Introduction
In recent years, studies based on animal and
clinical trials have demonstrated the potential value of bone
marrow-derived mesenchymal stem cell (BM-MSC) transplantation in
augmenting angiogenesis of ischemic tissue, such as in myocardial
infarction, stroke and skin flaps (1–5). In
ischemic tissue, oxygen concentration markedly decreases, and
influences the biological behavior of engrafted cells directly
(6–8).
BM-MSCs are multipotent cells that can be induced to
terminally differentiate into multiple lineages and secrete various
cytokines, such as vascular endothelial growth factor (VEGF),
epidermal growth factor and insulin-like growth factor (9,10). In
vivo, BM-MSCs are located near bone surfaces and perivascular
niches, both of which have low levels of oxygen supply (11,12).
Therefore, oxygen tension is currently recognized as a crucial
component of the stem-cell ‘niche’ that maintains the proliferative
capacity and functions of BM-MSCs. The effect of hypoxic culture
conditions may decrease the cell expansion time and induce the
differentiation of BM-MSCs when compared with the standard
protocols (13,14). In addition, BM-MSCs paracrine more
angiogenesis-associated cytokines subsequent to culturing under
hypoxic conditions, including basic fibroblast growth factor
(bFGF), VEGF, interleukin-6 (IL-6) and IL-8 (15). To date, the mechanism through which
hypoxia regulates self-renewal, differentiation and paracrine of
BM-MSCs remains unclear.
The phosphatidylinositol 3-kinases (PI3Ks) and their
downstream target AKT are a conserved family of signal transduction
enzymes that has been investigated extensively for its roles in
cell proliferation, cell transformation, paracrine function and
angiogenesis (16–18). Therefore, in the present study, the
activation of PI3K/AKT pathway in BM-MSCs cultured under hypoxic
conditions was detected. In addition, the PI3K/AKT pathway-mediated
cellular responses were examined, including proliferation,
differentiation into endothelial cells and paracrine function.
Materials and methods
Cell culture
All the animal procedures were approved under the
guidelines of Shanghai Jiao Tong University Medical Center and the
Institutional Animal Care and Use Committee (Shanghai, China). Ten
male Wistar rats (3-week-old; weight, 25–30 g) were sacrificed with
3% sodium pentobarbital (Xinya, Inc., Shanghai, China). Next, bone
marrow (BM) cells were flushed with 2 ml Dulbecco's modified
Eagle's medium (DMEM; Gibco-BRL; Thermo Fisher Scientific, Inc.,
Grand Island, NY, USA) from the femur and tibia of the rats. To
remove the red blood cells from the BM cells, red blood cell lysis
buffer (Sigma-Aldrich, St. Louis, MO, USA) was added for 10 min,
and centrifuged at 800 × g for 5 min at room temperature.
Then, 1×10 6 remaining BM cells were plated in a 100 mm
dish with 10 ml DMEM supplemented with 10% fetal bovine serum
(Gibco-BRL; Thermo Fisher Scientific, Inc.) and 1%
penicillin/streptomycin (Sigma-Aldrich) and cultivated in a
humidified atmosphere with 20% O2 and 5% CO2
at 37°C (Thermo Scientific BBD 6220 CO2 incubator;
Omnilab, Bremen, Germany). After 3 days of culture, the medium and
non-adherent cells were replaced, while adherent BM-MSCs were
further grown in fresh medium. When 80–90% confluence was reached,
adherent cells were trypsinized and expanded at a dilution of 1:3.
All cells used in the present study were of passages 3 to 4.
Cell morphology and characterization
of BM-MSCs expanded in vitro
The BM-MSCs at passages 3 or 4 were collected and
resuspended in phosphate-buffered saline containing 1% bovine serum
albumin (Sigma-Aldrich) at 1×106 cells/ml. Cell aliquots
were incubated with phycoerythrin-conjugated mouse anti-rat cluster
of differentiation (CD)90 at a 1:200 dilution (cat. no. 554898, BD
Biosciences, San Jose, CA, USA) and CD105 at a 1:150 dilution (cat.
no. 562380, BD Biosciences) and fluorescein
isothiocyanate-conjugated mouse anti-rat CD34 at a 1:100 dilution
(cat. no. 555005; BD Biosciences) and CD29 at a 1:200 dilution
(cat. no. 60942; BD Biosciences) at 37°C for 1 h. Labeled cells
were analyzed by flow cytometry and with FACSDiva Pro software
version 3.0 (BD Biosciences).
Cell treatments
BM-MSCs were plated on culture dish overnight with
complete medium in a humidified atmosphere with 20% O2.
Subsequently, the cells were transferred to be cultured under 2%
O2 in complete medium with or without 25 mM LY294002
(Sigma-Aldrich), which is a commonly used PI3K/AKT signaling
pathway inhibitor (19,20). The cells cultured in complete medium
at 20% O2 served as the control group. In total, there
were three study groups, including the normoxia (control), hypoxia
group and hypoxia+LY294002 groups.
Determination of AKT activation
Western blot analysis was performed in order to
detect the expression levels of AKT and phosphorylated AKT (p-AKT),
since the phosphorylation of AKT represents the activation of the
PI3K/AKT signaling pathway (19,21).
Briefly, 3×105 cells were treated as described above for
7 days, and 3×106 cells were harvested and lysed with
M-PER lysis buffer (Pierce Biotechnology, Inc., Rockford, IL, USA)
followed by centrifugation at 12,000 × g at 4°C for 10 min.
The supernatants were then collected, and protein concentration was
determined using a BCA assay kit (Invitrogen; Thermo Fisher
Scientific, Inc). In all cell groups, 20 mg cellular protein was
resolved to 10% SDS-PAGE and transferred onto polyvinylidene
difluoride membranes. The membranes were washed once with
Tris-buffered saline with 0.1% Tween 20 (TBST) then blocked for 1 h
at room temperature with 5% skim milk in Tris-buffered saline
containing 0.1% Tween 20. Then, membranes were probed with primary
antibodies against p-AKT (1:1,000 dilution; cat. no. 4060; Cell
Signaling Technology, Inc., Danvers, MA, USA), AKT (1:1,500
dilution; cat. no. 4691; Cell Signaling Technology, Inc.) and
β-actin (1:2,000 dilution; cat. no. 3700; Cell Signaling
Technology, Inc.) overnight at 4°C. The membranes were then washed
with TBST three times and incubated horseradish
peroxidase-conjugated mouse anti-rabbit IgG (1:3,000 dilution; cat.
no. 5127; Cell Signaling Technology, Inc.) for 1 h at room
temperature. The samples were then developed using
chemiluminescence substrates (EMD Millipore, Billerica, MA, USA).
Images of the membranes were captured using a Bio-Rad ChemiDoc XRS
system (Bio-Rad Laboratories, Inc., Hercules, CA, USA), and
quantified and analyzed using the Quantity One software (version
16.0; Bio-Rad Laboratories, Inc.).
Cell proliferation assay
Cell proliferation was assessed by cell counting
kit-8 (CCK-8) assay (Sigma-Aldrich) according to the manufacturer's
instructions. Briefly, BM-MSCs were seeded in a 96-well plate at a
density of 3,000 cells/well and treated under different conditions,
as described earlier. Subsequently, the cells were incubated with
CCK-8 solution for 2 h at 37°C. Absorbance of each well was
measured at 450 nm. The results were presented as the ration
OD450 of treated cells / OD450 of control
cells. Three independent experiments were performed.
Immunofluorescence staining
In order to investigate the expression of CD31 on
the cell surface in the various study groups, the treated cells
were grown on glass coverslips and fixed with 4% paraformaldehyde.
The cells (1×104) were then blocked with 10% bovine
serum albumin at 37°C for 1 h and incubated with rabbit anti-rat
CD31 antibody (1:100 dilution; cat. no. ab32457; Abcam, Cambridge,
UK) at 4°C overnight. Subsequent to washing, the cells were
incubated with the Alexa Fluor 555-conjugated goat anti-rabbit IgG
(1:100 dilution; cat. no. sc-3739; Santa Cruz Biotechnology, Inc.,
Dallas, TX, USA) for 1 h at 37°C. The nuclei of cells were then
counterstained with DAPI (Abcam). Fluorescence images of the cells
were acquired using a fluorescence microscope. The number of
CD31-positive cells in 10 random fields of view in the three groups
was counted in order to perform statistical analysis.
Gene expression determination
Quantitative polymerase chain reaction (qPCR) was
perform to detect the expression of specific genes of endothelial
cells, including fms related tyrosine kinase 1 (Flt-1), fetal liver
kinase 1 (Flk-1), von Willebrand factor (vWF) and vascular
endothelial (VE)-cadherin. In addition, qPCR was used to measure
the gene expression of VEGF, which is the most important
angiogenesis-associated cytokine (22). Following appropriate treatment for 7
days, 1×106 cells were collected from each group, and
total RNA was prepared from the cells using TRIzol reagent
(Invitrogen; Thermo Fisher Scientific, Inc.) according to the
manufacturer's instructions. Next, the total RNA was
reverse-transcribed into complementary DNA using GeneAmp RNA PCR
Core kit (Applied Biosystems; Thermo Fisher Scientific, Inc.,
Foster City, CA, USA). Quantitative gene expression was
subsequently determined with the Mastercycler Realplex S instrument
(Eppendorf, Hamburg, Germany) with the SYBR Green Realtime PCR
Master Mix (Toyobo, Osaka, Japan). The reaction was performed in a
20 µl system, including the following: 10 µl SYBR Premix Ex
Taq II, 1 µl cDNA, 2 µl primers, and 7 µl ultrapure water. All
primers were designed using primer 5.0 and synthesized by Shenggong
Biotech Co., Ltd. (Shanghai, China). The gene primer sequences are
shown in Table I. PCR amplification
was performed as follows: One cycle of denaturation at 94°C for 4
min, followed by 40 cycles of denaturation at 94°C for 30 sec,
annealing at the appropriate temperature for 30 sec, and
extension/fluorescence acquisition at 72°C for 30 sec. Absolute
gene transcription was normalized to GAPDH. The relative expression
level of the target mRNA was plotted as a fold change compared with
the control using the 2-ΔΔCq method (23).
 | Table I.Primer sequences and product size. |
Table I.
Primer sequences and product size.
| Gene | Primer sequence
(5′→3′) | Product size
(bp) |
|---|
| Flk-1 | Sense:
5′-CCCGCACGAATGATATCCCA-3′ | 136 |
|
| Anti-sense:
5′-TCCTGCAGTGCATAACCTGG-3′ |
|
| Flt-1 | Sense:
5′-ATCCCTCAGCCTACCATCAA-3′ | 303 |
|
| Anti-sense:
5′-AAAGCCGTTTGGCACATCT-3′ |
|
| vWF | Sense:
5′-GATGACCCTGATGCTGTCTG-3′ | 153 |
|
| Antisense:
5′-GTCTCCCTTGTTGCCATTGT-3′ |
|
| VE-cadherin | Sense:
5′-CGCTTCTACCACTTCCACCT-3′ | 305 |
|
| Anti-sense:
5′-GCGTTGTCATTCTCATCCAA-3′ |
|
| VEGF | Sense:
5′-CAGCGACAAGGCAGACTATT-3′ | 151 |
|
| Antisense:
5′-GTTGGCACGATTTAAGAGGG-3′ |
|
| GAPDH | Sense:
5′-CTCATGGCCTACATGGCCTC-3′ | 70 |
|
| Antisense:
5′-CTCATGGCCTACATGGCCTC-3 |
|
Statistical analysis
All values were expressed as the mean ± standard
deviation. Analysis of variance with Dunnett's test was used to
determine statistically significant differences in multiple
comparisons, which were indicated by values of P<0.05. All
statistical analyses were performed using SPSS software, version
16.0 (SPSS, Inc., Chicago, IL, USA).
Results
Characterization of BM-MSCs
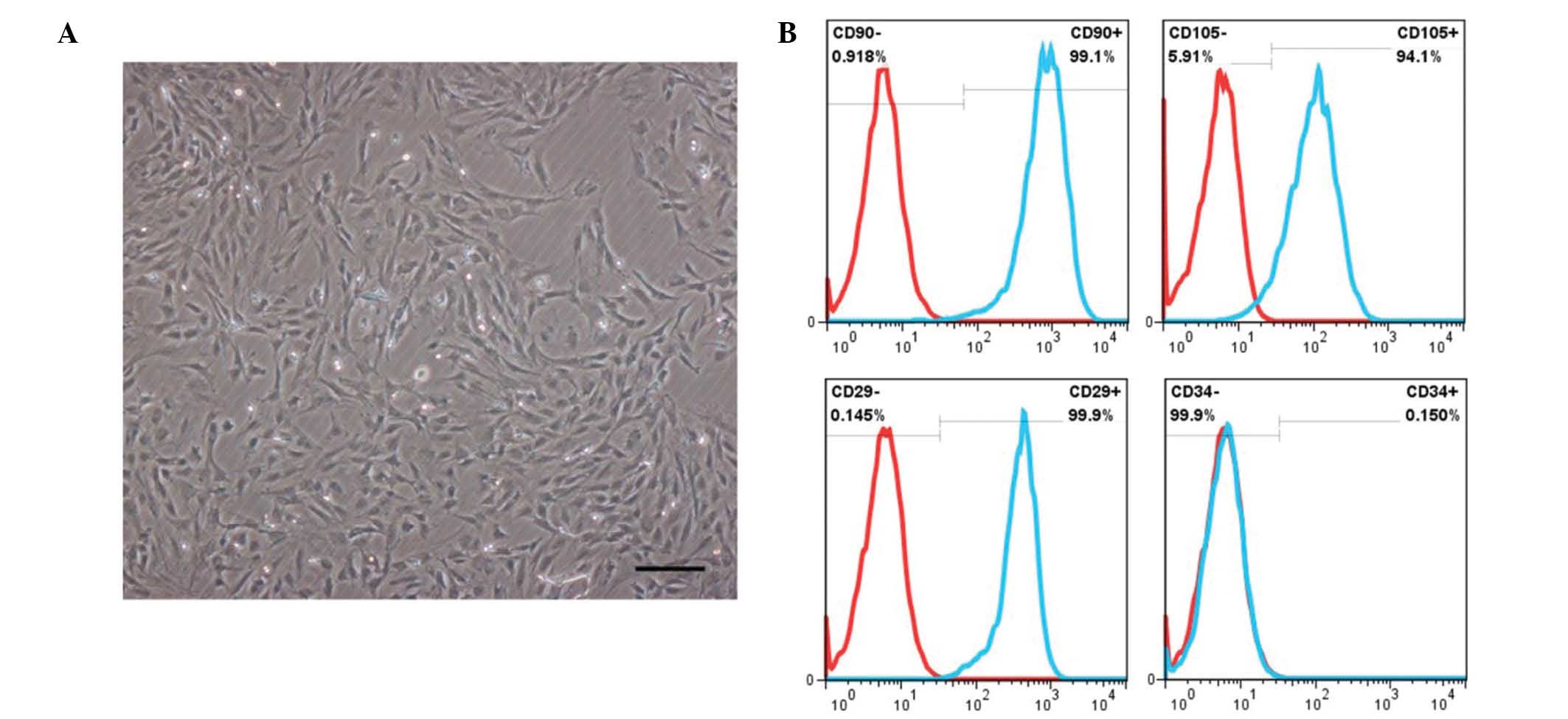
As shown in Fig. 1A,
rat BM-MSCs at passage 3 or 4 were demonstrated to have an
elongated fibroblast-like morphology. Fluorescence-activated cell
sorting analysis of BM-MSCs revealed that the majority of cells
were negative for the lineage cell marker CD34, whereas they
strongly expressed typical surface antigens of stem cells,
including CD90, CD105 and CD29 (Fig.
1B).
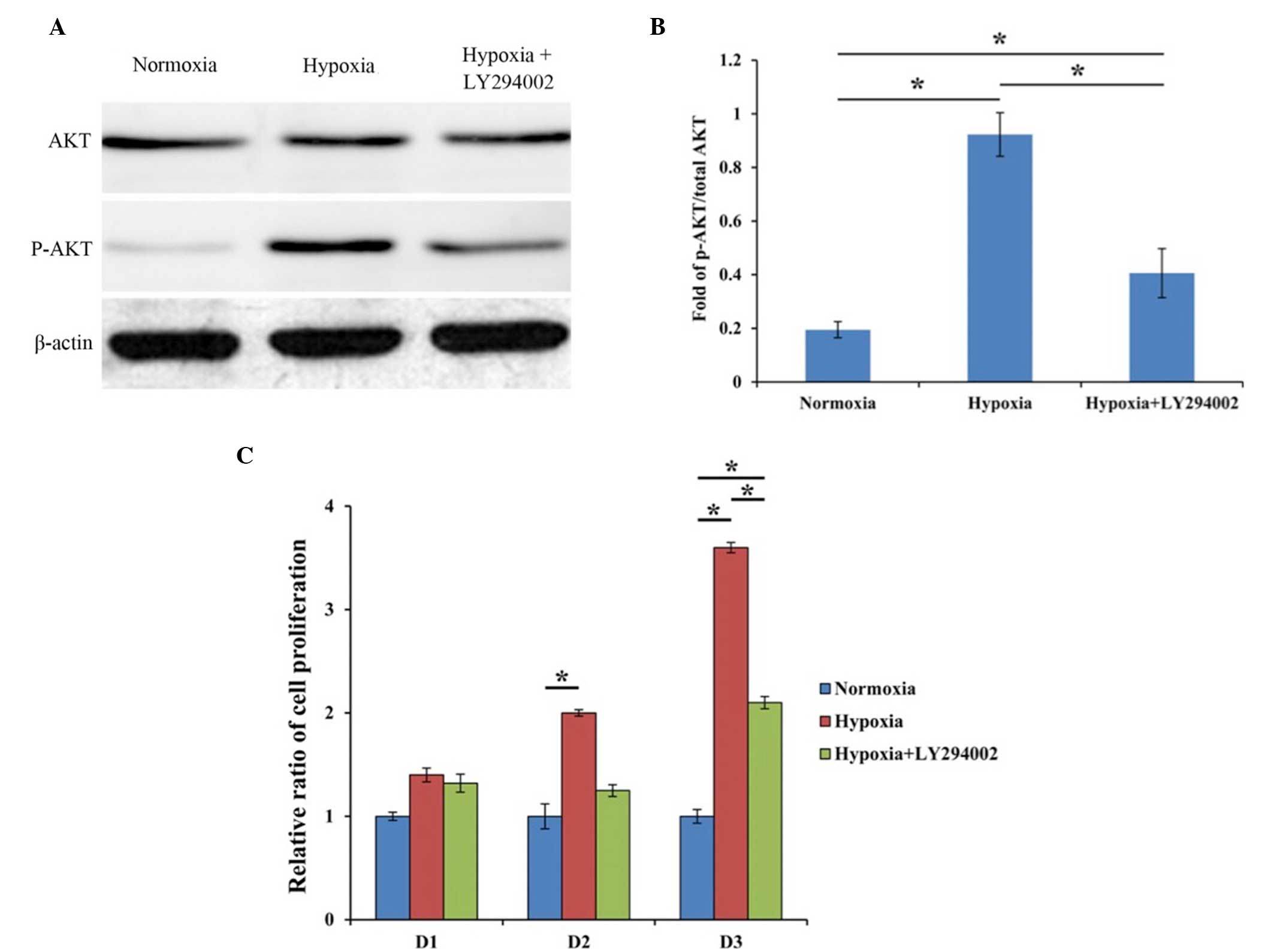
AKT activation detected by western
blot analysis
Western blot analysis was performed to analyze AKT
and p-AKT expression, and the result was displayed as the fold of
p-AKT to total AKT. β-actin was used as an internal control. In the
hypoxia group, higher expression of p-AKT was observed when
compared with the control group (P<0.001), suggesting that the
PI3K/AKT signaling pathway was activated. However, upon the
addition of LY294002, the expression of p-AKT was evidently
decreased as compared with the hypoxia group (P=0.04) (Fig. 2A and B). These findings indicated
that hypoxia was able to effectively activate the PI3K/AKT pathway,
and that LY294002 was a potent inhibitor of the PI3K/AKT pathway,
which is consistent with the results of previous studies (24,25).
Cell proliferation after different
treatments
As shown in Fig. 2C,
the proliferation of cultured BM-MSCs in the hypoxia group at days
2 and 3 was much higher compared with that in the normoxia group
(P=0.030 and P=0.017, respectively). In the hypoxia group treated
with LY294002, the cell proliferation at day 3 remained
significantly higher compared with that in cells cultured in
normoxia (P=0.026). These findings demonstrated that hypoxia was
able to significantly enhance the proliferation of cultured
BM-MSCs, and this effect was partly inhibited by PI3K/AKT pathway
inhibitor (Fig. 2C).
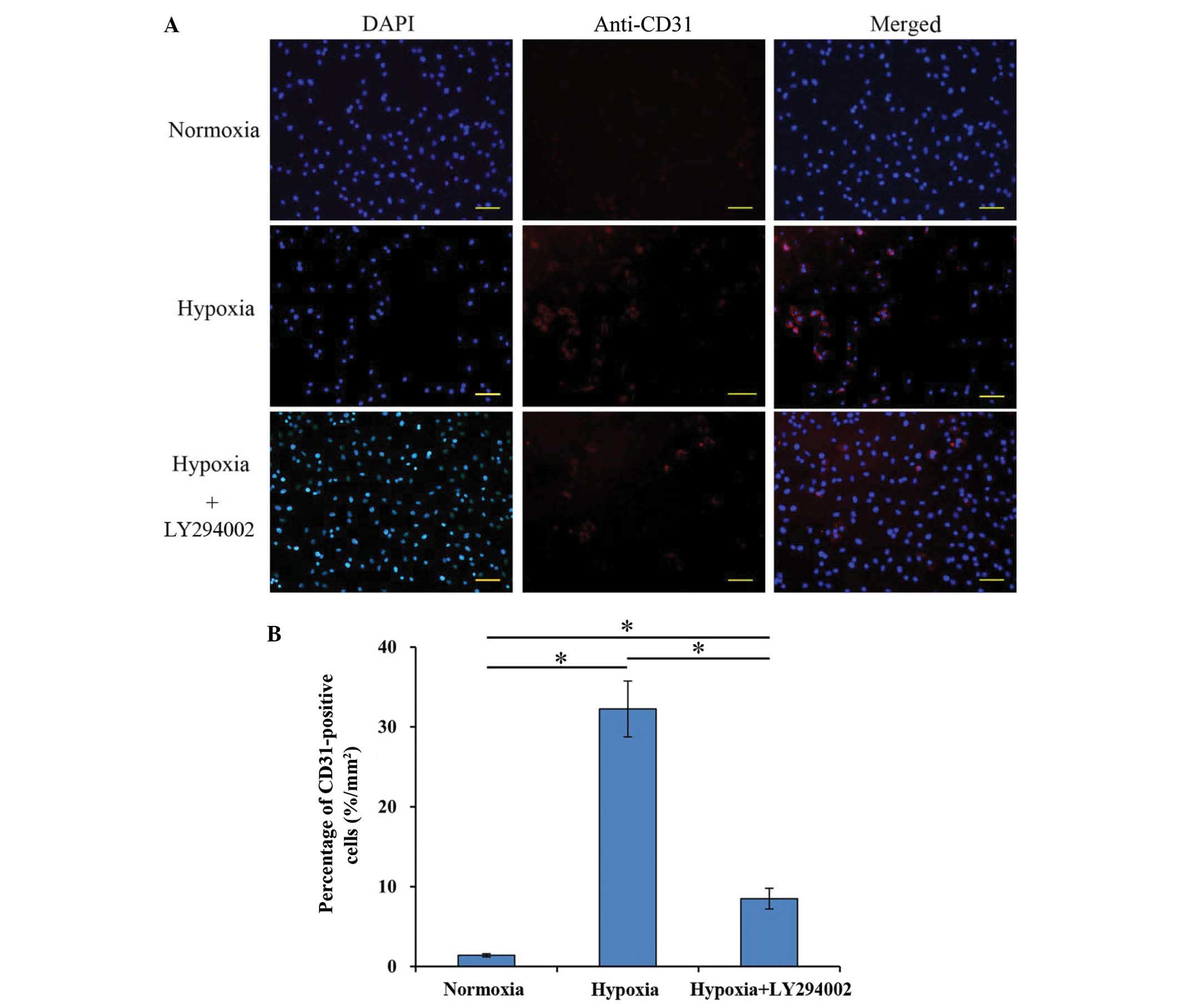
Endothelial cell differentiation of
rat BM-MSCs
After culturing under hypoxia for 7 days,
immunofluorescence staining results showed that approximately
32.25±3.5% BM-MSCs expressed CD31, a known marker of endothelial
cells, which was significantly higher compared with the percentage
in the normoxia group (1.4±0.2%; P<0.001; Fig. 3). In the hypoxia+LY294002 group, the
percentage of differentiated cells decreased to 8.47±1.2%, which
was significantly different compared with both the normoxia
(P=0.035) and hypoxia groups (P=0.024) (Fig. 3).
Hypoxia upregulates the expression of
endothelial cell-specific genes
qPCR analysis was performed to investigate the
relative mRNA expression of endothelial cell-specific genes in the
three study groups. The results demonstrated that hypoxia
significantly upregulated the mRNA expression levels of Flk-1
(4.98-fold), Flt-1 (3.29-fold), vWF (4.76-fold) and VE-cadherin
(5.08-fold) when compared with those in the normoxia group
(Fig. 4A). Following the addition of
LY294002 under hypoxia, the mRNA expression levels decreased for
Flk-1 (2.33-fold), Flt-1 (2.34-fold), vWF (1.52-fold) and
VE-cadherin (3.17-fold) when compared with those in the normoxia
group, with a significant decrease observed for all genes except
vWF. Furthermore, the mRNA expression of these genes was
significantly reduced in the hypoxia+LY294002 group when compared
with that in the hypoxia group, with the exception of Flt-1 that
did not present a significant decrease (Fig. 4A).
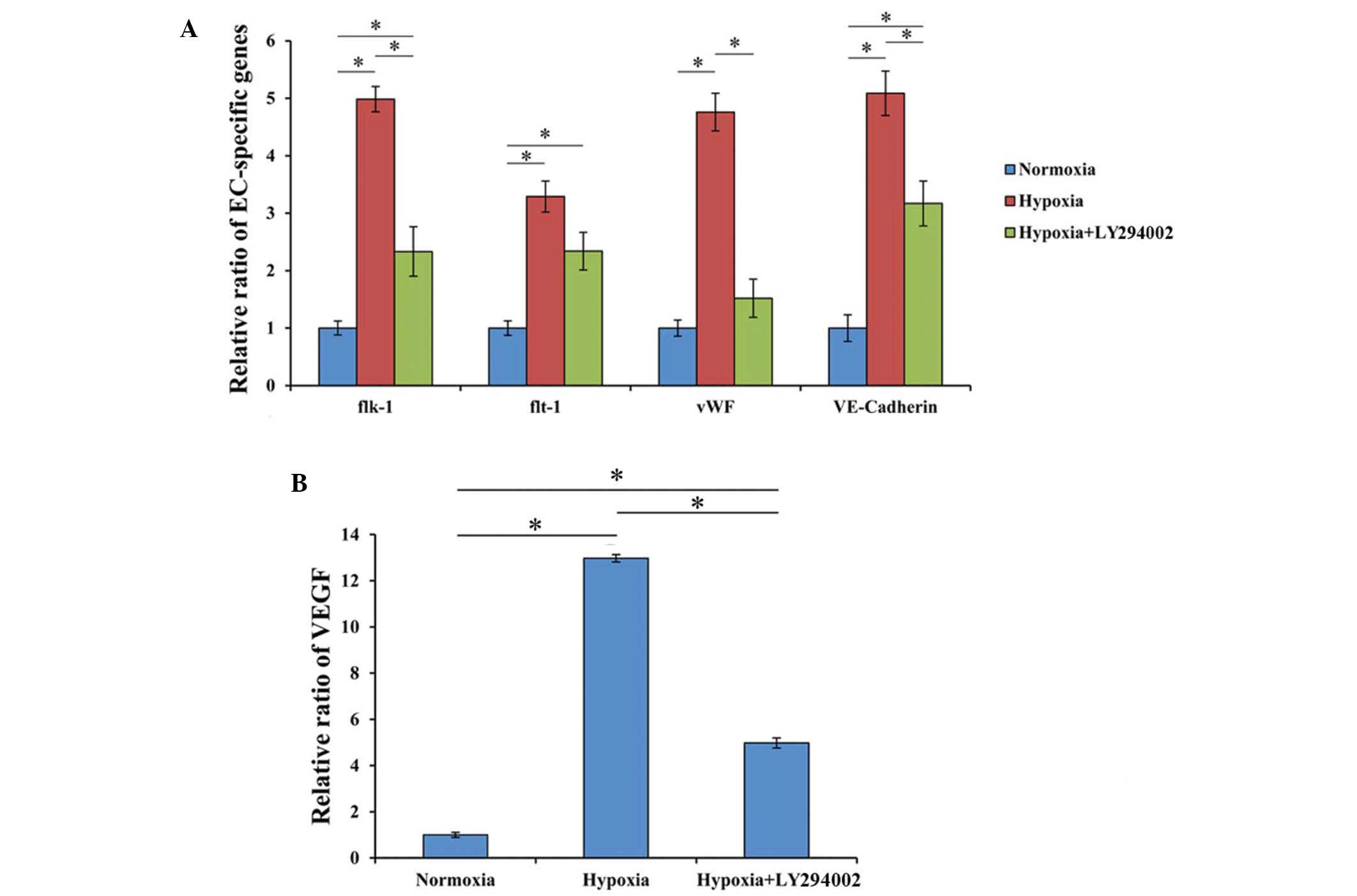 | Figure 4.Expression of endothelial
cell-specific genes and VEGF. (A) Hypoxia significantly increased
the expression of endothelial cell-specific genes in BM-MSCs,
including Flk-1, Flt-1, vWF and VE-cadherin, as compared with the
cells cultured under normoxic conditions. Following inhibition with
LY294002, the hypoxia-induced expression of the endothelial
cell-specific genes was significantly decreased. *P<0.05. (B)
The highest expression levels of VEGF were observed in the hypoxia
group, and LY294002 significantly decreased VEGF expression levels.
*P<0.05. BM-MSC, bone marrow-derived mesenchymal stem cell; EC,
endothelial cell; Flt-1, fms-related tyrosine kinase 1; Flk-1,
fetal liver kinase 1; vWF, von Willebrand factor; VE, vascular
endothelial; VEGF, vascular endothelial growth factor. |
VEGF expression induced by
hypoxia
In hypoxic condition, treated cells showed a
significantly higher VEGF gene expression (12.9±0.16-fold) compared
with that of cells cultured under normoxia (P<0.001).
Furthermore, after addition of LY294002, the expression of VEGF
gene in hypoxia was 4.85±0.43 times greater than that of the
normoxia group, and the difference was statistically significant
(P=0.040). There was also a statistical significant difference
between the hypoxia groups with and without LY294002 (P=0.003;
Fig. 4B).
Discussion
Stem cell transplantation represents an attractive
technique for use in tissue engineering and reparative medicine.
For the improvement of the therapeutic potential of BM-MSCs, a more
comprehensive understanding of the in vitro culture
parameters that can maintain their stem-cell phenotype and
multipotent capabilities during expansion is required (7). Oxygen has been demonstrated to be a
potent signaling molecule due to its ability to affect the
fundamental characteristics of various types of progenitor cells,
including their proliferation, differentiation and gene expression
(6,26).
In the present study, BM-MSCs were cultured under 2%
O2, which is considered as mild to moderate level of
hypoxia, for the investigation of cell biology and possible
underlying mechanisms. The results showed that the PI3K/AKT
signaling pathway was activated by hypoxia, as indicated by the
high expression of p-AKT, which is the activated state of AKT. In
addition, in order to examine the effect of PI3K/AKT pathway on the
influence of hypoxia on BM-MSCs, a PI3K/AKT inhibitor was used to
prevent the signaling of this pathway.
Low oxygen is a potent proliferation regulator of
numerous cell types (6,25). In the present study, the hypoxic
culture conditions evidently induced BM-MSC proliferation; however,
following treatment with LY294002, the proliferation decreased
markedly. This result indicated that the PI3K/AKT pathway served an
important role in the process of cell proliferation induced by
hypoxia. Numerous studies have focused on the role of PI3K/AKT in
cell proliferation. For instance, Watanabe et al (27) demonstrated that impaired PI3K/AKT
activation directly contributes to the effect of aging on
pancreatic acinar cell proliferation. In addition, Mangi et
al (28) genetically modified
MSCs with AKT using retroviruses and found that the engineered
AKT-MSCs were more resistant to hypoxic injury. The role of
PI3K/AKT in promoting cell proliferation may rely on
phosphorylating the pro-apoptotic protein Bad (29,30) or
caspase-9 (31), which may account
for the antiapoptotic effect of AKT, thereby inhibiting its
pro-apoptotic function.
Hypoxia is also a potent differentiation inducer
towards endothelial cell differentiation. In a previous study,
BM-MSCs were treated with VEGF under hypoxic conditions, and a
greater proportion of BM-MSCs differentiated into endothelial cells
when compared with those cultured under standard conditions
(8). Xiao et al (22) demonstrated that PI3K/AKT signaling
pathway served an important role in rat cardiac stem cell
differentiation into endothelial cells, while Wortmannin (a
PI3K/AKT signaling pathway inhibitor) was able to decrease this
differentiation. Other than its role in promoting differentiation
towards endothelial cells, the PI3K/AKT pathway has been shown to
participate in improving neovascularization of human umbilical vein
endothelial cells, and LY294002 has been demonstrated to abolish
this positive effect (32).
Therefore, the present study examined the role of PI3K/AKT pathway
in BM-MSC differentiation. The results revealed that the PI3K/AKT
signaling pathway inhibitor LY294002 decreased the differentiation
of BM-MSCs towards endothelial cells, which was induced by
hypoxia.
A previous in vitro study indicated that
conditional medium without stem cells attenuated myocardial
reperfusion injury, and the cardioprotection effect was mediated by
the activation of PI3K/AKT pathway through paracrine factors
(33). These observations suggested
the important role of PI3K/AKT pathway in the paracrine function of
BM-MSCs. In ischemia therapy, angiogenesis is crucial. Amongst all
the molecules participating in angiogenesis, VEGF is particularly
relevant since it modulates the function of vascular and
non-vascular cells (34) and
promotes every step of angiogenesis, in both physiological and
pathological conditions (35).
Therefore, in the current study, VEGF expression was detected to
represent the role of PI3K/AKT pathway in BM-MSC paracrine function
caused by hypoxia. Following treatment with the PI3K/AKT inhibitor
under hypoxia, VEGF expression in the BM-MSCs decreased
conspicuously. This result was consistent with the findings of a
previous study, which demonstrated that the migration ability and
cytokine paracrine function of BM-MSCs were attenuated by a
PI3K/AKT pathway inhibitor, leading to a decreased mobilization,
homing of BM-MSCs and angiogenesis (36).
In conclusion, stem cell transplantation is widely
applied in ischemia treatment; however, the effect of low oxygen on
the engrafted cells remains unclear. Improved understanding of the
effect of hypoxia is essential in order to improve the use of stem
cell-based therapy. The results in the present study indicated that
hypoxia promoted the proliferation, differentiation into
endothelial cells and VEGF expression of BM-MSCs, and thus the
PI3K/AKT signaling pathway may serve an important role in this
effect. The current study provides an insight into a potentially
intriguing pathway, which may assist further studies in stem
cell-based applications in ischemia therapy.
Acknowledgements
The current study was supported by the National
Natural Science Foundation of China (grant no. 8127129).
References
|
1
|
Chen J, Li Y, Wang L, Zhang Z, Lu D, Lu M
and Chopp M: Therapeutic benefit of intravenous administration of
bone marrow stromal cells after cerebral ischemia in rats. Stroke.
32:1005–1011. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tse HF, Siu CW, Zhu SG, Songyan L, Zhang
QY, Lai WH, Kwong YL, Nicholls J and Lau CP: Paracrine effects of
direct intramyocardial implantation of bone marrow derived cells to
enhance neovascularization in chronic ischaemic myocardium. Eur J
Heart Fail. 9:747–753. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Uysal CA, Ogawa R, Lu F, Hyakusoku H and
Mizuno H: Effect of mesenchymal stem cells on skin graft to flap
prefabrication: An experimental study. Ann Plast Surg. 65:237–244.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cao F, Lin S, Xie X, Ray P, Patel M, Zhang
X, Drukker M, Dylla SJ, Connolly AJ, Chen X, et al: In vivo
visualization of embryonic stem cell survival, proliferation and
migration after cardiac delivery. Circulation. 113:1005–1014. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Horwitz EM, Prockop DJ, Fitzpatrick LA,
Koo WW, Gordon PL, Neel M, Sussman M, Orchard P, Marx JC, Pyeritz
RE and Brenner MK: Transplantability and therapeutic effects of
bone marrow-derived mesenchymal cells in children with osteogenesis
imperfecta. Nat Med. 5:309–313. 1999. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhu LL, Wu LY, Yew DT and Fan M: Effects
of hypoxia on the proliferation and differentiation of NSCs. Mol
Neurobiol. 31:231–242. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Grayson WL, Zhao F, Bunnell B and Ma T:
Hypoxia enhances proliferation and tissue formation of human
mesenchymal stem cells. Biochem Biophys Res Commun. 358:948–953.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ren H, Cao Y, Zhao Q, Li J, Zhou C, Liao
L, Jia M, Zhao Q, Cai H, Han ZC, et al: Proliferation and
differentiation of bone marrow stromal cells under hypoxic
conditions. Biochem Biophys Res Commun. 347:12–21. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chen L, Tredget EE, Wu PY and Wu Y:
Paracrine factors of mesenchymal stem cells recruit macrophages and
endothelial lineage cells and enhance wound healing. PloS One.
3:e18862008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Han SK, Chun KW, Gye MS and Kim WK: The
effect of human bone marrow stromal cells and dermal fibroblasts on
angiogenesis. Plast Reconstr Surg. 117:829–835. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mohyeldin A, Garzón-Muvdi T and
Quiñones-Hinojosa A: Oxygen in stem cell biology: A critical
component of the stem cell niche. Cell Stem Cell. 7:150–161. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yew TL, Chang MC, Hsu YT, He FY, Weng WH,
Tsai CC, Chiu FY and Hung SC: Efficient expansion of mesenchymal
stem cells from mouse bone marrow under hypoxic conditions. J
Tissue Eng Regen Med. 7:984–993. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hung SP, Ho JH, Shih YR, Lo T and Lee OK:
Hypoxia promotes proliferation and osteogenic differentiation
potentials of human mesenchymal stem cells. J Orthop Res.
30:260–266. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cicione C, Muiños-López E, Hermida-Gómez
T, Fuentes-Boquete I, Díaz-Prado S and Blanco FJ: Effects of severe
hypoxia on bone marrow mesenchymal stem cells differentiation
potential. Stem Cells Int. 2013:2328962013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen L, Xu Y, Zhao J, Zhang Z, Yang R, Xie
J, Liu X and Qi S: Conditioned medium from hypoxic bone
marrow-derived mesenchymal stem cells enhances wound healing in
mice. PLoS One. 9:e961612014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jung F, Haendeler J, Goebel C, Zeiher AM
and Dimmeler S: Growth factor-induced phosphoinositide 3-OH
kinase/Akt phosphorylation in smooth muscle cells: Induction of
cell proliferation and inhibition of cell death. Cardiovasc Res.
48:148–157. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ma J, Sawai H, Ochi N, Matsuo Y, Xu D,
Yasuda A, Takahashi H, Wakasugi T and Takeyama H: PTEN regulates
angiogenesis through PI3K/Akt/VEGF signaling pathway in human
pancreatic cancer cells. Mol Cell Biochem. 331:161–171. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang Z, Zhao C, Liu B, Liang D, Qin X, Li
X, Zhang R, Li C, Wang H, Sun D and Cao F: Inositol pyrophosphates
mediate the effects of aging on bone marrow mesenchymal stem cells
by inhibiting Akt signaling. Stem Cell Res Ther. 5:332014.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhou N, Lu F, Liu C, Xu K, Huang J, Yu D
and Bi L: IL-8 induces the epithelial-mesenchymal transition of
renal cell carcinoma cells through the activation of AKT signaling.
Oncol Lett. 12:1915–1920. 2016.PubMed/NCBI
|
|
20
|
Huang P, Li Y, Lv Z, Wang J, Zhang Q, Yao
X, Corrigan CJ, Huang K, Wang W and Ying S: Comprehensive
attenuation of IL-25-induced airway hyperresponsiveness,
inflammation and remodelling by the PI3K inhibitor LY294002.
Respirology. 24–Aug;2016.(Epub ahead of print).
|
|
21
|
Tong X and Pelling JC: Targeting the
PI3K/Akt/mTOR axis by apigenin for cancer prevention. Anticancer
Agents Med Chem. 13:971–978. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Xiao N, Qi XY, Tang LN, Tan LL, Chen YQ
and Zhao HM: VEGF promotes cardiac stem cells differentiation into
vascular endothelial cells via the PI3K/Akt signaling pathway.
Artif Cells Nanomed Biotechnol. 42:400–405. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2-ΔΔCt method. Methods. 25:402–408. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chen L, Tao Y and Jiang Y: Apelin
activates the expression of inflammatory cytokines in microglial
BV2 cells via PI-3K/Akt and MEK/Erk pathways. Sci China Life Sci.
58:531–540. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Caraci F, Battaglia G, Busceti C, Biagioni
F, Mastroiacovo F, Bosco P, Drago F, Nicoletti F, Sortino MA and
Copani A: TGF-beta 1 protects against Abeta-neurotoxicity via the
phosphatidylinositol-3-kinase pathway. Neurobiol Dis. 30:234–242.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Csete M: Oxygen in the cultivation of stem
cells. Ann N Y Acad Sci. 1049:1–8. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Watanabe H, Saito H, Ueda J and Evers BM:
Regulation of pancreatic duct cell differentiation by
phosphatidylinositol-3 kinase. Biochem Biophys Res Commun.
370:33–37. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Mangi AA, Noiseux N, Kong D, He H, Rezvani
M, Ingwall JS and Dzau VJ: Mesenchymal stem cells modified with Akt
prevent remodeling and restore performance of infarcted hearts. Nat
Med. 9:1195–1201. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
29
|
Datta SR, Dudek H, Tao X, Masters S, Fu H,
Gotoh Y and Greenberg ME: Akt phosphorylation of BAD couples
survival signals to the cell-intrinsic death machinery. Cell.
91:231–241. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Bai HZ, Pollman MJ, Inishi Y and Gibbons
GH: Regulation of vascular smooth muscle cell apoptosis. Modulation
of bad by a phosphatidylinositol 3-kinase-dependent pathway. Circ
Res. 85:229–237. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Cardone MH, Roy N, Stennicke HR, Salvesen
GS, Franke TF, Stanbridge E, Frisch S and Reed JC: Regulation of
cell death protease caspase-9 by phosphorylation. Science.
282:1318–1321. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Xiao X, Wang W, Liu D, Zhang H, Gao P,
Geng L, Yuan Y, Lu J and Wang Z: The promotion of angiogenesis
induced by three-dimensional porous beta-tricalcium phosphate
scaffold with different interconnection sizes via activation of
PI3K/Akt pathways. Sci Rep. 5:94092015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Angoulvant D, Ivanes F, Ferrera R,
Matthews PG, Nataf S and Ovize M: Mesenchymal stem cell conditioned
media attenuates in vitro and ex vivo myocardial reperfusion
injury. J Heart Lung Transplant. 30:95–102. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Fox SB, Gatter KC and Harris AL: Tumour
angiogenesis. J Pathol. 179:232–237. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Hicklin DJ and Ellis LM: Role of the
vascular endothelial growth factor pathway in tumor growth and
angiogenesis. J Clin Oncol. 23:1011–1027. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
He H, Zhao ZH, Han FS, Wang XF and Zeng
YJ: Activation of protein kinase C ε enhanced movement ability and
paracrine function of rat bone marrow mesenchymal stem cells partly
at least independent of SDF-1/CXCR4 axis and PI3K/AKT pathway. Int
J Clin Exp Med. 8:188–202. 2015.PubMed/NCBI
|