Introduction
Inflammatory bowel disease (IBD) is a group of
common chronic inflammatory gastrointestinal disorders, including
Crohn's disease and ulcerative colitis (1,2). IBD
usually occurs in young adults and is closely associated with
inheritance, infection and immune function (3). IBD is harmful to health with
characteristics including a high recurrence rate, high canceration
rate and poor prognosis (4,5). Further investigation of the molecular
mechanisms of IBD may provide valuable information for the
treatment of IBD.
The pathogenesis of IBD is complex. Intestinal
mucosal inflammation can lead to sustained and irreversible damage
to the digestive tract and produce serious clinical consequences
(6). A variety of inflammatory
mediators such as interleukin (IL)-4, IL-1, tumor necrosis factor
and transforming growth factor (TGF)-β, as well as several signal
pathways such as the nuclear factor-κB signaling pathway,
participate in the damage of the digestive tract (7–9).
Immunosuppressant therapy is an important method for the treatment
of IBD, as it is able to relieve intestinal inflammation and
results in significant treatment efficiency (10). Autophagy is an important cellular
pathway for the maintenance of homeostasis. It degrades damaged
organelles, misfolded proteins and damaged DNA to provide energy
that allows the cells to respond to adverse environments (11,12).
Autophagy is closely associated with inflammation (13) and is induced by a variety of
inflammatory factors. Conversely it is also able to downregulate
inflammation (14). However, the
exact molecular mechanisms require further investigation.
Decorin (DCN) is a small leucine-rich proteoglycan
with glycosaminoglycan chains attached to a core protein and is one
of the important components of the extracellular matrix (15). Abnormal expression of DCN or
alteration of the structure of DCN correlates closely with a
variety of pathological processes including inflammation and cancer
(16,17). DCN has a number of biological
functions, including inhibition of the proliferation, invasion and
metastasis of a variety of tumors and is a potential target protein
for cancer therapy (18,19). In inflammation, DCN expression
negatively correlates with TGF-1 and IL-1, and positively
correlates with IL-4, indicating that DCN is involved in the
occurrence and development of inflammation (20). Currently, the expression and function
of DCN in IBD remains unclear. In the present study, IBD mouse
models were produced and the expression of DCN, as well as
autophagy-associated proteins in the intestinal tissues of the IBD
mice was examined. To further investigate the biological functions
of DCN in IBD, the effects of DCN on autophagy regulation in normal
human colon mucosal epithelial cells (NCM460 cells) were
studied.
Materials and methods
Reagents
M3 medium was purchased from Gibco (Thermo Fisher
Scientific, Inc., Waltham, MA, USA). 3-Methyladenine (3-MA) was
purchased from Sigma-Aldrich (Merck Millipore, Darmstadt, Germany).
Fluorescein isothiocyanate (FITC) Annexin V Apoptosis Detection kit
I was purchased from BD Biosciences (Franklin Lakes, NJ, USA).
Rabbit anti-human/mouse DCN (#P07585) and beclin 1 (#Q14457)
antibodies, and mouse anti-human/mouse GAPDH (#O14556) antibody
were purchased from Bioworld Technology, Inc. (St. Louis Park, MN,
USA). Rabbit anti-human/mouse p62 antibody (#8025) was purchased
from Cell Signaling Technology, Inc. (Boston, MA, USA). Rabbit
anti-human/mouse LC3B antibody (#AL221) was purchased from Beyotime
Institute of Biotechnology (Beijing, China). DCN expression plasmid
(pEGFP-N1-DCN) was purchased from Ruino (Guangzhou, China).
Lipofectamine 2000 transfection reagent was purchased from
Invitrogen (Thermo Fisher Scientific, Inc.).
IBD mouse model
The study was approved by the Ethics Committee of
the General Hospital of Chinese People's Liberation Army (Beijing,
China). A total of 60 healthy adult male BALB/c mice weighing
21±1.34 g were purchased from Chengdu DOSSY Experimental Animal
Co., Ltd. (Chengdu, China) and kept under specific-pathogen-free
conditions. The animals were housed under a 12-h light/dark cycle
and allowed ad libitum access to rodent chow and water. The
room temperature was maintained at 22°C and 50% relative humidity.
The mice were randomly divided into three groups (n=20 per group),
namely the normal group, the control group and the IBD group. In
the IBD group, the mice were anesthetized with pentobarbital (50
mg/kg; Shanghai Chemical Reagent Company, Shanghai, China) after
defecation and kept with the head down in a vertical position. A
polyethylene pipe with a diameter of 0.2 µm was inserted into the
colon (4 cm proximal to the anus) of each mouse and a dose of 200
mg/kg trinitrobenzene sulfonic acid (TNBS; Sigma-Aldrich) solution
in 50% ethanol was injected intrarectally. After that, the mice
were fed normally. TNBS administration was performed once a day for
7 days and 24 h after the last administration, the mice were
sacrificed after anesthetization with 50 mg/kg pentobarbital. In
the control group, the mice received only 50% ethanol. In the
normal group, the mice were kept under normal conditions without
treatment. The colon tissues of the mice were removed and fixed
with formaldehyde or stored in liquid nitrogen.
Cell culture
NCM460 cells were purchased from American Type
Culture Collection (Manassas, VA, USA). The cells were cultured in
M3 medium supplemented with 10% fetal bovine serum (FBS; Gibco).
The media was changed every other day and the cells were passaged
using trypsin digestion on reaching 80–90% confluence.
Hematoxylin and eosin (H&E)
staining
H&E staining was performed on the colon tissues
of the mice. Briefly, the colon tissues were fixed with 10%
formaldehyde for 24 h, rehydrated in graded alcohols, hyalinized
with xylene, embedded in paraffin and cut into 2-µm tissue
sections. The tissue sections were dewaxed in xylene, stained with
hematoxylin, stained with eosin, rehydrated in graded alcohols and
hyalinized with xylene. After that, the sections were mounted with
neutral gum and observed using an optical miscroscope (BX50;
Olympus Corporation, Tokyo, Japan.
Immunohistochemistry
The expression of DCN, beclin 1 and LC3B in the
colon tissues of the mice was detected using immunohistochemistry.
The colon tissues were fixed with 10% formaldehyde, embedded in
paraffin and cut into 4-µm sections. The sections were dewaxed and
rehydrated in graded xylene and alcohols. The sections were then
incubated with 3% H2O2 at room temperature
for 10 min to inactivate endogenous peroxidase and processed for
antigen retrieval using microwave heating at 400 W for 15 min.
Nonspecific binding was blocked with 10% goat serum (diluted with
PBS) at room temperature for 10 min. The sections were incubated
with anti-DCN antibody (1:200), anti-beclin 1 antibody (1:200) and
anti-LC3B antibody, respectively, at room temperature for 1 h, then
with goat anti-rabbit IgG H&L biotinylated secondary
antibody(1:200; #ab97049; Abcam, Cambridge, MA, USA) at 37°C for 30
min. The sections were then developed with 3,3′-diaminobenzidine
chromogenic reagent. Finally, the sections were counterstained with
hematoxylin for ~30 sec. Following hydrochloric acid
differentiation and hyalinization with xylene, the sections were
mounted with neutral gum and observed under an optical
microscope.
Positive cells were defined as cells with brown
staining in the cytoplasm or on the cell membrane. For each
section, five fields were randomly taken under a high power field
(HPF). The positive cells and negative cells were counted in each
field. The positive rate of each field was the percentage of the
ratio of the number of positive cells to the number of total cells
(the sum of positive and negative cells). The positive rate of each
tissue section was expressed as the mean of the positive rate of
the five fields. The expression of the proteins was scored
according to both the positive rate and the degree of staining. The
positive rate was scored as following: 0%, score 0; 1–25%, score 1;
26–50%, score 2 and 51–100%, score 3. The degree of staining was
scored as following: Without staining, score 0; with light yellow
staining, score 1; with light brown staining, score 2 and with
brown staining, score 3. The final score of the expression of the
proteins was obtained by multiplying the score of the positive rate
with the score of the degree of staining and was graded as
following: Score 0 or 1, negative; score 2 or 3, weakly positive;
score 4–6, positive and score >6, strongly positive.
Transmission electron microscopy
observation
NCM460 cells were seeded into 10-mm culture plates.
After culturing for 24 h, the culture medium was discarded and
cells were washed with PBS twice. Then, the cells were fixed in
2.5% glutaric dialdehyde for 30 min at 4°C. Cells were collected
into 1.5-ml EP tubes by scraping. Then, 2.5% glutaric dialdehyde
was added and incubated at 4°C overnight. The subsequent procedures
were performed by Shanghai Fucheng Biological Technology Co. Ltd.
(Shanghai, China).
Western blot analysis
The colon tissues of the mice, ground into a powder
in liquid nitrogen, were lysed with radioimmunoprecipitation assay
buffer containing phenylmethanesulfonyl fluoride. The lysates were
subjected to electrophoresis on 11% SDS-PAGE gels. The proteins
were then transferred to poly(vinylidene fluoride) membranes for
western blot analysis. After blocking with 5% non-fat milk for 1 h
at room temperature, the membranes were incubated overnight with
anti-DCN, anti-beclin 1, anti-LC3B antibody, anti-p62 (all 1:1,000)
or anti-GAPDH (1:10,000) antibodies at 4°C overnight. After
washing, the membrane was incubated with goat anti-mouse (1:3,000;
#ab6789) and anti-rabbit (1:4,000; #ab6721) horseradish
peroxidase-conjugated secondary antibody (Abcam)at room temperature
for 1 h. Bound antibodies were detected using an enhanced
chemiluminescence kit (Amersham; GE Healthcare, Little Chalfont,
UK). The mean normalized optical density (OD) of the DCN, beclin 1,
LC3B or p62 protein band relative to the OD of GAPDH band from the
same sample was calculated using Quantity One software, version
4.62 (Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Cell transfection
NCM460 cells in the logarithmic growth phase were
seeded at 2×105 cells/well in 24-well plates. On the next day,
NCM460 cells were transfected with 1 µg DCN expression plasmid
(pEGFP-N1-DCN) using Lipofectamine 2000 according to the
manufacturer's instructions. At 48 h after transfection, the cells
were harvested and lysed for western blot analysis as described
above. Cells without transfection or transfected with empty plasmid
were used as controls.
Apoptosis assays
NCM460 cells were transfected with DCN expression
plasmid as above described. The autophagy inhibitor 3-MA (5 µM) was
added and incubated for 24 h. Then, cells were cultured with fresh
glucose-free medium in a three-gas incubator (5% CO2, 1%
O2 and 94% N2) with saturated humidity. After
24 h, cells were collected for apoptosis analysis. Apoptosis assays
were performed using the FITC Annexin V Apoptosis Detection kit I
according to the manufacturer's protocol. Cells that are in early
apoptosis are Annexin V positive and propidium iodide (PI)
negative, cells that are in necrosis are PI positive and Annexin V
negative, and cells that are in late apoptosis are Annexin V and PI
positive.
Statistical analysis
All data were processed using the SPSS version 17.0
statistical package (SPSS, Inc., Chicago, IL, USA). Data are
presented as means ± standard deviation. Statistical significance
was determined using the Student's t-test. P<0.05 was considered
to indicate a statistically significant result.
Results
Successful modeling of IBD in mice.
The IBD mouse model was generated by intrarectal injection of
TNBS
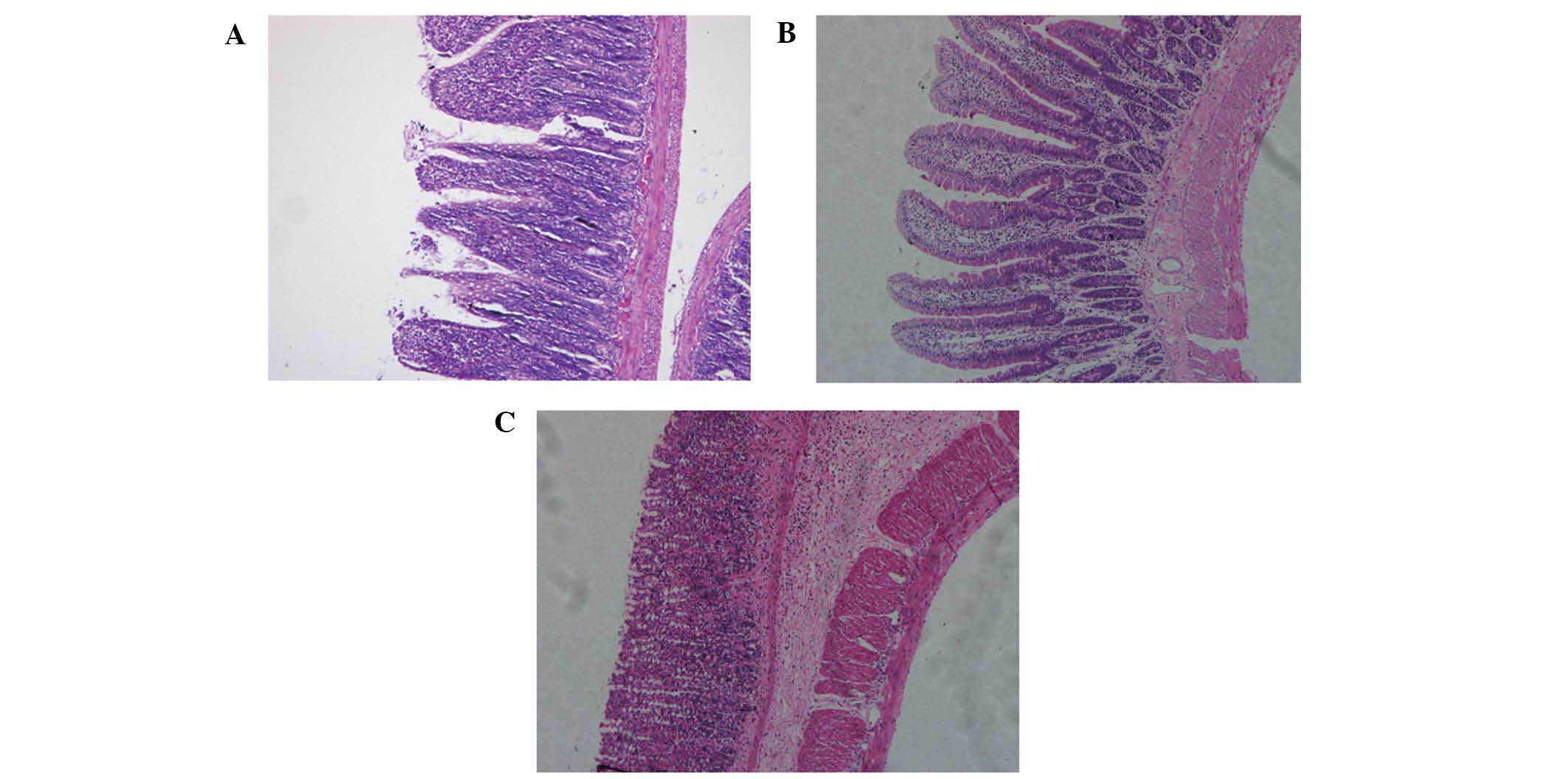
The pathology of colon tissues in the mice was
examined using H&E staining. In the normal group and the
control group, the mice were in good spirits with normal weight and
stools. The hair of the mice was smooth and flat. In the IBD group,
the mice remained alive throughout the whole experiment, until
sacrifice. However, they were lethargic with reduced physical
activity and yellow mushy stools. In some mice, the stools
contained mucus, blood and pus and their hair stood on end. H&E
staining showed mucosal erosion, inflammatory cell infiltration,
thickening of the intestinal wall and ulceration in the colon
tissues of mice in the IBD group but not in the normal group
(Fig. 1).
Altered expression of DCN and
autophagy-associated proteins in the intestinal tissues of IBD
mice
To investigate the expression of DCN, as well as
autophagy-associated proteins in the intestinal tissues of IBD
model mice, immunohistochemical assays and western blot analysis
were performed. As shown in Fig. 2,
in the IBD group, DCN expression could be observed in the cytoplasm
and nucleus of all cells in the intestinal tissue. The strong
positive rate was 48%. In the normal group and the control group,
DCN expression was negative or weakly positive. The difference was
statistically significant (P<0.05). The positive rates of
autophagy-associated proteins beclin 1 and LC3B in the IBD group
were significantly higher than those in the control group
(P<0.05).
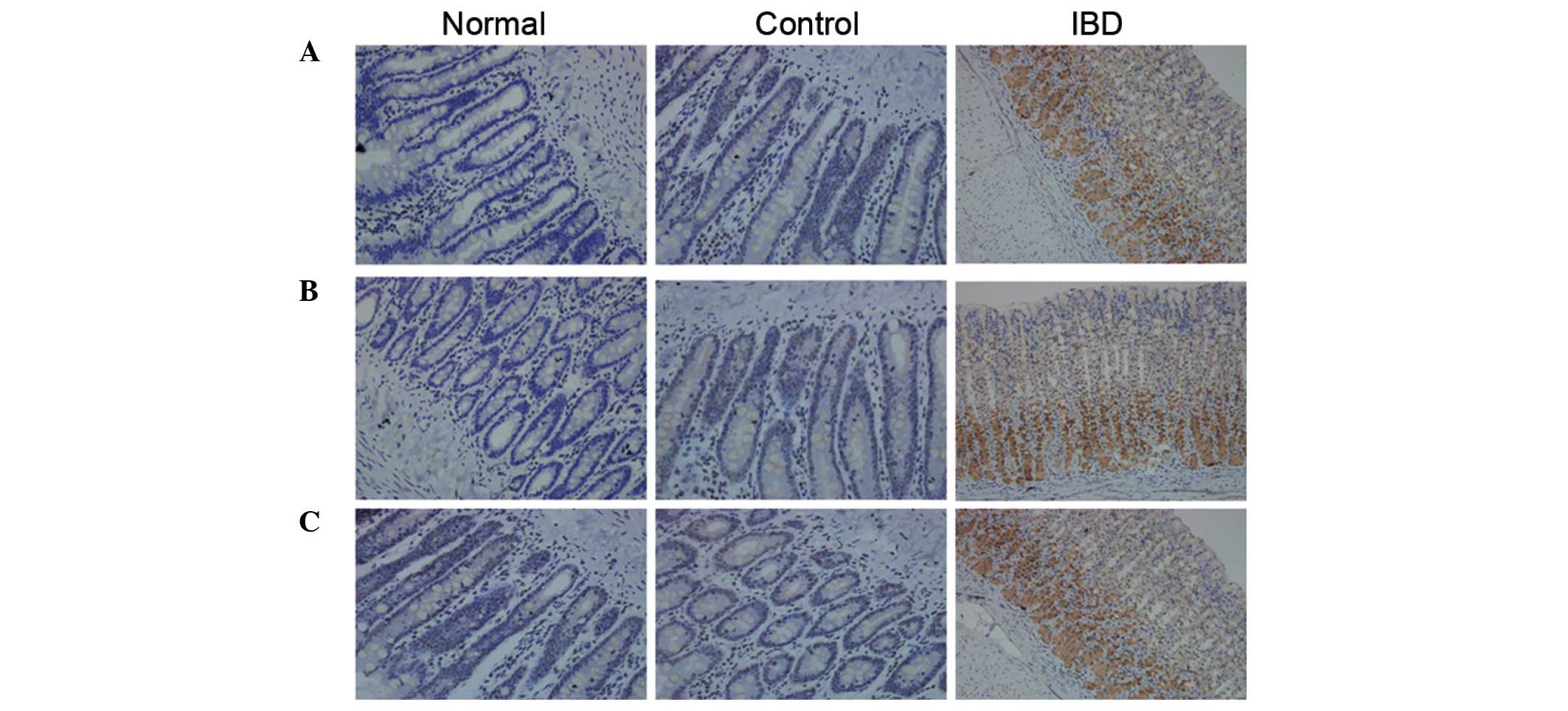 | Figure 2.Expression of DCN and
autophagy-associated proteins in the intestinal tissues detected
using immunohistochemical assay (magnification, ×200).
Representative immunocytochemical staining results of (A) DCN, (B)
beclin 1 and (C) LC3B are shown. Positive cells were stained brown.
(A) DCN expression was negative in the normal group, weak in the
control group and significantly increased in the IBD group. (B) In
the normal and control groups, beclin 1 expression was negative or
weakly positive. There was no significant difference in beclin 1
expression between these two groups. In the IBD group, beclin 1
expression significantly increased. (C) In the normal and control
groups, LC3B expression was negative or weakly positive. There was
no significant difference in LC3B expression between these two
groups. In the IBD group, LC3B expression significantly increased.
Normal, normal group; control, control group; IBD, inflammatory
bowel disease group; DCN, decorin. |
The expression levels of DCN, beclin 1, LC3B and p62
in the intestinal tissues of the mice were also examined using
western blot analysis. As shown in Fig.
3, there were no significant differences in the expression of
DCN, beclin 1, LC3B and p62 between the normal group and the
control group. The expression of DCN, beclin 1 and LC3B was
significantly higher in the IBD group than in the normal group
(P<0.05). The expression of p62 was significantly lower in the
IBD group than in the normal group (P<0.05). These results
suggest that autophagy is induced in the intestinal tissues of the
mice with IBD.
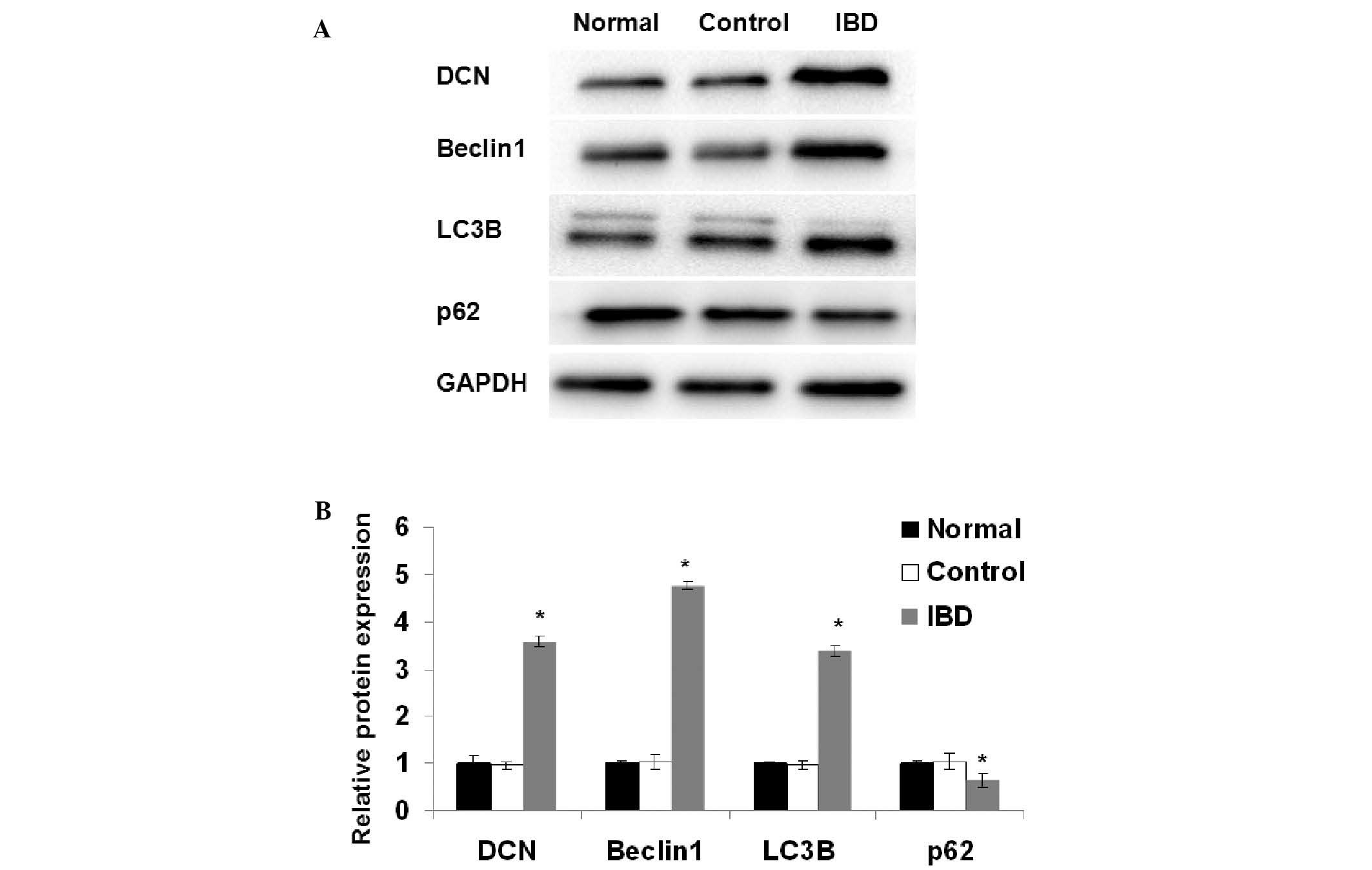 | Figure 3.Expression of DCN and
autophagy-associated proteins in the intestinal tissues detected
using western blot analysis. (A) Representative results for DCN,
beclin 1, LC3B, p62 and GAPDH are shown. GAPDH was used as an
internal control. (B) Quantitative comparison of DCN, beclin 1,
LC3B and p62 expression is shown. Data were derived from three
independent experiments as in (A). The mean normalized optical
density of DCN, beclin 1, LC3B and p62 protein bands relative to
that of GAPDH bands from the same sample was calculated. Expression
levels of DCN, beclin 1, LC3B and p62 are expressed as fold changes
compared with the normal group. Data are expressed as mean ±
standard deviation. *P<0.05 vs. the normal group Student's
t-test. Normal, normal group; control, control group; IBD,
inflammatory bowel disease group; DCN, decorin. |
Increased expression of
autophagy-associated proteins in NCM460 cells transfected with DCN
expression plasmid
To further investigate the correlation of the
expression of DCN with autophagy-associated proteins, NCM460 cells
were transfected with DCN expression plasmid, and the expression of
DCN and autophagy-associated proteins was detected using western
blot analysis. As shown in Fig. 4,
compared with NCM460 cells without transfection and NCM460 cells
transfected with empty plasmid, the expression of DCN was
significantly increased in the cells transfected with DCN
expression plasmid (P<0.05). The expression of beclin 1 and LC3B
was also significantly increased in the cells transfected with DCN
expression plasmid (P<0.05). There were no significant
differences in the expression of p62 between the cells transfected
with DCN expression plasmid and the cells without transfection or
transfected with empty plasmid. These results indicate that DCN may
promote autophagy.
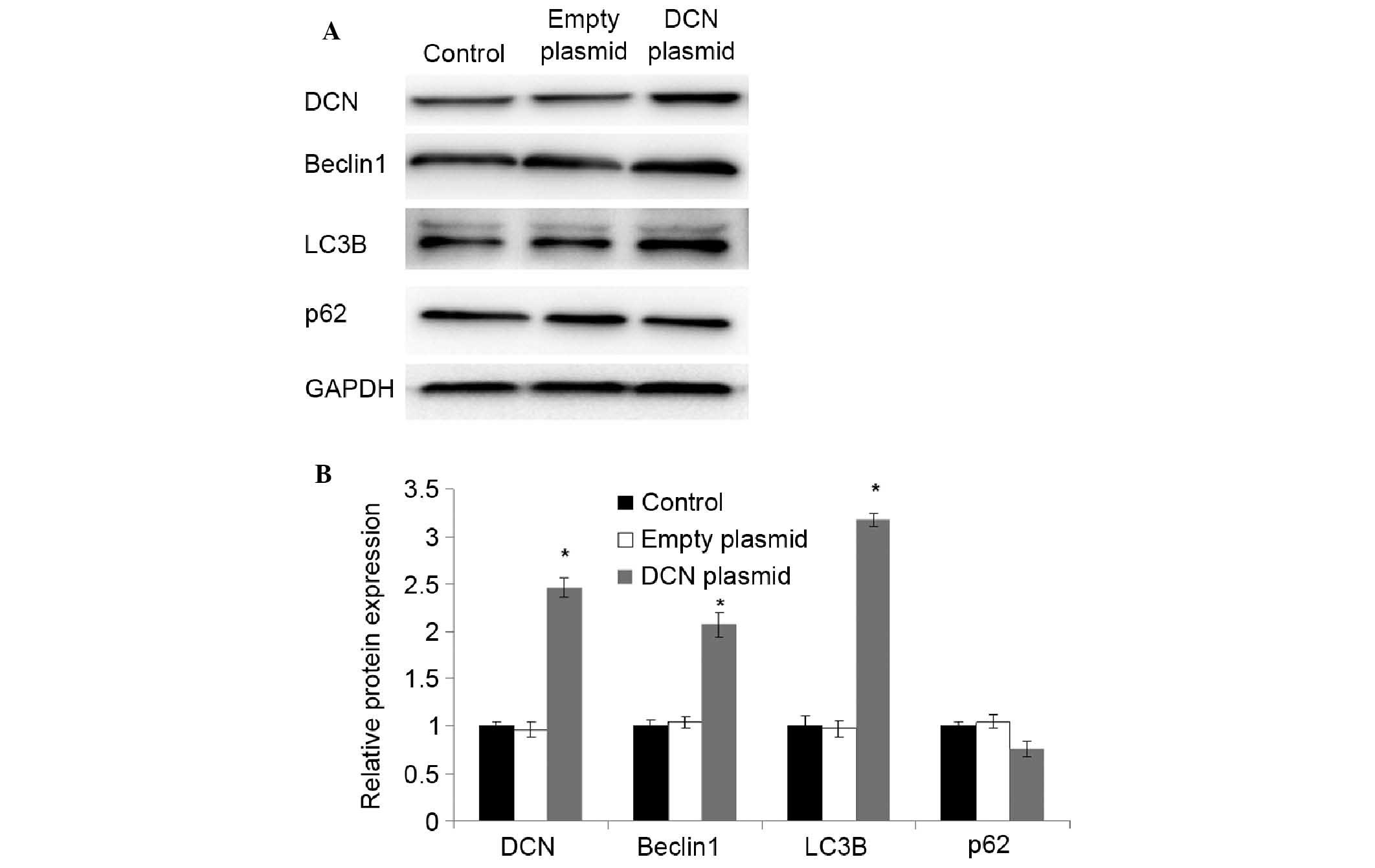 | Figure 4.Increased expression of
autophagy-associated proteins in NCM460 cells transfected with DCN
expression plasmid. (A) Expression of DCNand autophagy-associated
proteins was detected using western blot analysis. Representative
results of DCN, beclin 1, LC3B, p62 and GAPDH are shown. GAPDH was
used as an internal control. (B) Quantitative comparison of DCN,
beclin 1, LC3B and p62 expression. Data were derived from three
independent experiments as in (A). The mean normalized optical
density of DCN, beclin 1, LC3B and p62 protein bands relative to
that of GAPDH bands from the same sample was calculated. Expression
levels of DCN, beclin 1, LC3B and p62 are expressed as fold changes
compared with the normal group. Data are expressed as mean ±
standard deviation. *P<0.05 vs. the control group (Student's
t-test). Control, NCM460 cells without transfection; empty plasmid,
NCM460 cells transfected with empty plasmid; DCN plasmid, NCM460
cells transfected with DCN expression plasmid; DCN, decorin. |
Increased autophagy in NCM460 cells
transfected with DCN expression plasmid
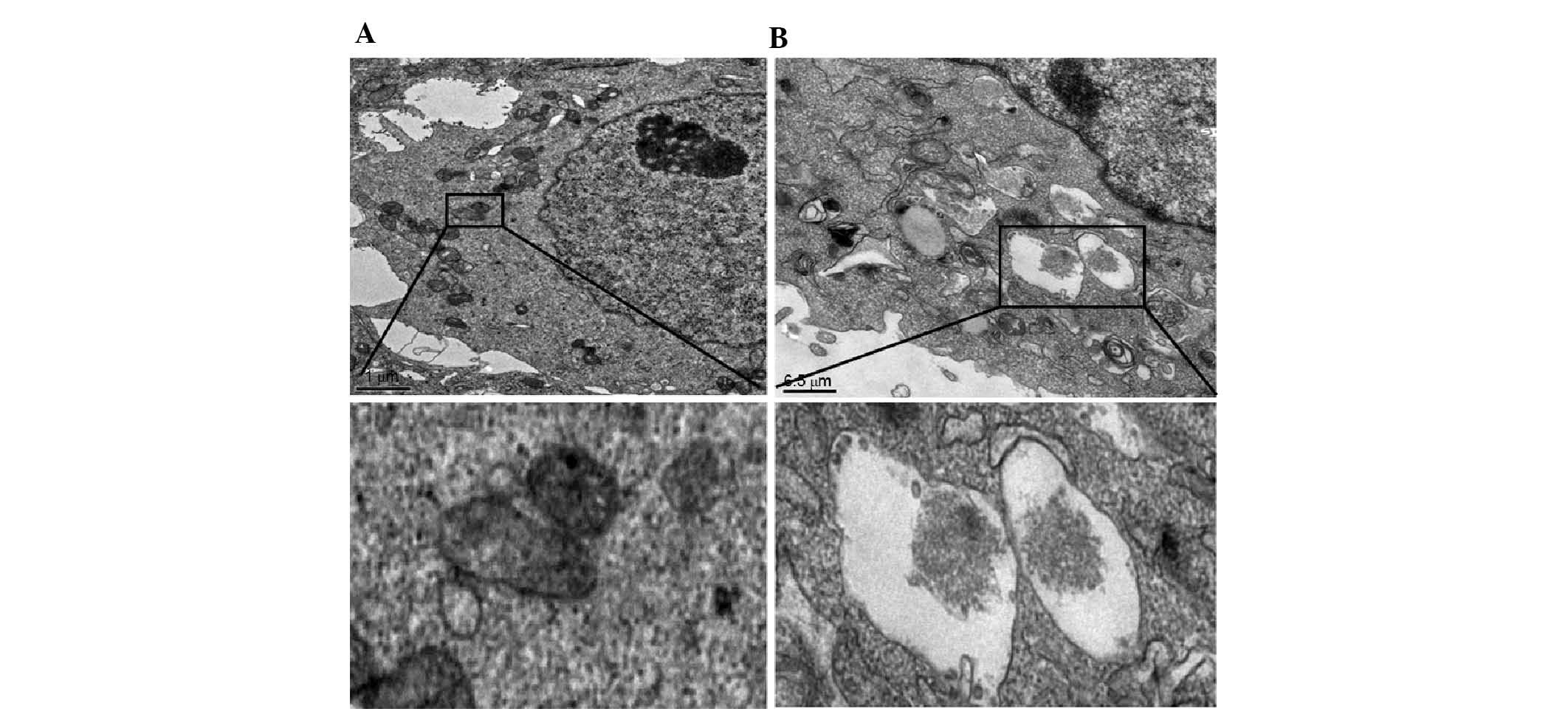
To investigate the effects of DCN on autophagy,
NCM460 cells were transfected with DCN expression plasmid and
intracellular autophagosomes were observed using transmission
electron microscopy. As shown in Fig.
5, a small number of autophagosomes with clear borders and
undigested contents were observed in the NCM460 cells transfected
with empty plasmid. In the NCM460 cells transfected with DCN
expression plasmid, the number of autophagosomes increased
significantly, which were vacuole-shaped with fully digested
contents. These results indicate that autophagy was increased in
NCM460 cells transfected with DCN expression plasmid.
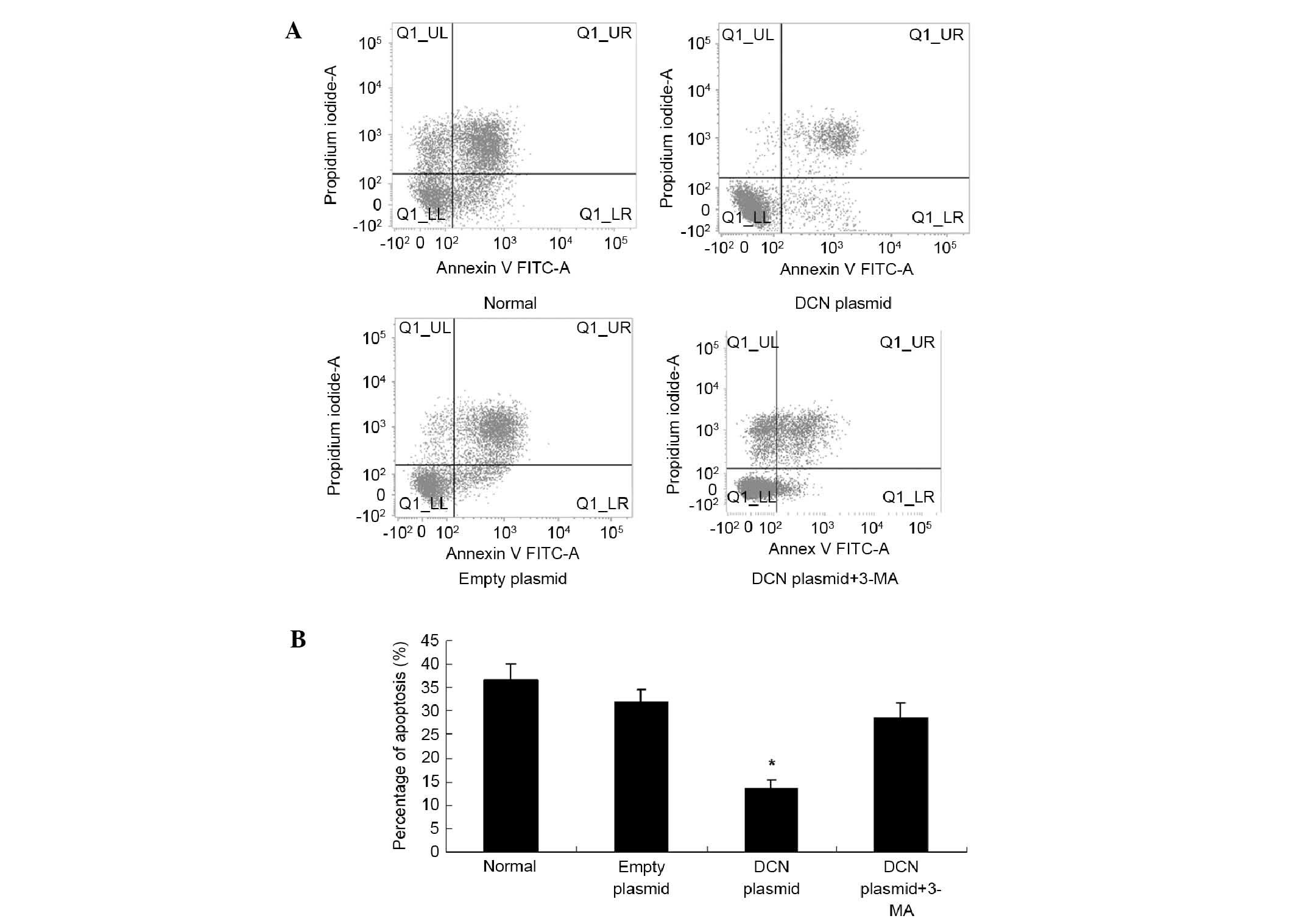
Decreased apoptosis in NCM460 cells
transfected with DCN expression plasmid
As mentioned above, DCN expression was positively
associated with the expression of autophagy-associated proteins.
Under stress, autophagy can provide energy and protect cells
(21). To investigate whether DCN
protects the cells by regulating autophagy, NCM460 cells were
transfected with DCN expression plasmid, cultured under oxygen
glucose deprivation (OGD) conditions and treated with autophagy
inhibitor 3-MA (5 µM). Cell apoptosis was studied using an Annexin
V apoptosis detection kit. As shown in Fig. 6, compared with the NCM460 cells
without transfection or transfected with empty plasmid, apoptosis
was significantly decreased in the cells transfected with DCN
expression plasmid. Furthermore, the autophagy inhibitor 3-MA
attenuated the effects of DCN overexpression on apoptosis.
Discussion
IBD is an autoimmune disease involving numerous
inflammatory factors (22). It has
been reported that inflammation induces autophagy, which degrades
damaged organelles to provide energy and protect cells from
apoptosis (23). It was thus
hypothesized that autophagy may protect intestinal cells during the
development of IBD. DCN is capable of negatively regulating the
proliferation of tumor cells (24).
In recent years, the effects of DCN on the occurrence and
development of inflammation have aroused much attention. DCN may
regulate the expression of a number of genes and is closely
associated with inflammatory signaling pathways (25). In the present study, the expression
of DCN as well as autophagy-associated proteins in IBD was examined
for the first time, to the best of our knowledge. The correlation
of DCN with autophagy and biological functions was also
examined.
TNBS is able to induce colitis in mice with a high
success rate, low mortality and pathological features similar to
those of IBD in humans (26). Thus,
in this study, an IBD mouse model produced by the intrarectal
injection of TNBS was used. Immunohistochemical assays showed
increased expression of DCN as well as autophagy-associated
proteins in the intestinal tissues of the IBD mice. These results
were further confirmed by western blot analysis. It appears that
during the development of IBD, increased DCN expression is
associated with autophagy and DCN participates in the regulation of
autophagy. To test this hypothesis, NCM460 cells were transfected
with DCN expression plasmid. The results showed significantly
increased expression of autophagy-associated proteins and increased
amounts of autophagosomes in the NCM460 cells with DCN
overexpression. Furthermore, under OGD conditions, the apoptosis of
NCM460 cells transfected with DCN expression plasmid was
significantly lower than that of the control group. In addition,
autophagy inhibitor 3-MA treatment increased the apoptosis of these
NCM460 cells with DCN overexpression.
In conclusion, these results indicate that DCN
regulates autophagy and protects cells from apoptosis during the
development of IBD. Thus, DCN may serve as a potential new target
for IBD therapy.
Acknowledgements
This study was supported by the National High
Technology Research and Development Program of China (grant no.
2012AA02A504) and Army Medical Science Youth Development Project
(grant no. 13QNP185).
References
|
1
|
Agilli M, Aydin FN, Cayci T and Kurt YG:
Assessment of active mucosal inflammation in IBD patients in
clinical remission. J Gastrointestin Liver Dis. 23:462–463.
2014.PubMed/NCBI
|
|
2
|
Dunkin D, Mehandru S and Colombel JF:
Immune cell therapy in IBD. Dig Dis. 32:(Suppl 1). S61–S66. 2014.
View Article : Google Scholar
|
|
3
|
Hildner K, Punkenburg E, Abendroth B and
Neurath MF: Immunopathogenesis of IBD: Batf as a Key Driver of
Disease Activity. Dig Dis 34 (Suppl 1). 40–47. 2016.
|
|
4
|
De Salvo C, Ray S and Pizarro TT:
Mechanisms and models for intestinal fibrosis in IBD. Dig Dis.
32:(Suppl 1). S26–S34. 2014. View Article : Google Scholar
|
|
5
|
Latella G, Di Gregorio J, Flati V, Rieder
F and Lawrance IC: Mechanisms of initiation and progression of
intestinal fibrosis in IBD. Scand J Gastroenterol. 50:53–65. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bryant RV, Brain O and Travis SP:
Conventional drug therapy for inflammatory bowel disease. Scand J
Gastroenterol. 50:90–112. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chen M, Yi F, Zhou F, Huang M, Li J, Yan
W, Li L and Xia B: Risk factors for initial surgery in patients
with Crohn's disease in Central China. Surg Today. 45:1002–1008.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Connelly TM, Koltun WA, Sangster W, Berg
AS, Hegarty JP, Harris L III, Deiling S and Stewart DB: An
interleukin-4 polymorphism is associated with susceptibility to
Clostridium difficile infection in patients with inflammatory bowel
disease: Results of a retrospective cohort study. Surgery.
156:769–774. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Katz LH, Kopylov U, Fudim E, Yavzori M,
Picard O, Ungar B, Eliakim R, Ben-Horin S and Chowers Y: Expression
of IL-2, IL-17 and TNF-alpha in patients with Crohn's disease
treated with anti-TNF antibodies. Clin Res Hepatol Gastroenterol.
38:491–498. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zatorski H, Marynowski M and Fichna J: Is
insulin-like growth factor 1 (IGF-1) system an attractive target
inflammatory bowel diseases? Benefits and limitation of potential
therapy. Pharmacol Rep. 68:809–815. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Nata T, Fujiya M, Ueno N, Moriichi K,
Konishi H, Tanabe H, Ohtake T, Ikuta K and Kohgo Y: MicroRNA-146b
improves intestinal injury in mouse colitis by activating nuclear
factor-kB and improving epithelial barrier function. J Gene Med.
15:249–260. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chen JY, Hour TC, Yang SF, Chien CY, Chen
HR, Tsai KL, Ko JY and Wang LF: Autophagy is deficient in nasal
polyps: Implications for the pathogenesis of the disease. Int Forum
Allergy Rhinol. 5:119–123. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liu H, He Z and Simon HU: Protective role
of autophagy and autophagy-related protein 5 in early
tumorigenesis. J Mol Med (Berl). 93:159–164. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Annese V, Duricova D, Gower-Rousseau C,
Jess T and Langholz E: Impact of New Treatments on Hospitalisation,
Surgery, Infection, and Mortality in IBD: A Focus Paper by the
Epidemiology Committee of ECCO. J Crohns Colitis. 10:216–225. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Murthi P, van Zanten DE, Eijsink JJ, Borg
AJ, Stevenson JL, Kalionis B, Chui AK, Said JM, Brennecke SP and
Erwich JJ: Decorin expression is decreased in first trimester
placental tissue from pregnancies with small for gestation age
infants at birth. Placenta. 45:58–62. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Nemani N, Santo L, Eda H, Cirstea D,
Mishima Y, Patel C, O'Donnell E, Yee A and Raje N: Role of decorin
in multiple myeloma (MM) bone marrow microenvironment. J Bone Miner
Res. 30:465–470. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Esmaeili M, Berry M, Logan A and Ahmed Z:
Decorin treatment of spinal cord injury. Neural Regen Res.
9:1653–1656. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kasamatsu A, Uzawa K, Minakawa Y, Ishige
S, Kasama H, Endo-Sakamoto Y, Ogawara K, Shiiba M, Takiguchi Y and
Tanzawa H: Decorin in human oral cancer: A promising predictive
biomarker of S-1 neoadjuvant chemosensitivity. Biochem Biophys Res
Commun. 457:71–76. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Araki K, Wakabayashi H, Shintani K,
Morikawa J, Matsumine A, Kusuzaki K, Sudo A and Uchida A: Decorin
suppresses bone metastasis in a breast cancer cell line. Oncology.
77:92–99. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Reed CC, Waterhouse A, Kirby S, Kay P,
Owens RT, McQuillan DJ and Iozzo RV: Decorin prevents metastatic
spreading of breast cancer. Oncogene. 24:1104–1110. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Obba S, Hizir Z, Boyer L, Selimoglu-Buet
D, Pfeifer A, Michel G, Hamouda MA, Gonçalvès D, Cerezo M,
Marchetti S, et al: The PRKAA1/AMPKα1 pathway triggers autophagy
during CSF1-induced human monocyte differentiation and is a
potential target in CMML. Autophagy. 11:1114–1129. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cheng X, Zhang X, Su J, Zhang Y, Zhou W,
Zhou J, Wang C, Liang H, Chen X, Shi R, et al: miR-19b
downregulates intestinal SOCS3 to reduce intestinal inflammation in
Crohn's disease. Sci Rep. 5:103972015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zeng TS, Liu FM, Zhou J, Pan SX, Xia WF
and Chen LL: Depletion of Kupffer cells attenuates systemic insulin
resistance, inflammation and improves liver autophagy in high-fat
diet fed mice. Endocr J. 62:615–626. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mauviel A, Santra M, Chen YQ, Uitto J and
Iozzo RV: Transcriptional regulation of decorin gene expression.
Induction by quiescence and repression by tumor necrosis
factor-alpha. J Biol Chem. 270:11692–11700. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Schaefer L, Macakova K, Raslik I, Micegova
M, Gröne HJ, Schönherr E, Robenek H, Echtermeyer FG, Grässel S,
Bruckner P, et al: Absence of decorin adversely influences
tubulointerstitial fibrosis of the obstructed kidney by enhanced
apoptosis and increased inflammatory reaction. Am J Pathol.
160:1181–1191. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tomasello G, Sinagra E, Raimondo D,
Palumbo VD, Puleio R, Cottone M, Damiani P, Traina G, Abruzzo A,
Damiani F, et al: Validation of a modified model of TNBS-induced
colitis in rats. How to induce a chemical colitis in rats. Acta
Biomed. 86:92–96. 2015.PubMed/NCBI
|