATG-3′ |
|
| Reverse |
5′-CCAGGTGGATGGGGATTAGCAGTAG-3′ |
| SENP5 | Forward |
5′-TGCTAGATCACCTCGTCTTCA-3′ |
|
| Reverse |
5′-AGTGCTTAGTGGTTTTCATGATA-3′ |
| SUMO1 | Forward |
5′-GGATCCATGGCCGACGAAAAGCCCAAG-3′ |
|
| Reverse |
5′-CCCGGGTCAGTAGACACCTCCCGTCTG-3′ |
| SUMO2 | Forward |
5′-GACGAGAAACCCAAGGA-3′ |
|
| Reverse |
5′-CTGCCGTTCACAATAGG-3′ |
| SUMO3 | Forward |
5′-GGATCCATGTCCGAGGAGAAGCCCAAG-3′ |
|
| Reverse |
5′-CCCGGGCTAGAAACTGTGCCCTGCCAG-3′ |
| SAE1 | Forward |
5′-AGACACAGATGAATTGGCCAA-3′ |
|
| Reverse |
5′-TCTGTGTCTACTTAACCGGAA-3′ |
| SAE2 | Forward |
5′-GAGGTGACTGTGCGGCTGAATG-3′ |
|
| Reverse |
5′-TCTTGAGCTGTGGAGGTGGAGG-3′ |
| UBC9 | Forward |
5′-CAGCCATTACCATCAAACAGA-3′ |
|
| Reverse |
5′-GTCGGTAATGGTAGTTTGTCT-3′ |
Animal studies and xenograft tumor
formation assays
Four-week-old female BALB/c nu/nu mice (n=5 per
group; weight, 14.5±1.87 g) were purchased from the Laboratory
Animal Center of the Chinese Academy of Science (Shanghai, China)
and maintained in a pathogen-free animal facility. Animals were
placed under controlled environmental conditions in iron cages (n=5
in each cage) at 23±1°C (60% humidity) and a 12-h dark/light cycle.
Animals were subjected to acclimatization for at least one week
before the experiment. They were provided with commercially
available laboratory rodent diet and water was provided ad
libitum throughout the period of the study. All experimental
procedures involving animals were approved by the Animal Care and
Use Committee of Zhongshan Hospital of Fudan University. CAL27
cells were collected and resuspended in DMEM, prior to subcutaneous
injection into the right flank of BALB/c nude mice at a density of
5×106 cells in 200 µl DMEM. When the tumors were
palpable, the mice were randomly divided into control or
VPA-treated groups. Control group mice received DMSO only and the
VPA-treated group received 300 mg/kg VPA via intraperitoneal
injection (19–21) administered daily for 28 days. Tumor
volumes were regularly measured using Vernier calipers, twice per
week, and calculated according to the formula: length × width ×
height × 0.52 (mm3). Following treatment, the mice were
immediately euthanized via cervical dislocation and tumors were
excised. Tumors were washed with PBS and fixed with 4%
paraformaldehyde for immunohistochemical examination and terminal
deoxynucleotidyl transferase dUTP nick-end labeling (TUNEL) assay
analysis.
Immunohistochemical staining
Tumor specimens from the xenograft models were cut
into 5 µm slices, fixed in 10% neutral buffered formalin, and
embedded in paraffin. Slides were preincubated in 5% goat serum in
PBS and immunostained with anti-Ki67 (ab15580) and
anti-proliferating cell nuclear antigen (PCNA; ab29) primary
antibodies (both Abcam, Cambridge, UK) diluted 1:100 at 4°C
overnight. Slides were treated with hematoxylin for 30 sec for
visualization under a light microscope.
TUNEL assay
Half of each xenograft tumor was fixed in 4%
paraformaldehyde and embedded in paraffin. Slides were washed after
deparaffinization and the sections were permeabilized with 0.1%
Triton X-100 for 5 min on ice. Sections were subsequently incubated
with proteinase K (Sigma-Aldrich; Merck Millipore) for 10 min at
room temperature. Following this, TUNEL staining was performed
using an in situ apoptosis detection kit (KeyGen Biotech
Co., Ltd., Nanjing, China), according to the manufacturer's
instructions. Sections were counterstained with hematoxylin, and
TUNEL-positive cells were counted at ×100 magnification in 10
randomly chosen fields using a light microscope. Results were
expressed as the mean percentage of apoptotic cells.
Statistical analysis
SPSS 16.0 software (SPSS Inc., Chicago, IL, USA) was
used for statistical analyses. Comparisons among the CAL27 cell
cycle data sets following VPA treatment were performed using
nonparametric statistical tests. Comparisons among the CAL27 cell
apoptosis data were conducted using one-way analysis of variance,
and post-hoc comparisons between the groups were analyzed by the
least significant test. Data were expressed as the mean ± standard
deviation and P<0.05 was considered to indicate a statistically
significant difference.
Results
VPA decreases the growth of CAL27
cells in vitro
The anti-proliferative effect of VPA was examined by
incubating CAL27 cells with increasing concentrations of VPA. Cell
viability, as assessed by MTT assay, demonstrated appropriate
concentration- and time-dependent inhibitory effects by VPA as
displayed in Fig. 1. The
concentrations and incubation times of VPA required for significant
inhibition of cell viability (P<0.05) were >0.5 mmol/l and 24
h, respectively. A pronounced dose-dependent decrease in viability
was observed at VPA concentrations of 0.5–3.0 mm/l at each time
point from 24–120 h.
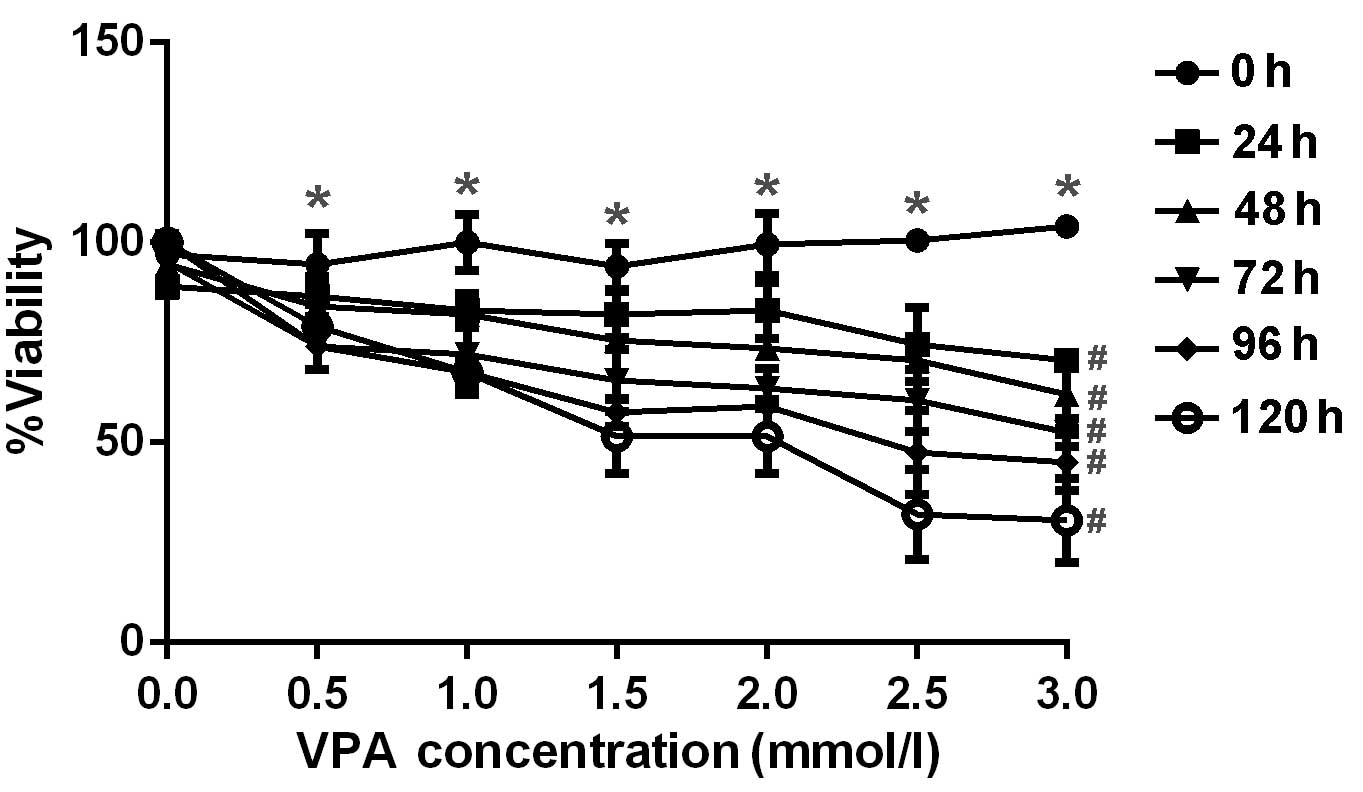 | Figure 1.Inhibitory effect of various doses of
VPA on CAL27 cell proliferation. CAL27 cells were treated with 0.5,
1.0, 1.5, 2.0, 2.5 and 3.0 mm/l VPA for 24, 48, 72, 96 and 120 h,
respectively, in vitro. Cell viability was determined using
MTT assay and analyzed as the percentage of the absorbance value
compared with control. *P<0.05 vs. 0.0 mmol/l;
#P<0.05 vs. 0 h. Error bars represent the standard
error of the mean. VPA, valproic acid. |
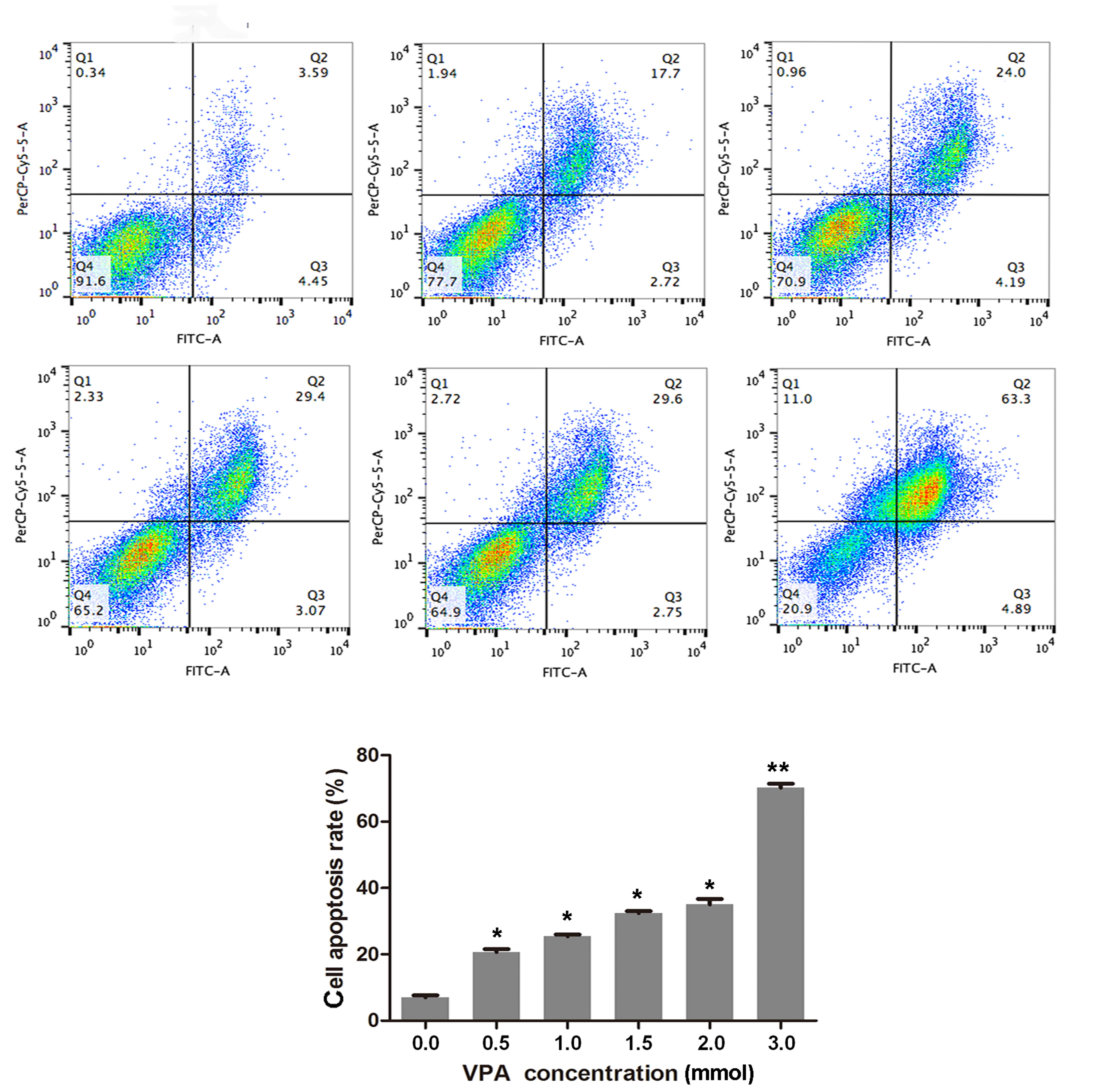
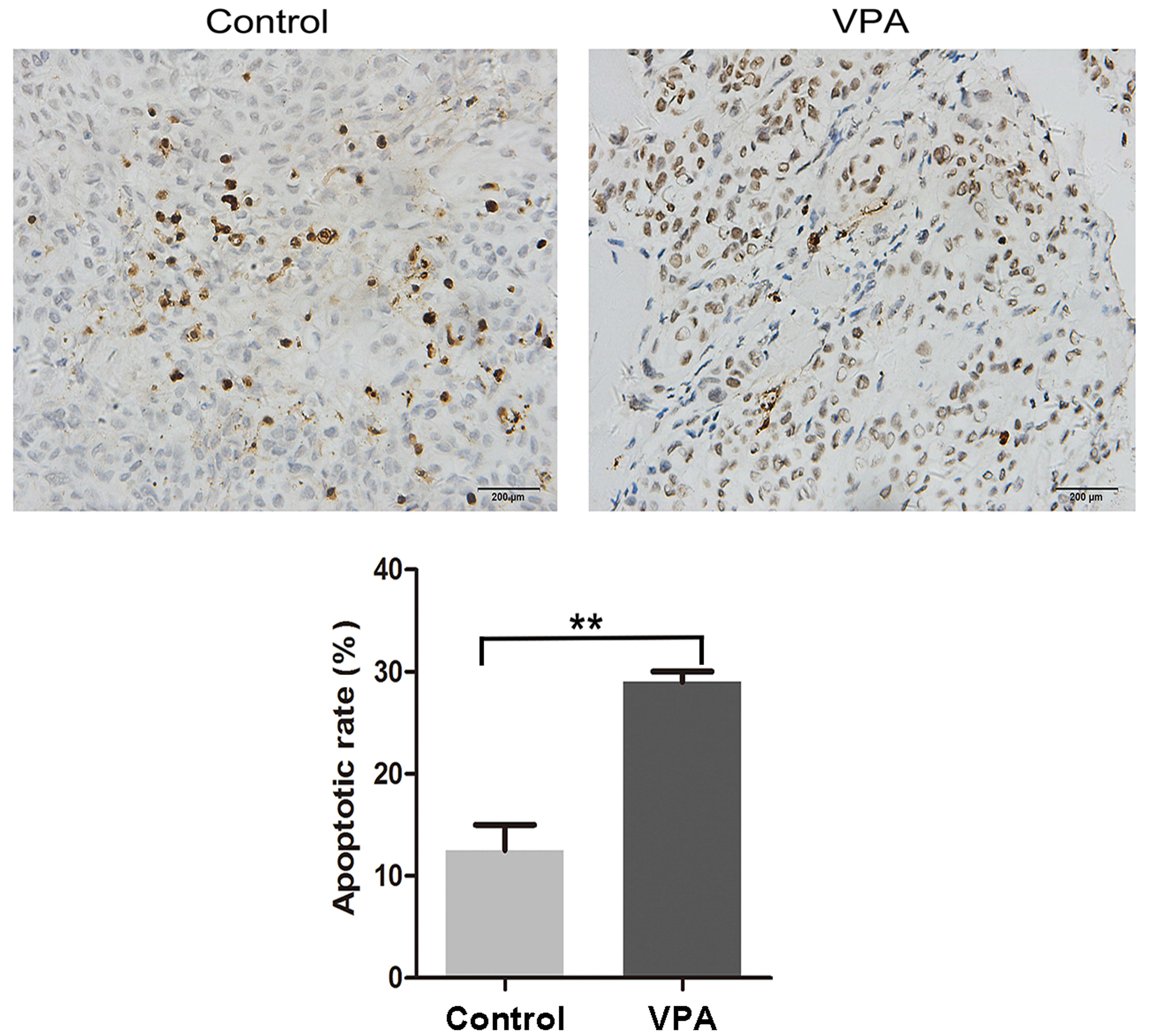
VPA induces apoptosis
The findings of the MTT assay showed that VPA
reduced the viability of CAL27 cells, which suggested a
relationship between cell growth and the cell cycle and/or cell
apoptosis (Fig. 1). Consequently,
the effects of VPA on CAL27 apoptosis were analyzed using Annexin V
and PI staining assays (Fig. 2) with
the same dosage range as used in the previous experiments. Cells
that were positive for both Annexin V and PI positive were deemed
to be in the later stages of apoptosis, while Annexin V-positive
cells were in the earlier stages of apoptosis. The present findings
demonstrated that CAL27 cells underwent apoptosis after 48 h of
incubation with VPA in a significant and dose-dependent manner, as
compared with the control (P<0.05).
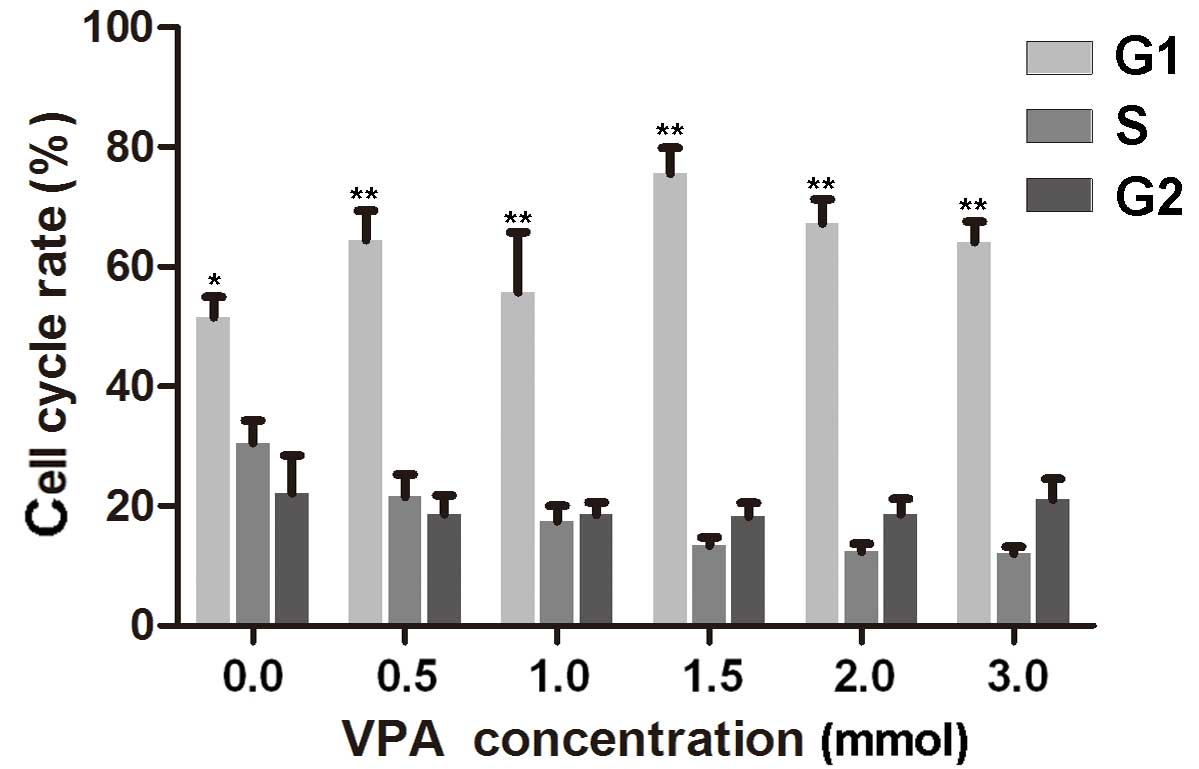
VPA-induces cell cycle arrest
To investigate the effect of VPA on the cell cycle
of CAL27 cells, flow cytometry experiments were performed. The
results indicated that cells treated with 0.5, 1.0, 1.5, 2.0 and
3.0 mmol/l VPA, respectively, were in the S phase for less time
than the control cells. G1 cell populations increased
significantly at these concentrations of VPA, compared with the
control cells (P<0.05; Fig. 3;
Table II).
 | Table II.Alterations in the cell cycle
progression of CAL27 cells after treatment with VPA for 24 h. |
Table II.
Alterations in the cell cycle
progression of CAL27 cells after treatment with VPA for 24 h.
| Phase | Control group | 0.5 mm/l VPA | 1 mm/l VPA | 1.5 mm/l VPA | 2 mm/l VPA | 3 mm/l VPA | P-value |
|---|
| G0/G1 | 51.53±5.99 | 64.43±8.89 | 62.24±6.76 | 75.63±7.38 | 67.66±7.07 | 64.31±6.26 | <0.05 |
| S | 30.66±6.56 | 21.5±6.92 | 17.42±4.70 | 13.3±2.57 | 12.41±2.34 | 12.25±2.56 | <0.05 |
| G2/M | 22.43±11.00 | 18.85±5.76 | 18.71±3.27 | 21.65±7,14 | 18.86±4.46 | 21.18±5.99 | >0.05 |
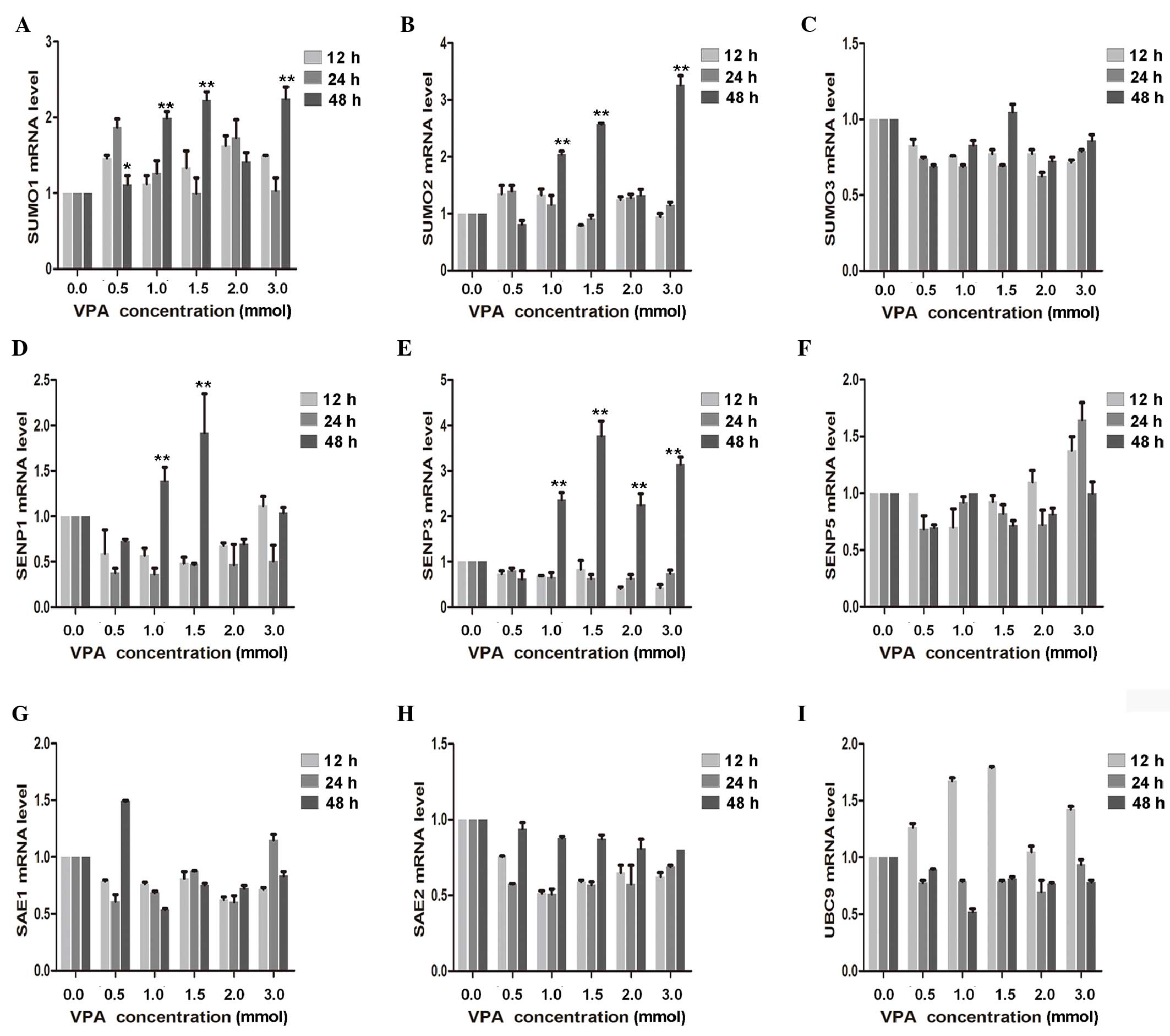
VPA increases the mRNA expression
levels of various SUMOylation genes
To determine the effect of VPA on CAL27 inhibition,
RT-qPCR was used to demonstrate that SUMO1 and SUMO2 mRNA
expression significantly increased with the increasing VPA dosage
(P<0.01). A significant increase in SENP3 was also detected
after 48-h treatment with 1.0, 1.5, 2.0 and 3.0 mmol/l VPA
(P<0.01). In contrast, expression of SENP1 initially decreased,
declining to a minimum at 24 h, and subsequently exhibited a
significant increase at 24 h with 1.0 and 1.5 mmol/l VPA
(P<0.01). mRNA expression levels of SAE1, SAE2, SUMO3, SENP5 and
UBC9 were also measured; however, but no significant dose- and/or
time-dependent effects were observed (Fig. 4).
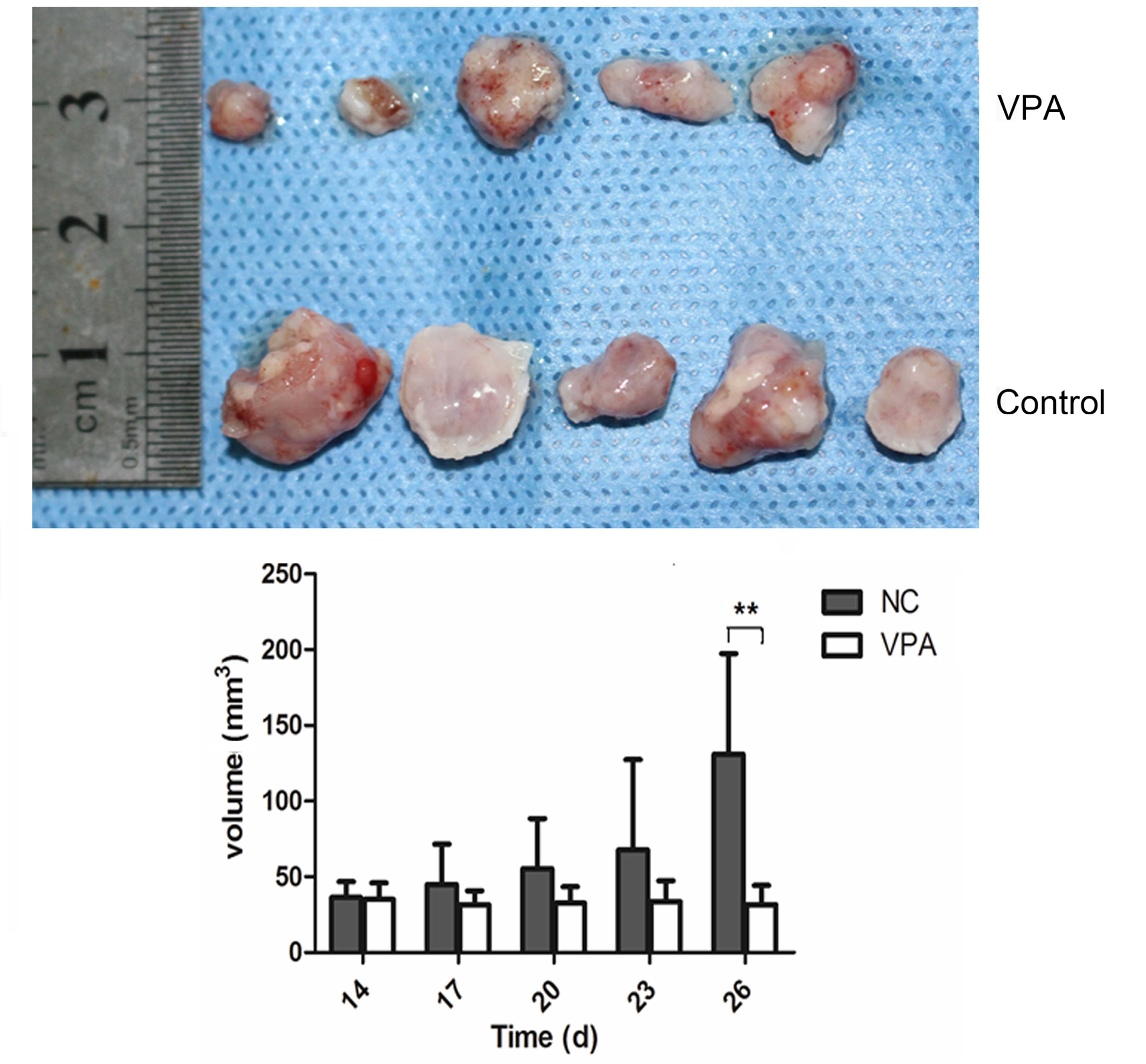
VPA reduces the speed of tumor growth
in vivo
To further evaluate the therapeutic potential of VPA
in vivo, a xenograft model was established in nude mice via
subcutaneous injection of CAL27 cells. The volumes of established
tumors were examined every four days, and the time course of
changes in xenografted tumor volumes are shown in Fig. 5. Following treatment with VPA, tumors
generated from CAL27 cells grew more slowly than control tumors
treated with the vehicle only.
VPA decreases proliferation potential
and increases apoptosis in xenograft tumors in vivo
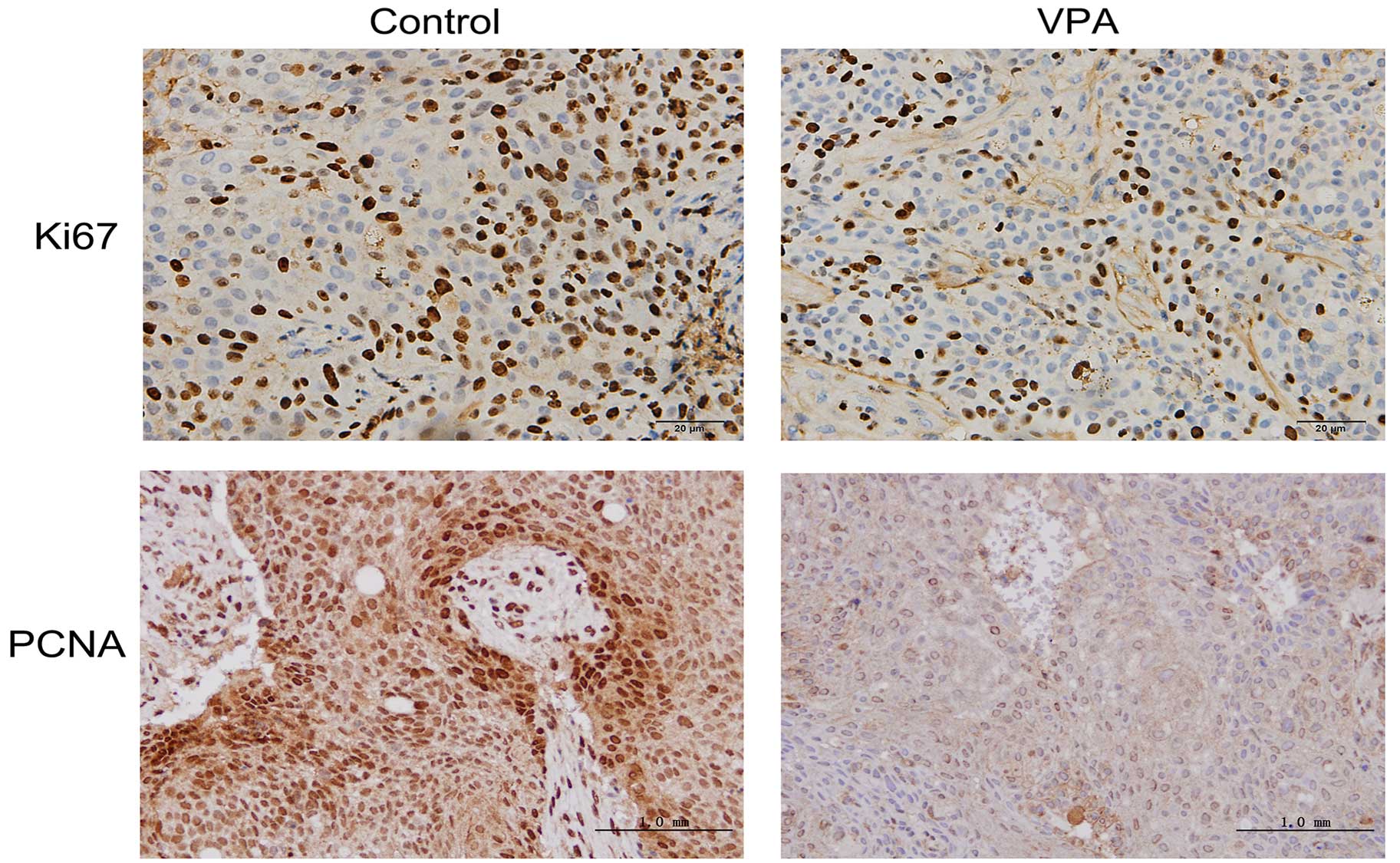
Ki67 and PCNA are considered to be pivotal markers
of tumor proliferation (22,23). Xenograft tumors were fixed with
formalin and embedded in paraffin, followed by immunohistochemistry
to visualize the expression levels of both markers. Representative
images of the immunohistochemical findings for Ki67 and PCNA are
shown in Fig. 6, indicating the
decreased expression of both proteins in the tumors of VPA-treated
mice. The effect of VPA on apoptosis in xenograft tumors
originating from CAL27 cells was also characterized by a TUNEL
assay. Representative results are presented in Fig. 7, demonstrating increased DNA
fragmentation in the sections from VPA-treated mice as a result of
activated apoptotic signaling cascades.
Discussion
The function of VPA in various malignant tumors has
been widely studied in recent years. As such, it has been
demonstrated that treatment with VPA induces a significant
reduction in tumor proliferation through the induction of S phase
arrest (24), and this anticancer
effect may have been acquired through the alteration of cell cycle
proteins, including cyclins D1 and D3 and cyclin-dependent kinase 4
(25). Previous studies have also
indicated that VPA markedly increased the enrichment of reactive
oxygen species in leukemia cells, which led to the upregulation of
growth inhibitory pathway (26,27).
Ki67, as a functional component of proliferating cells during
mitosis, was reduced by VPA in murine neural stem cells, resulting
in growth inhibitory effects (28).
In addition, VPA also induced a significant reduction in tumor
proliferation through the induction of apoptosis (29). Previous investigation in leukemia
cells demonstrated that inhibition of HDAC activity by VPA led to
the induction of apoptosis via the disturbance of normal epigenetic
processes (29). Furthermore,
studies have confirmed that VPA treatment resulted in the induction
of differentiation in murine neural stem cells and some malignant
cells via HDAC inhibition (30–32).
Thus, the anticancer effects of VPA have been well-documented.
Oral squamous cell carcinoma is the most frequently
occurring malignant oral tumor in the oral cancer group (2); however, to the best of our knowledge,
the effectiveness of VPA on OSCC has not been widely investigated.
In the present study, the effects of VPA on OSCC were examined
in vivo and in vitro. Treatment with VPA inhibited
the growth of CAL27 cells and xenografted OSCC. This effect may be
attributed to VPA-induced increases in G1 phase arrest
rates as well as elevated rates of apoptosis in carcinoma cells.
The inhibitory effects of VPA on OSCC were manifested by its
effects on the viability, differentiation, and apoptosis of cancer
cells, which is consistent with previous research (33).
Post-translational modification has emerged as an
important method of regulating the extent of cellular signaling by
pathway components (34). For
example, the specific acetylation activity pattern of SUMO was
shown to alter protein phosphorylation patterns, thus affecting the
activity of certain regulatory proteins (35). It has been hypothesized that SENPs
modulate the cell cycle and tumorigenesis, utilizing divergent
mechanisms (36–38). In the present study, SUMO
modification of mRNA expression levels in CAL27 cells differed
according to the VPA concentration. The mRNA expression level sof
SUMO ligases, SAE1 and SAE2, exhibited no significant alterations.
Moreover, the mRNA expression levels of SUMO1 and SUMO2 in CAL27
cells exposed to different concentrations of VPA did not initially
change after 24 h exposure, but rose rapidly after 48 h with 1.0
and 1.5 mmol/l VPA. Treatment with VPA at 1.0, 1.5, and 3.0 mmol/l
produced fold changes of 1.8, 2.2, 2.1 and 2.1, 2.6, 3.0-fold in
SUMO1 and SUMO2 mRNA expression levels, respectively,. At 1.0, 1.5
and 3.0 mmol/l VPA, the changes in the mRNA expression levels of
SUMO1 and SUMO2 were statistically significant. Regarding SENP mRNA
expression, the findings of the present study showed that SENP1
mRNA expression levels transiently declined 12 h after VPA exposure
regardless of the concentration used. In contrast, mRNA expression
levels of SENP3 were significantly increased following 48 h
exposure to 1.0, 1.5, 2.0 and 3.0 mmol/l VPA. SENP5 mRNA expression
levels were not demonstrated to be statistically correlated with
VPA concentration or exposure time.
The alterations in SUMO and SENP expression levels
observed in the present study suggest that SUMO modifications are
not involved in the early differentiation of OSCC induced by VPA.
One possible reason is that SUMO modification and conversion occur
on different proteins. While some proteins may be selectively
SUMOylated, SUMO modifications do not increase overall and SENP1
and SENP3 may have important roles in regulating the timing of SUMO
modifications. However, position changes of SUMO proteins and
SUMO-related proteins SENP1, SENP3, SENP5 were not examined in the
present study. Therefore, the cellular location and volume changes
of SUMO-related proteins should be examined in future studies in
order to fully elucidate the specific mechanisms.
In conclusion, VPA, which is a HDACi, was
demonstrated to be associated with cell cycle inhibition and
apoptosis induction in OSCC. Targeted therapy with VPA may decrease
cell viability and induce significant antitumor effects by
promoting the differentiation of OSCC. These findings indicate that
VPA may be useful for the clinical treatment of OSCC, and that
alterations in SUMO-related pathway may mediate the anti-cancer
effect of VPA.
References
|
1
|
Carvalho AL, Nishimoto IN, Califano JA and
Kowalski LP: Trends in incidence and prognosis for head and neck
cancer in the United States: A site-specific analysis of the SEER
database. Int J Cancer. 114:806–816. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chu W, Song X, Yang X, Ma L, Zhu J, He M,
Wang Z and Wu Y: Neuropilin-1 promotes epithelial-to-mesenchymal
transition by stimulating nuclear factor-kappa B and is associated
with poor prognosis in human oral squamous cell carcinoma. PLoS
One. 9:e1019312014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lafon-Hughes L, Di Tomaso MV, Méndez-Acuña
L and Martinez-López W: Chromatin-remodelling mechanisms in cancer.
Mutat Res. 658:191–214. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Backs J and Olson EN: Control of cardiac
growth by histone acetylation/deacetylation. Circ Res. 98:15–24.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ito K, Ito M, Elliott WM, Cosio B,
Caramori G, Kon OM, Barczyk A, Hayashi S, Adcock IM, Hogg JC and
Barnes PJ: Decreased histone deacetylase activity in chronic
obstructive pulmonary disease. N Engl J Med. 352:1967–1976. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Brandl A, Wagner T, Uhlig KM, Knauer SK,
Stauber RH, Melchior F, Schneider G, Heinzel T and Krämer OH:
Dynamically regulated sumoylation of HDAC2 controls p53
deacetylation and restricts apoptosis following genotoxic stress. J
Mol Cell Biol. 4:284–293. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Xu WS, Parmigiani RB and Marks PA: Histone
deacetylase inhibitors: Molecular mechanisms of action. Oncogene.
26:5541–5552. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li Y, Liu T, Ivan C, Huang J, Shen DY,
Kavanagh JJ, Bast RC Jr, Fu S, Hu W and Sood AK: Enhanced cytotoxic
effects of combined valproic acid and the aurora kinase inhibitor
VE465 on gynecologic cancer cells. Front Oncol. 3:582013.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wedel S, Hudak L, Seibel JM, Makarević J,
Juengel E, Tsaur I, Wiesner C, Haferkamp A and Blaheta RA: Impact
of combined HDAC and mTOR inhibition on adhesion, migration and
invasion of prostate cancer cells. Clin Exp Metastasis. 28:479–491.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Dong LH, Cheng S, Zheng Z, Wang L, Shen Y,
Shen ZX, Chen SJ and Zhao WL: Histone deacetylase inhibitor
potentiated the ability of MTOR inhibitor to induce autophagic cell
death in Burkitt leukemia/lymphoma. J Hematol Oncol. 6:532013.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mazurkiewicz-Beldzińska M, Szmuda M and
Matheisel A: Long-term efficacy of valproate versus lamotrigine in
treatment of idiopathic generalized epilepsies in children and
adolescents. Seizure. 19:195–197. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ullmann R, Chien CD, Avantaggiati ML and
Muller S: An acetylation switch regulates SUMO-dependent protein
interaction networks. Mol Cell. 46:759–770. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wilkinson KA and Henley JM: Mechanisms,
regulation and consequences of protein SUMOylation. Biochem J.
428:133–145. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Drag M and Salvesen GS: DeSUMOylating
enzymes-SENPs. IUBMB Life. 60:734–742. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Katayama A, Ogino T, Bandoh N, Takahara M,
Kishibe K, Nonaka S and Harabuchi Y: Overexpression of small
ubiquitin-related modifier-1 and sumoylated Mdm2 in oral squamous
cell carcinoma: Possible involvement in tumor proliferation and
prognosis. Int J Oncol. 31:517–524. 2007.PubMed/NCBI
|
|
16
|
Ding X, Sun J, Wang L, Li G, Shen Y, Zhou
X and Chen W: Overexpression of SENP5 in oral squamous cell
carcinoma and its association with differentiation. Oncol Rep.
20:1041–1045. 2008.PubMed/NCBI
|
|
17
|
Sun Z, Hu S, Luo Q, Ye D, Hu D and Chen F:
Overexpression of SENP3 in oral squamous cell carcinoma and its
association with differentiation. Oncol Rep. 29:1701–1706.
2013.PubMed/NCBI
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2-ΔΔCt method. Methods. 25:402–408. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Xing B, Liang XP, Liu P, Zhao Y, Chu Z and
Dang YH: Valproate inhibits methamphetamine induced hyperactivity
via glycogen synthase kinase 3β signaling in the nucleus accumbens
core. PLoS One. 10:e1280682015. View Article : Google Scholar
|
|
20
|
Xing B, Zhao Y, Zhang H, Dang Y, Chen T,
Huang J and Luo Q: Microinjection of valproic acid into the
ventrolateral orbital cortex exerts an antidepressant-like effect
in the rat forced swim test. Brain Res Bull. 85:153–157. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhao Y, Xing B, Dang YH, Qu CL, Zhu F and
Yan CX: Microinjection of valproic acid into the ventrolateral
orbital cortex enhances stress-related memory formation. PLoS One.
8:e526982013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Garcia H, Fleyshman D, Kolesnikova K,
Safina A, Commane M, Paszkiewicz G, Omelian A, Morrison C and
Gurova K: Expression of FACT in mammalian tissues suggests its role
in maintaining of undifferentiated state of cells. Oncotarget.
2:783–796. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tripi TR, Bonaccorso A, Rapisarda E and
Bartoloni G: Proliferative activity in periapical lesions. Aust
Endod J. 29:31–33. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Praefcke GJ, Hofmann K and Dohmen RJ: SUMO
playing tag with ubiquitin. Trends Biochem Sci. 37:23–31. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Juengel E, Makarević J, Tsaur I, Bartsch
G, Nelson K, Haferkamp A and Blaheta RA: Resistance after chronic
application of the HDAC-inhibitor valproic acid is associated with
elevated Akt activation in renal cell carcinoma in vivo. PLoS One.
8:e531002013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Miller CP, Ban K, Dujka ME, McConkey DJ,
Munsell M, Palladino M and Chandra J: NPI-0052, a novel proteasome
inhibitor, induces caspase-8 and ROS-dependent apoptosis alone and
in combination with HDAC inhibitors in leukemia cells. Blood.
110:267–277. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Boku S, Nakagawa S, Masuda T, Nishikawa H,
Kato A, Takamura N, Omiya Y, Kitaichi Y, Inoue T and Kusumi I:
Valproate recovers the inhibitory effect of dexamethasone on the
proliferation of the adult dentate gyrus-derived neural precursor
cells via GSK-3beta and beta-catenin pathway. Eur J Pharmacol.
723:425–430. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Brunn J, Wiroth V, Kowalski M, Runge U and
Sabolek M: Valproic acid in normal therapeutic concentration has no
neuroprotective or differentiation influencing effects on long term
expanded murine neural stem cells. Epilepsy Res. 108:623–633. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Valdés-Mora F, Song JZ, Statham AL,
Strbenac D, Robinson MD, Nair SS, Patterson KI, Tremethick DJ,
Stirzaker C and Clark SJ: Acetylation of H2A.Z is a key epigenetic
modification associated with gene deregulation and epigenetic
remodeling in cancer. Genome Res. 22:307–321. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Liu S, Klisovic RB, Vukosavljevic T, Yu J,
Paschka P, Huynh L, Pang J, Neviani P, Liu Z, Blum W, et al:
Targeting AML1/ETO-histone deacetylase repressor complex: A novel
mechanism for valproic acid-mediated gene expression and cellular
differentiation in AML1/ETO-positive acute myeloid leukemia cells.
J Pharmacol Exp Ther. 321:953–960. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Leiva M, Moretti S, Soilihi H, Pallavicini
I, Peres L, Mercurio C, Dal Zuffo R, Minucci S and de Thé H:
Valproic acid induces differentiation and transient tumor
regression, but spares leukemia-initiating activity in mouse models
of APL. Leukemia. 26:1630–1637. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Driever PH, Wagner S, Hofstädter F and
Wolff JE: Valproic acid induces differentiation of a supratentorial
primitive neuroectodermal tumor. Pediatr Hematol Oncol. 21:743–751.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Gan CP, Hamid S, Hor SY, Zain RB, Ismail
SM, Mustafa W, Mahadzir W, Teo SH, Saunders N and Cheong SC:
Valproic acid: Growth inhibition of head and neck cancer by
induction of terminal differentiation and senescence. Head Neck.
34:344–353. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Suzuki T, Yokozaki H, Kuniyasu H, Hayashi
K, Naka K, Ono S, Ishikawa T, Tahara E and Yasui W: Effect of
trichostatin A on cell growth and expression of cell cycle- and
apoptosis-related molecules in human gastric and oral carcinoma
cell lines. Int J Cancer. 88:992–997. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Cappadocia L, Mascle XH, Bourdeau V,
Tremblay-Belzile S, Chaker-Margot M, Lussier-Price M, Wada J,
Sakaguchi K, Aubry M, Ferbeyre G and Omichinski JG: Structural and
functional characterization of the phosphorylation-dependent
interaction between PML and SUMO1. Structure. 23:126–138. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Sharma P, Yamada S, Lualdi M, Dasso M and
Kuehn MR: Senp1 is essential for desumoylating Sumo1-modified
proteins but dispensable for Sumo2 and Sumo3 deconjugation in the
mouse embryo. Cell Rep. 3:1640–1650. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Madu IG, Namanja AT, Su Y, Wong S, Li YJ
and Chen Y: Identification and characterization of a new chemotype
of noncovalent SENP inhibitors. ACS Chem Biol. 8:1435–1441. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wen D, Xu Z, Xia L, Liu X, Tu Y, Lei H,
Wang W, Wang T, Song L, Ma C, et al: Important role of SUMOylation
of Spliceosome factors in prostate cancer cells. J Proteome Res.
13:3571–3582. 2014. View Article : Google Scholar : PubMed/NCBI
|