Introduction
Endothelial progenitor cells (EPCs) are important in
maintaining endothelial homeostasis (1,2). In a
variety of pathological processes such as atherosclerosis, acute
myocardial infarction and percutaneous coronary intervention, EPCs
migrate to the site of endothelial injury or tissue ischemia, and
promote endothelial repair and neovascularization (3). Numerous previous studies have reported
a number of factors, including smoking, hypertension and diabetes
mellitus, that reduce the number of circulating EPCs and impair
their function (4,5).
Smoking is a factor that demonstrates a marked
correlation with cardiovascular diseases and associated mortality.
In 2000, >1 in 10 cardiovascular deaths were attributable to
smoking (6). However, multiple
previous studies focused on the association between smoking and
endothelial cells, reporting that smoking markedly impaired
endothelial function (4) and
integrity (7). More recently,
reports documented that smoking has detrimental effects on EPCs: In
2001, Vase et al (8)
identified that smoking was an important risk factor for a
reduction in circuiting EPC number. Subsequent studies demonstrated
that the number of circulating EPCs was reduced in chronic smokers,
and that reduced EPC levels are restored following smoking
cessation (9,10).
Notably, nicotine may have benefits for EPCs. Wang
et al (11) reported that low
concentrations of nicotine increase the EPC count and their
proliferative, migratory, adhesive and vasculogenic capacity.
Furthermore, nicotine administration improved the blood flow in a
rat ischemic hindlimb model by enhancing the function of
transplanted EPCs (12). A
hypothesized explanation of these effects was that nicotine reduced
EPC senescence by activating telomerase through the PI3K/Akt
pathway (13). However, these
conclusions were made from short-term experiments (>4 weeks),
and the effect of long-term nicotine administration on EPCs remains
unclear. As most smokers are long-term consumers of tobacco and
cigarettes, the present study aimed to determine the long-term
effects of nicotine on EPCs.
Materials and methods
Animal experiments
All animal experiments conformed to the Guide for
the Care and Use of Laboratory Animals (14) and the protocol was approved by the
Experimental Animal and Ethics Committee of the Third Military
Medical University (Chongqing, China).
A total of 120 C57BL/6 male mice (age, 8 weeks;
weight, 28±1.32 g) were purchased from the Animal Centre at the
Medical Department of Beijing University (Beijing, China) and
maintained in 22°C with a 12 h light/dark cycle, specific
pathogen-free conditions and ad libitum access to water.
Prior to experimental treatment, all mice were maintained on a
normal chow diet for 1 week. Mice were randomly allocated into 6
groups (n=20 mice per group): Control group mice sacrified at 1, 3
or 6 months (abbreviated as C-1 M, C-3 M and C-6 M, respectively);
and nicotine-treated groups sacrificed at 1, 3 or 6 months
(abbreviated as N-1 M, N-3 M and N-6 M, respectively). In
nicotine-treated groups, 100 ng/ml nicotine (Jingkehuaxue,
Shanghai, China) diluted in 2% sucrose water was administered
orally, on a daily basis. The control groups received 2% sucrose
water.
Murine EPC culture and
identification
Murine EPCs were isolated and cultured as previously
described (15). Briefly, mice were
sacrificed by cervical dislocation following anesthetization with
an overdose of sodium pentobarbitone (intraperitoneal injection, 60
mg/kg). Bone marrow were obtained by flushing mice femurs, then the
flushing fluid was subjected to density gradient centrifugation in
order to isolate mononuclear cells. The cells were resuspended in
endothelial cell growth medium (EGM-2MV BulletKit medium; Lonza,
Basel, Switzerland) containing 5% fetal bovine serum (FBS) and
supplemented with recombinant human (rh) epidermal growth factor,
rh fibroblast growth factor-B, rh insulin-like growth factor-1, rh
vascular endothelial cell growth factor (VEGF), ascorbic acid and
heparin. Equal cell densities (4.106
cells/cm2) were seeded in fibronectin-coated culture
plates and cultured in an incubator at 37°C and 5% CO2.
The culture medium was changed after 48 h following seeding, and
every 3 days subsequent to this. In order to identify EPCs, cells
were subjected to fluorescent labeling with acetylated low density
lipoprotein (DiI-ac-LDL) and fluorescein isothiocyanate-conjugated
Ulex europaeus agglutinin (FITC-UEA-I); the double-positive
cells were identified as EPCs.
EPC number and proliferation
assay
After culturing for 7 days, cells were stained with
DiI-ac-LDL and FITC-UEA-I, and the number of double-positive cells
was counted in six randomly selected fields of view. FITC-labelled
bromodeoxyuridine (BrdU) incorporation was used to assess EPC
proliferation activity according to the manufacturer's
instructions. Briefly, 10 mM BrdU was added to cells cultured in
96-well plates, and these were incubated for 24 h. The cells were
fixed with 4% paraformaldehyde and DAPI stained. Fluorescent cells
were observed with a fluorescence microscope, and images were
analyzed using Image J software (version 1.50i;
imagej.nih.gov/ij/). The proliferative ratio was calculated using
the equation: Proliferative ratio (%) = (BrdU-positive cells/DAPI
positive cells) × 100.
EPC migration assay
The migration of EPCs was assessed within a modified
Boyden chamber assay. In brief, after culturing for 7 days, EPCs
were resuspended in serum-free EGM-2 medium, and 5×104
cells were placed in the upper chamber, whilst the lower chamber
was filled with medium containing 5% FBS and 20 ng/ml VEGF. After
incubation for 12 h, the upper side of the membrane was wiped with
a cotton swab, and the membranes were collected and fixed with 4%
paraformaldehyde, which was followed by crystal violet staining.
The stained cells were counted in six random fields of view using a
light microscope.
EPC senescence assay
EPC senescence was assessed using a Senescence
β-galactosidase Staining kit (Beyotime Institute of Biotechnology,
Haimen, China) according to the manufacturer's instructions.
Briefly, cells were cultured in 6-well plates for 14 days, were
washed with phosphate-buffered saline, fixed with 4%
paraformaldehyde, then cells were incubated overnight with the kit
staining mixture at 37°C. The positive (blue) cells were observed
under a light microscope.
Telomerase activity assay
Quantitative polymerase chain reaction (qPCR) of
telomerase reverse transcriptase (TERT) expression was used to
assess telomerase activity. Total RNA was extracted and purified
using RNAiso Plus (cat. no. 9018; Takara Biotechnology Co., Ltd.,
Dalian, China). Single cDNAs were synthesized from RNA using a
PrimeScript™ RT reagent Kit (TaKaRa, China, Cat number: RR037A)
according to the manufacturer's instruction A qPCR kit to detect
mouse TERT (KeyGen Biotech. Co. Ltd., Nanjing, China) was used
according to the manufacturer's instructions. The specific primers
for TERT and GAPDH (as the reference gene) were supplied in the
kit. The thermal cycling conditions were as follows: Initial
denaturation at 95°C for 10 sec, 40 cycles of denaturation at 95°C
for 15 sec, then annealing at 60°C for 60 sec. The expression level
of TERT mRNA was calculated by the ΔCq method (16).
Furthermore, the telomerase activity was also
assessed by the telomeric repeat amplification protocol assay
(17). The telomerase reaction
product was amplified by PCR using the TeloTAGGG Telomerase PCR
ELISAPLUS kit (cat. no. 11854666910; Roche Diagnostics, Basel,
Switzerland) according to the manufacturer's instructions.
Western blot analysis
EPCs were solubilized using a lysis buffer (Beyotime
Institute of Biotechnology), supplemented with 0.5 mM
phenylmethylsulfonyl fluoride and centrifuged at 12,000 x g
for 15 min. Protein concentrations were assayed using a
bicinchoninic acid assay kit (Beyotime Institute of Biotechnology),
30 µg total protein from each group was separated by sodium dodecyl
sulfate-polyacrylamide gel (10% for nAChR and 5% for SIRT1)
electrophoresis and these proteins were transferred onto
polyvinylidene fluoride membranes. The membranes were blocked with
5% bovine serum albumin, and probed with antibodies against sirtuin
1 (SIRT1; dilution, 1:200; cat. no. sc-15404; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA), alpha-7 nicotinic receptor
(nAChR-α7; dilution, 1:500; cat. no. AB15332; Merck Millipore,
Darmstadt, Germany) and β-actin (dilution, 1:1,000; cat. no. AA128;
Beyotime Institute of Biotechnology) at 4°C overnight. Following
this, horseradish peroxidase-conjugated goat anti-rabbit IgG
secondary antibodies (dilution, 1:1,000; Beyotime Institute of
Biotechnology) were incubated at 37°C for 1 h, and bands were
detected using an enhanced chemiluminescence kit (cat. no. 32109;
Thermo Fisher Scientific, Inc., Waltham, MA, USA). Densiometry
signals were quantified by ImageQuant TL software (GE Healthcare
Life Sciences, Chalfont, UK).
Statistical analysis
Data is presented as the mean ± standard error.
Intergroup comparisons were performed by one-way analysis of
variance test accompanied by post-hoc least significant difference
test. P-values <0.05 were considered to indicate a statistically
significant difference.
Results
Long-term nicotine exposure reduces
EPC number
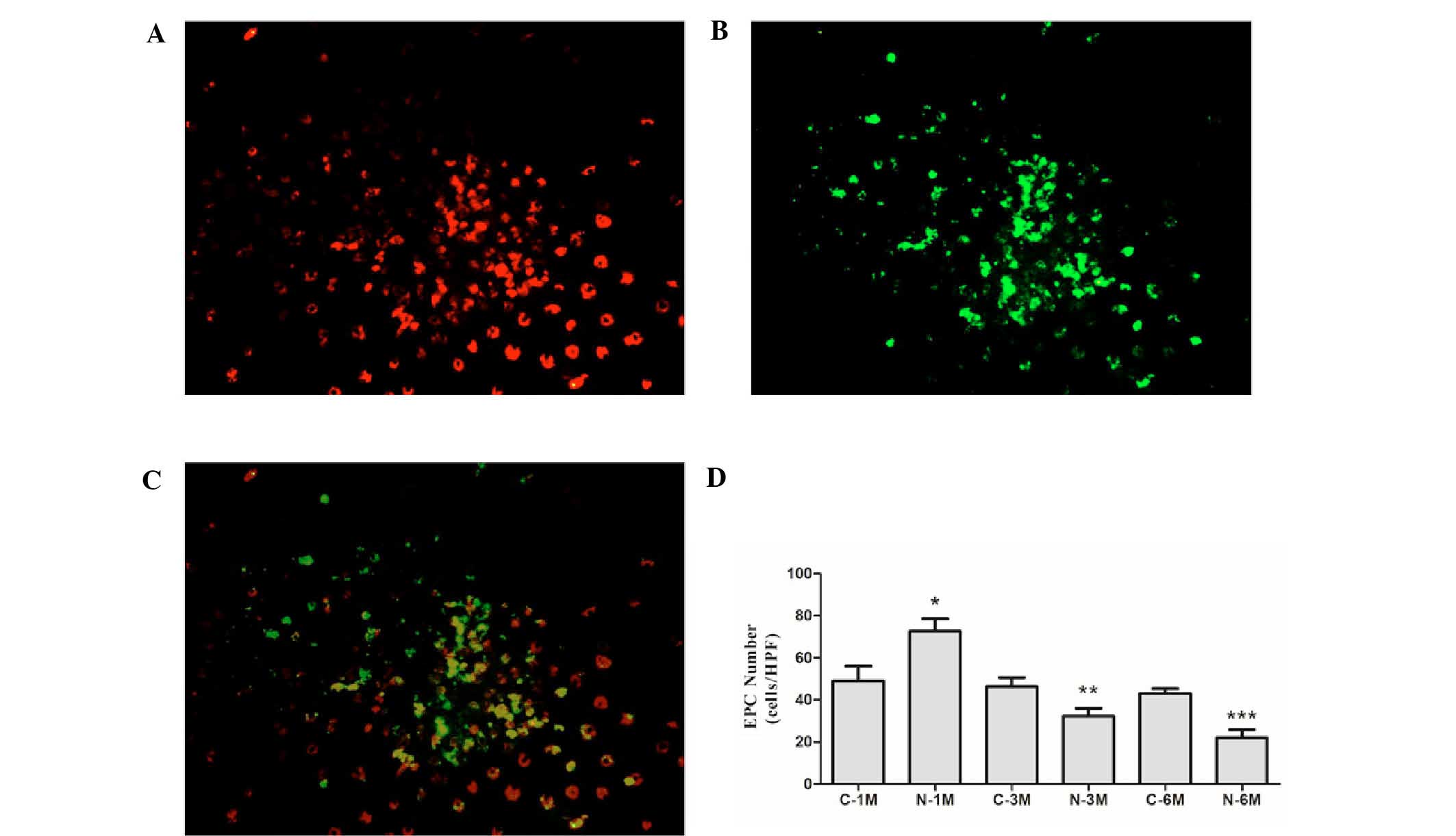
EPCs derived from mice in the different groups were
cultured in the same conditions for 7 days. At this stage, the
percentage of cells that double positive for the uptake of
DiI-ac-LDL and binding of UEA-1 were 85.3±3.4, 84.2±2.6, 82.9±3.8,
86.1±4.3, 79.6±3.5 and 82.3±5.9% in C-1M, N-1M, C-3M, N-3M, C-6M
and N-6M groups, respectively (Fig.
1A-C). There was no significant difference in the number of
EPCs between the control groups at the different time points.
Short-term nicotine exposure (1 month) significantly increased the
EPC number; however, as duration of nicotine exposure increased,
the EPC number was gradually reduced compared with the
corresponding control group (Fig.
1D).
Long-term nicotine exposure reduces
EPC proliferation and migration
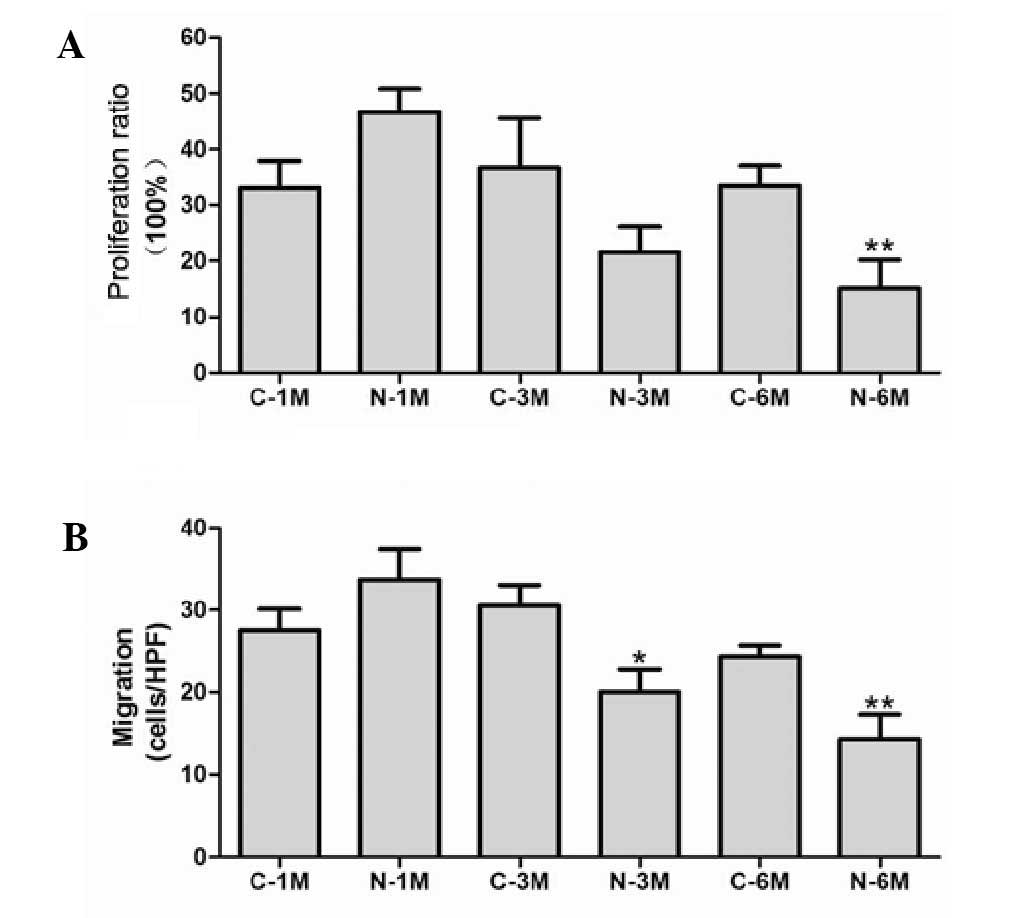
The effect of nicotine on EPC proliferation was
evaluated by BrdU incorporation assay. Short-term nicotine exposure
non-significantly increased EPC proliferation compared with the
matched control group. However, EPC proliferative activity was
significantly reduced compared with the corresponding control group
following exposure to nicotine for 6 months (Fig. 2A). Next, migratory activity of EPCs
was determined using a modified Boyden chamber assay. There was no
significant difference between the number of migrating cells in
control groups, however, long-term nicotine exposure (6 months)
significantly reduced EPC migration (Fig. 2B).
Long-term nicotine exposure promotes
EPC senescence
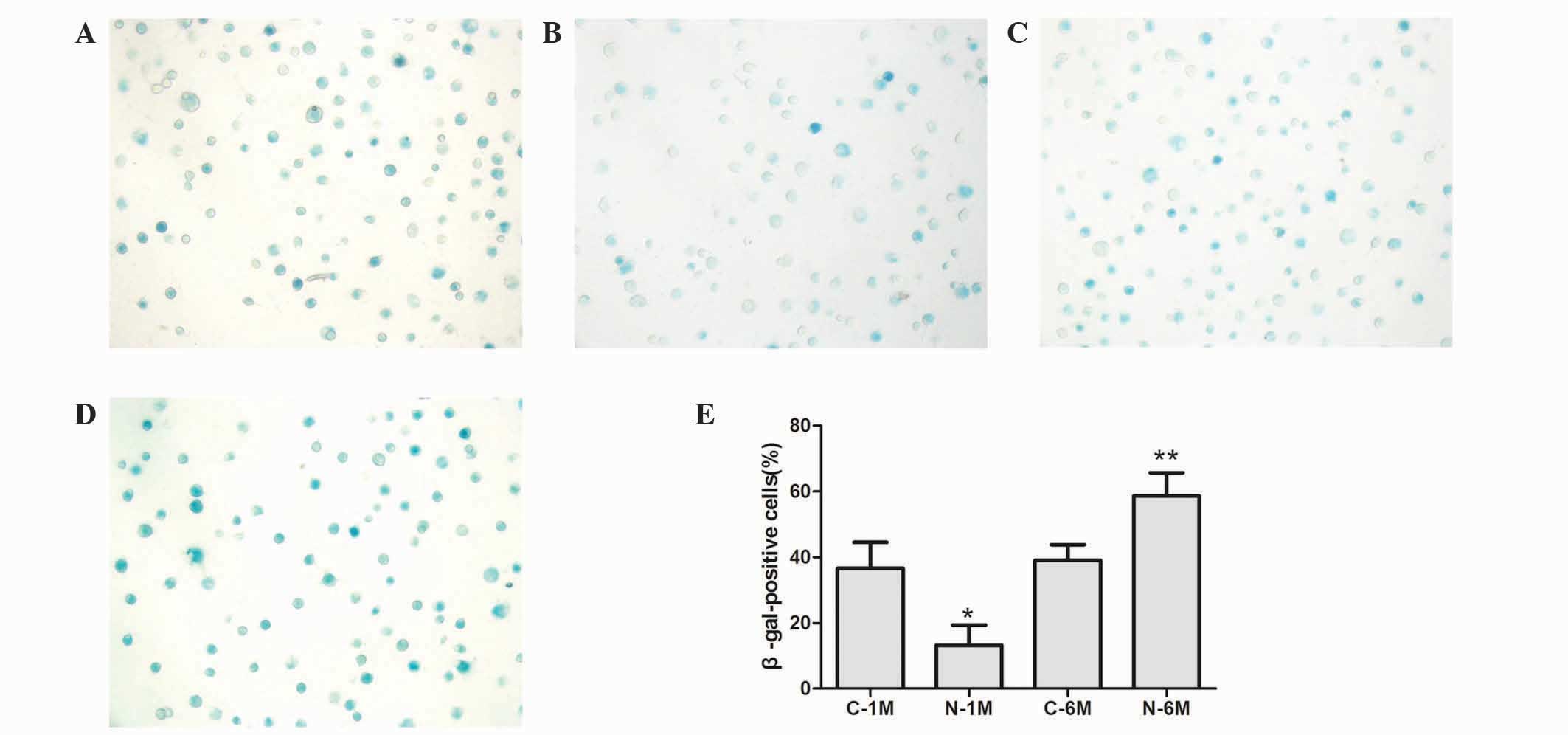
To assess EPC senescence, cells were cultured for 14
days and β-galactosidase was detected to determine the onset of
cellular senescence. Short-term nicotine exposure significantly
decreased the number of β-gal-positive, senescent cells compared
with the control group; however, long-term exposure to nicotine
significantly increased the number of β-gal-positive, senescent
cells (Fig. 3).
Long-term nicotine exposure reduces
EPC telomerase activity
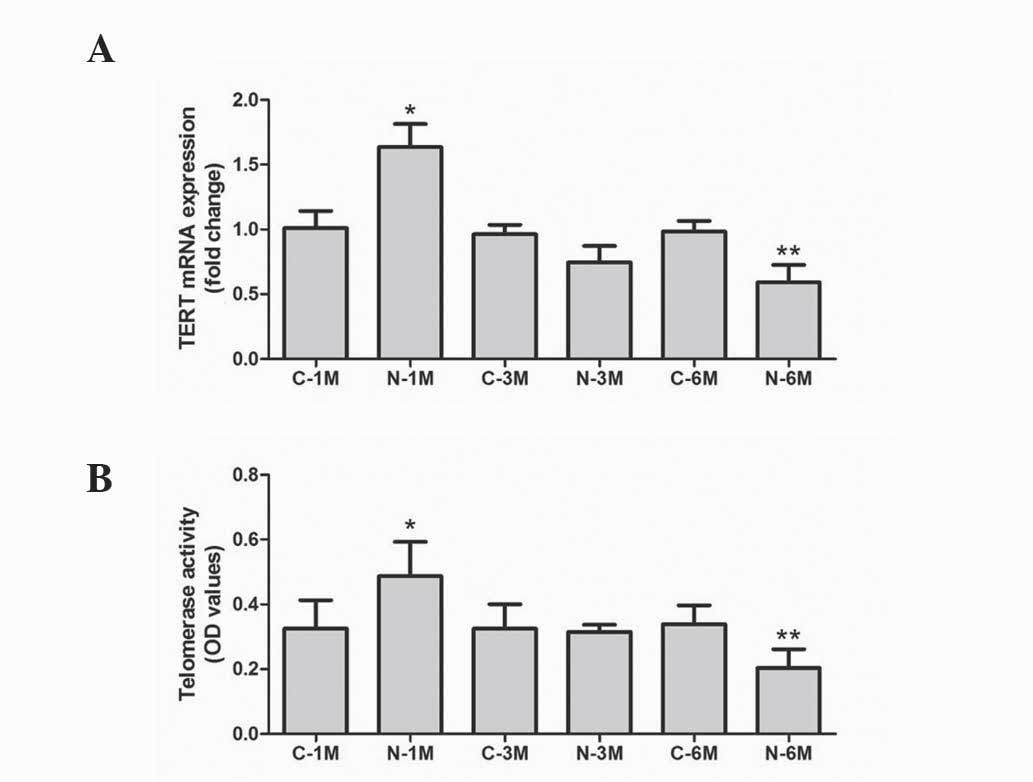
In the present study, the mRNA level of TERT was
used to evaluate telomerase activity. As reported in Fig. 4A, expression of TERT was similar
across the control groups. Short-term exposure to nicotine
significantly increased TERT mRNA expression, but long-term
exposure decreased this compared with corresponding control groups.
Furthermore, telomerase activity was also determined, using a
TeloTAGGG Telomerase PCR ELISA kit. As demonstrated in Fig. 4B, short-term exposure to nicotine
significantly increased the telomerase activity but long-term
exposure decreased this when compared with matched control groups.
These results indicate that long-term nicotine exposure reduces EPC
telomerase activity.
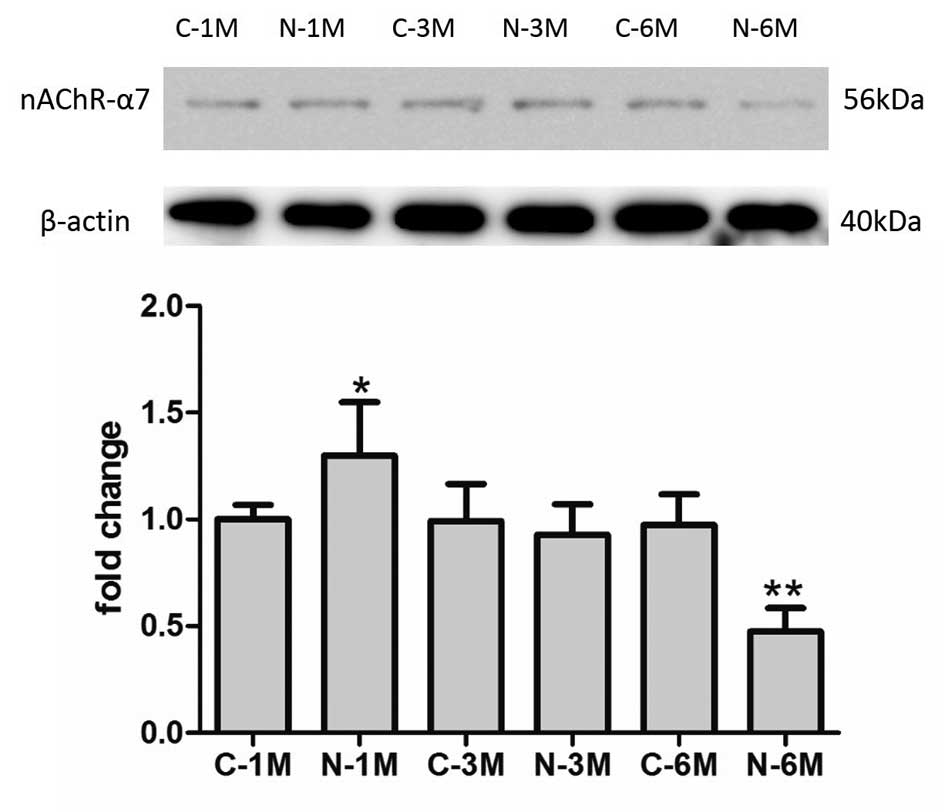
Long-term nicotine exposure reduces
the expression of nAChRs
The effect of nicotine is reliant on nAChR activity.
In the current study, the expression of nAChR-α7 was detected by
western blotting in EPCs. As demonstrated in Fig. 5, short-term nicotine exposure
increased the expression of nAChR-α7 compared with the
corresponding control group. However, following long-term exposure
to nicotine, the expression of nAChR-α7 decreased compared with
expression in control group mice.
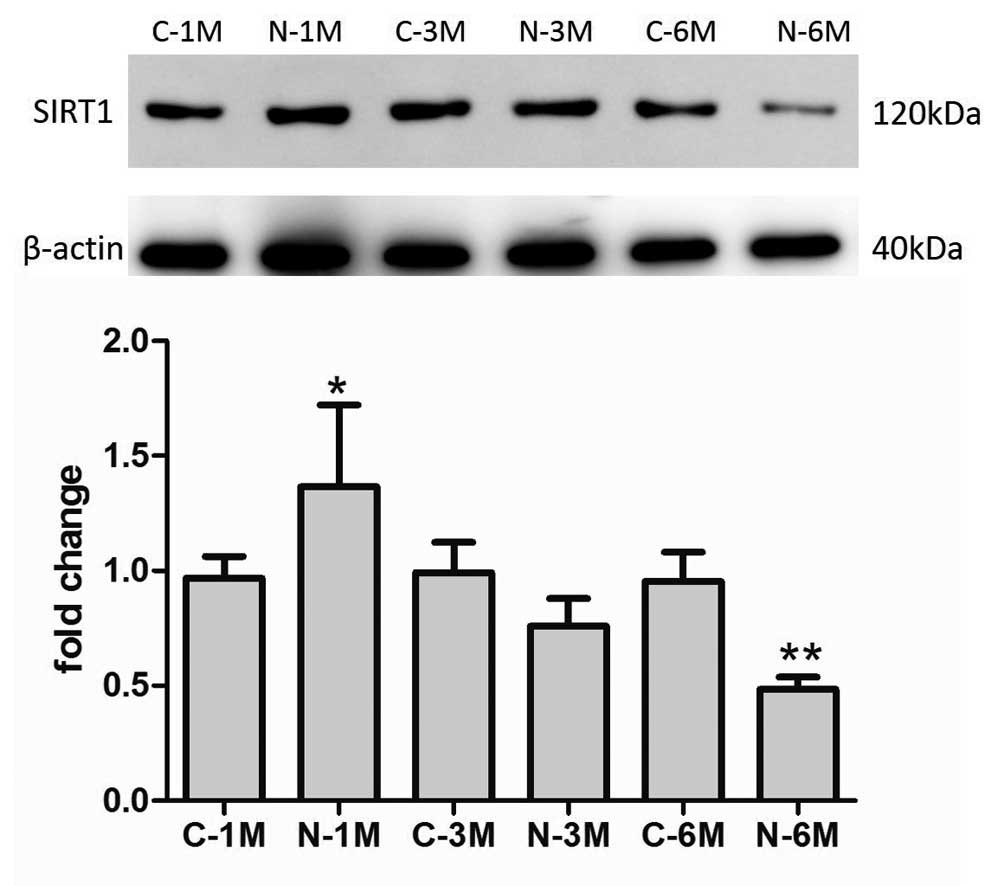
Long-term nicotine exposure reduced
expression of SIRT1 protein
SIRT1 protein exerts protective effects against EPC
senescence (18). In the present
study, the effects of nicotine exposure on SIRT1 expression were
examined. As indicated in Fig. 6,
the expression of SIRT1 increased compared with that of control
group mice following short-term nicotine exposure; however,
subsequent to increased duration of nicotine exposure, the
expression of SIRT1 decreased compared with the matched control
group.
Discussion
EPC dysfunction is common in chronic smokers, but
increasing evidence suggests that short-term nicotine exposure
presents benefits to EPCs (12). In
the present study, the long-term effects of nicotine on EPCs were
investigated. Short-term nicotine exposure was revealed to increase
EPC number and to inhibit EPC senescence. However, long-term
exposure to nicotine reduced EPC number and impaired EPC
proliferation and migration, in addition to promoting EPC
senescence. These effects appear to correlate with the change in
SIRT1 protein expression.
Cigarette smoke is a complex mixture of >4,000
chemical constituents. The most impactful constituents of these are
nicotine, carbon monoxide, aldehydes and sulfur compounds (19). Nicotine was previously demonstrated
to have complex pharmacological effects on endothelial cells, and
most of these effects were deleterious. For example, a previous
study investigating coronary heart disease identified smoking as
one of the five key risk factors in development of atherosclerosis
(20). In animal models, chronic
nicotine treatment also increased the rate of development of
atherosclerosis in arteries (21).
Furthermore, nicotine in similar concentrations to those
experienced by smokers impaired the structure and function of
vascular smooth muscle and endothelial cells (22,23).
Notably, a previous study also reported that short-term exposure to
nicotine enhanced EPC function. In the present study, it was
similarly demonstrated that short-term nicotine exposure increased
EPC number, consistent with the previous report. However,
short-term nicotine exposure did not affect the proliferation and
migration of these cells. Contrastingly, long-term nicotine
exposure significantly decreased EPC number and impaired the
proliferation and migration of EPCs.
The exposure time difference may explain the
conflicting effects of nicotine on EPCs. Short-term nicotine
exposure may act as a stimulus, promoting EPC functions such as
migration, chemoattraction and proliferation (11). These effects have been observed in
some pathological conditions; for example, patients undergoing
percutaneous coronary intervention demonstrated increases in
circulating EPC number (24,25). In the present study, it was
contrastingly demonstrated that long-term nicotine exposure
impaired EPC function. This impairment was similar to that observed
in relation to other risk factors to EPCs, such as cholesterol
(26), proinflammatory factors and
reactive oxygen species, which result in EPC dysfunction through
their chronic effects on these cells (27).
Besides the effects on the proliferative and
migratory capacity of EPCs, long-term nicotine exposure also
accelerated EPC senescence. Telomerase length and activity is a
recognized marker of the cellular life span (28). Factors such as oxidization of low
density lipoprotein, angiotensin II activity and high glucose are
established to be involved in promoting EPC senescence by
inhibiting telomerase activity (29–31).
These findings are consistent with the results in the present
study: Short-term nicotine exposure increased the telomerase
activity and resulted in decreased numbers of senescent cells,
whilst long-term nicotine exposure resulted in the opposite effect.
These results suggested that the mechanism by which nicotine
exposure affects EPCs senescence appears to involve telomerase
activity.
It is established that the effects of nicotine
depend on the signaling via nAChRs. Therefore, the current study
evaluated the expression of nAChR-α7 in EPCs, revealing that
short-term nicotine exposure increases the expression of nAChR-α7,
but that long-term exposure decreased expression of these
receptors. It is hypothesized that this may be a negative feedback
mechanism to alleviate the detrimental effects of nicotine.
However, additional research is required to determine the specific
association between nicotine exposure and the expression of
nAChR-α7 and other nAChRs.
In order to investigate the mechanisms by which
nicotine affected telomerase activity, the expression of SIRT1 was
evaluated in this study. Previous studies have demonstrated that
SIRT1 exerts protective effects following stress-induced
endothelial cell (32–34) and EPC (18,35)
senescence. In the present study, nicotine exposure similarly
affected the expression of SIRT1 protein, which was correlated and
consistent with alterations to telomerase activity and EPC
senescence. These results suggested that the effects of nicotine
exposure on telomerase activity was mediated, at least in part, by
regulation of SIRT1 expression.
In conclusion, the results of the present study
suggest that long-term nicotine exposure induces dysfunction and
senescence of EPCs, which may be associated with impairment of
telomerase activity through the downregulation of SIRT1. These
results additionally emphasize the necessity of smoking cessation
in order to prevent EPC dysfunction and other tobacco-associated
disease.
References
|
1
|
Urbich C and Dimmeler S: Endothelial
progenitor cells: Characterization and role in vascular biology.
Circ Res. 95:343–353. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Werner N, Kosiol S, Schiegl T, Ahlers P,
Walenta K, Link A, Böhm M and Nickenig G: Circulating endothelial
progenitor cells and cardiovascular outcomes. N Engl J Med.
353:999–1007. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Aicher A, Zeiher AM and Dimmeler S:
Mobilizing endothelial progenitor cells. Hypertension. 45:321–325.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Vasa M, Fichtlscherer S, Aicher A, Adler
K, Urbich C, Martin H, Zeiher AM and Dimmeler S: Number and
migratory activity of circulating endothelial progenitor cells
inversely correlate with risk factors for coronary artery disease.
Circ Res. 89:E1–E7. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tepper OM, Galiano RD, Capla JM, Kalka C,
Gagne PJ, Jacobowitz GR, Levine JP and Gurtner GC: Human
endothelial progenitor cells from type II diabetics exhibit
impaired proliferation, adhesion, and incorporation into vascular
structures. Circulation. 106:2781–2786. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ezzati M, Henley SJ, Thun MJ and Lopez AD:
Role of smoking in global and regional cardiovascular mortality.
Circulation. 112:489–497. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Powell JT: Vascular damage from smoking:
Disease mechanisms at the arterial wall. Vasc Med. 3:21–28. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bondjers G, Hansson G, Olsson G and
Pettersson K: Smoking, catecholamines and their effects on
endothelial cell integrity. Adv Exp Med Biol. 273:51–59. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kondo T, Hayashi M, Takeshita K, Numaguchi
Y, Kobayashi K, Iino S, Inden Y and Murohara T: Smoking cessation
rapidly increases circulating progenitor cells in peripheral blood
in chronic smokers. Arterioscler Thromb Vasc Biol. 24:1442–1447.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Puls M, Schroeter MR, Steier J, Stijohann
L, Hasenfuss G, Konstantinides S and Schäfer K: Effect of smoking
cessation on the number and adhesive properties of early outgrowth
endothelial progenitor cells. Int J Cardiol. 152:61–69. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang X, Zhu J, Chen J and Shang Y: Effects
of nicotine on the number and activity of circulating endothelial
progenitor cells. J Clin Pharmacol. 44:881–889. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sugimoto A, Masuda H, Eguchi M, Iwaguro H,
Tanabe T and Asahara T: Nicotine enlivenment of blood flow recovery
following endothelial progenitor cell transplantation into ischemic
hindlimb. Stem Cells Dev. 16:649–656. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Junhui Z, Xiaojing H, Binquan Z, Xudong X,
Junzhu C and Guosheng F: Nicotine-reduced endothelial progenitor
cell senescence through augmentation of telomerase activity via the
PI3K/Akt pathway. Cytotherapy. 11:485–491. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
National Institutes of Health (NIH), .
Guide for the Care and Use of Laboratory Animals. 7th. NIH;
Bethesda, MD, USA: Publication No. 85–23. 1996
|
|
15
|
Li W, Wang H, Kuang CY, Zhu JK, Yu Y, Qin
ZX, Liu J and Huang L: An essential role for the
Id1/PI3K/Akt/NFkB/survivin signalling pathway in promoting the
proliferation of endothelial progenitor cells in vitro. Mol Cell
Biochem. 363:135–145. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Reed J, Gunaratnam M, Beltran M, Reszka
AP, Vilar R and Neidle S: TRAP-LIG, a modified telomere repeat
amplification protocol assay to quantitate telomerase inhibition by
small molecules. Anal Biochem. 380:99–105. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Vassallo PF, Simoncini S, Ligi I, Chateau
AL, Bachelier R, Robert S, Morere J, Fernandez S, Guillet B,
Marcelli M, et al: Accelerated senescence of cord blood endothelial
progenitor cells in premature neonates is driven by SIRT1 decreased
expression. Blood. 123:2116–2126. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Stedman RL: The chemical composition of
tobacco and tobacco smoke. Chem Rev. 68:153–207. 1968. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kannel WB, McGee D and Gordon T: A general
cardiovascular risk profile: The Framingham Study. Am J Cardiol.
38:46–51. 1976. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Strohschneider T, Oberhoff M, Hanke H,
Hannekum A and Karsch KR: Effect of chronic nicotine delivery on
the proliferation rate of endothelial and smooth muscle cells in
experimentally induced vascular wall plaques. Clin Investig.
72:908–912. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Leake DS and Bowyer DE: Quantitative
studies of pinocytosis by arterial endothelial and smooth muscle
cells in culture. Exp Mol Pathol. 35:84–97. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zimmerman M and McGeachie J: The effect of
nicotine on aortic endothelial cell turnover. An autoradiographic
study. Atherosclerosis. 58:39–47. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Banerjee S, Brilakis E, Zhang S, Roesle M,
Lindsey J, Philips B, Blewett CG and Terada LS: Endothelial
progenitor cell mobilization after percutaneous coronary
intervention. Atherosclerosis. 189:70–75. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hristov M, Zernecke A, Liehn EA and Weber
C: Regulation of endothelial progenitor cell homing after arterial
injury. Thromb Haemost. 98:274–277. 2007.PubMed/NCBI
|
|
26
|
Hill JM, Zalos G, Halcox JP, Schenke WH,
Waclawiw MA, Quyyumi AA and Finkel T: Circulating endothelial
progenitor cells, vascular function and cardiovascular risk. N Engl
J Med. 348:593–600. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lin CP, Lin FY, Huang PH, Chen YL, Chen
WC, Chen HY, Huang YC, Liao WL, Huang HC, Liu PL and Chen YH:
Endothelial progenitor cell dysfunction in cardiovascular diseases:
Role of reactive oxygen species and inflammation. Biomed Res Int.
2013:8450372013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Counter CM: The roles of telomeres and
telomerase in cell life span. Mutat Res. 366:45–63. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Imanishi T, Hano T, Sawamura T and Nishio
I: Oxidized low-density lipoprotein induces endothelial progenitor
cell senescence, leading to cellular dysfunction. Clin Exp
Pharmacol Physiol. 31:407–413. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Imanishi T, Hano T and Nishio I:
Angiotensin II accelerates endothelial progenitor cell senescence
through induction of oxidative stress. J Hypertens. 23:97–104.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kuki S, Imanishi T, Kobayashi K, Matsuo Y,
Obana M and Akasaka T: Hyperglycemia accelerated endothelial
progenitor cell senescence via the activation of p38
mitogen-activated protein kinase. Circ J. 70:1076–1081. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ota H, Akishita M, Eto M, Iijima K, Kaneki
M and Ouchi Y: Sirt1 modulates premature senescence-like phenotype
in human endothelial cells. J Mol Cell Cardiol. 43:571–579. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zu Y, Liu L, Lee MY, Xu C, Liang Y, Man
RY, Vanhoutte PM and Wang Y: SIRT1 promotes proliferation and
prevents senescence through targeting LKB1 in primary porcine
aortic endothelial cells. Circ Res. 106:1384–1393. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wan YZ, Gao P, Zhou S, Zhang ZQ, Hao DL,
Lian LS, Li YJ, Chen HZ and Liu DP: SIRT1-mediated epigenetic
downregulation of plasminogen activator inhibitor-1 prevents
vascular endothelial replicative senescence. Aging Cell.
13:890–899. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lemarié CA, Shbat L, Marchesi C, Angulo
OJ, Deschênes ME, Blostein MD, Paradis P and Schiffrin EL: Mthfr
deficiency induces endothelial progenitor cell senescence via
uncoupling of eNOS and downregulation of SIRT1. Am J Physiol Heart
Circ Physiol. 300:H745–H753. 2011. View Article : Google Scholar : PubMed/NCBI
|