Introduction
Fructus sophorae is the dried ripe fruit of
Styphnolobium japonicum (L.). It has traditionally been used
for its anti-inflammatory properties, cooling blood and stopping
bleeding (using blood cooling hemostatics) in Korean and Chinese
medicine (1). Several studies have
demonstrated that Fructus sophorae has a preventative effect
against osteoclastogenesis and bone loss (2,3) and an
inhibitory effect on the production of inflammatory cytokines in
osteoblast-like cells (4). However,
few attempts have been made to observe the effects of Fructus
sophorae on rheumatoid arthritis.
The incidence of rheumatoid arthritis (RA) is ~1% of
the current adult population, and the incidence is increasing in
industrialized countries, especially in women >65 years of age
(5).
RA is characterized by symptoms such as persistent
synovitis and systemic inflammation, and consequently leads to the
destruction of cartilage and bone. RA is a an autoimmune disease,
yet the pathogenic causes of RA have yet to be elucidated.
An important factor that accelerates the
pathogenesis of RA is the proinflammatory mediators that are
overproduced by infiltrating inflammatory cells in inflamed joints.
Within the inflamed RA joint, tumour necrosis factor (TNF)-α is one
of the predominant proinflammatory cytokines, which provokes the
release of several other proinflammatory cytokines, chemokines,
prostaglandin E (PGE)2 and nitric oxide (NO), and has diverse
pathologic effects relevant to RA (6–10).
A major factor that promotes bone loss in RA is
osteoclastogenesis by the binding of the receptor activator of
nuclear factor-κB ligand (RANKL) to its receptor RANK on osteoclast
precursor cells. RANKL serves an important role in osteoclast
differentiation and induces the activation of matrix
metalloproteinases (MMPs), which lead to the degradation of the
extracellular matrix, loss of cartilage and formation of
osteoclastic bone resorption pits within the joint (11–13).
Collagen-induced arthritis (CIA) is the most widely
investigated autoimmune arthritis model, and has provided a
valuable experimental model for assessing the pathogenic mechanisms
of human RA (14).
In the present study using the CIA mouse model, the
anti-inflammatory properties and protective effects against bone
and cartilage damage of Fructus sophorae were investigated.
Materials and methods
Animals and Fructus sophorae extract
(FSE) preparation
Male BALB/c mice (age, 6 weeks; weight, 20–22 g)
were purchased from SAMTAKO Bio Korea (Osan, Korea). Mice were
housed in a temperature-controlled room (at 23±1°C) with a 12:12-h
dark:light cycle, in polystyrene cages at Dongeui University and
provided with standard rodent chow and water ad libitum.
Mice were cared for according to the Guide for the Care and Use of
Laboratory Animals (National Academy Press, Washington, DC, USA).
The experimental protocol was approved by the Institutional Animal
Research Committee of Dongeui University on Animal Care and Use
(approval no. DEU-R2014-008), and all efforts were made to minimize
animal suffering and reduce the number of animals used for the
experiments. FSE containing 20% isoflavone was provided by NOVAREX
(Ochang, Gyeongsangbuk-do, Korea; www.novarex.co.kr). The extract was isolated from the
dried ripe fruits of S. japonicum (L.) with 60% ethanol and
was stored at −20°C until required for experiments. Briefly, 10 g
of Fructus sophorae (Jesung Pharmaceutical Co., Kyungdong
Market, Korea) was crushed into 30-mesh size by using a dry
pulverizer. Distilled water was added to the crushed Fructus
sophorae to dilute it 10X, and the solution was heated at 121°C
for 2 h. Subsequently, the solution was cooled to 50°C, followed by
filtering with a 200-mesh filter cloth to remove precipitate and
obtain the filtrate. Amylase (Pectinase 100 l, Novo Nordisk A/S,
Copenhagen, Denmark) was added to the filtrate at a concentration
of 0.5% (v/v), and an enzyme reaction was performed at 50°C for 16
h. The reaction solution was centrifuged to recover precipitate 350
× g for 10 min at room temperature, and 60% ethanol was
added to the recovered precipitate. The ethanol mixture was mixed
for 30 min until the precipitate was completely dispersed. After
mixing, the solution was left for 1 h, then centrifuged to remove
precipitate and recover supernatant. The supernatant was
concentrated to the volume of 1:10 using a concentrator, and the
concentrate was powdered using a spray dryer to obtain 0.3 g of
Fructus sophorae extract powder (yield to crushed material
of Fructus sophorae: 3%). The voucher specimens (accession
no. DEU-5) have been deposited at a publicly available Natural
Resource Bank of Dongeui University College of Koreanl
Medicine.
Induction of CIA and FSE
treatment
Bovine type II collagen (CII) (Chondrex, Inc,
Redmond, WA, USA) was dissolved overnight at 4°C in 0.05 M acetic
acid at 4 mg/ml, then the solution was emulsified with an equal
volume of complete Freund's adjuvant (CFA; Sigma-Aldrich, St.
Louis, MO, USA) in an ice-cold water bath. Arthritis was induced
using an intradermal injection of 0.05 ml of the cold emulsion into
the base of the tail. Two weeks later, the mice were administered a
booster immunization with CII emulsified in CFA in the same
manner.
Following the onset of CIA, mice were randomized
into four groups (n=5/group) as follows: i) Non-immunized (normal
control), ii) CII-immunized (CIA control), and CII-immunized FSE
treated groups administered iii) 70 or iv) 350 mg/kg FSE. FSE
dissolved in saline was administered perorally (p.o.) to two
different groups (70 and 350 mg/kg) once daily for 2 weeks after
immunization. The normal control and CIA control mice were
administered the same volume of saline.
Assessment of CIA
The clinical severity of CIA was quantified by three
observers according to a graded scale from 0–4, as displayed in
Table I. Each paw and tail were
scored and yielded a maximum possible score of 19 per animal.
 | Table I.Clinical scores of CIA. |
Table I.
Clinical scores of CIA.
| Grade | Clinical
features |
|---|
| Paw |
|
| 0 | No swelling and
redness |
| 1 | Slight swelling and
redness in small jointsa or large jointsb |
| 2 | Moderate swelling
and redness in ≥1 joints |
| 3 | Severe swelling and
redness in large joints and moderate swelling and redness in small
joints |
| 4 | Most severe
swelling and redness in large joints and severe swelling and
redness in small joints |
| Tail |
|
| 0 | No swelling and
inflammation |
| 1 | Slight swelling at
the injection site and surrounding tissue |
| 2 | Inflammation or
necrosis ≤25% of tail |
| 3 | Swelling and
necrosis >25% of tail |
Paw swelling was measured by average hind paw volume
via water plethysmography (Model 7150; Ugo Basile Srl, Varese,
Italy) on day 0 (D0) and day 35 after immunization. The increase in
paw swelling was calculated by using the following formula: Paw
swelling increase (%) = (paw swelling on D35 after immunization) -
(paw swelling on D0 prior to immunization)/(paw swelling prior to
immunization) × 100
Histopathological assessment of ankle
joint
The ankle joints were removed on day 35 and fixed
for 24 h in 4% paraformaldehyde. The fixed tissues were decalcified
in Calci-Clear Rapid (National Diagnostics, Atlanta, GA, USA),
embedded in paraffin and sectioned (4 µm) using a microtome. Tissue
sections were stained with hematoxylin and eosin and Safranin
O/Fast Green staining (Sigma-Aldrich) to assess cartilage
destruction. Bone destruction, vascular proliferation, synovial
hyperplasia and inflammatory cell infiltration were assessed.
Spleen and thymus indexes
Mice were sacrificed by cervical dislocation after
sampling for serum on day 35 after immunization. The wet spleen and
thymus were weighed immediately following dissection. The indexes
of the spleen and thymus were calculated by using the following
formula: Index = spleen and thymus weight of mouse / body weight of
mouse.
Measurement of proinflammatory
cytokines and IgG2a levels
Levels of total tumor necrosis factor TNF-α,
interleukin (IL)-1β, IL-6 and immunoglobulin G (IgG)2a in the serum
of mice were investigated using commercially available
enzyme-linked immunosorbent assay (ELISA) kits (cat nos. 560478,
559603, 555240 and 552576, respectively; BD Biosciences Pharmingen,
San Diego, CA, USA). The aforementioned proinflammatory cytokines
and IgG2a were measured according to the manufacturer's
instructions, and concentrations were recalculated from their
respective standard curves.
Measurement of prostaglandin E2 (PGE2)
and nitric oxide (NO) levels
The level of PGE2 production in the serum of mice
was measured using a commercially available ELISA kit (cat no.
514010; Cayman Chemical, Ann Arbor, MI, USA) according to the
manufacturer's instructions.
Nitrite accumulation in the serum of mice was
measured colorimetrically by the Griess reaction using a Griess
reagent (Sigma-Aldrich). For the assay, equal volumes of the serum
of CIA mice and Griess reagent were mixed, and the absorption
coefficient was calibrated using a sodium nitrite solution standard
(Sigma-Aldrich). The absorbance of each sample after the Griess
reaction was determined using a microplate reader at 540 nm.
Reverse transcription-polymerase chain
reaction (RT-PCR)
Total RNA was extracted from joint tissues using
TRIzol reagent (Invitrogen, Carlsbad, CA, USA) according to the
manufacturer's instructions. The primers used to amplify TNF-α,
IL-1β, IL-6, interferon (IFN)-γ, inducible NO synthase (iNOS),
cyclooxygenase (COX)-2, RANKL, MMP-2, MMP-9, MMP-13 and
glyceraldehyde-3-phosphate dehydrogenase (GAPDH) are shown in
Table II.
 | Table II.Primers used in the current
study. |
Table II.
Primers used in the current
study.
| Gene | Sequences
(5′-3′) |
|---|
| TNF-α |
GCGACGTGGAACTGGCAGAAG |
|
|
TCCATGCCGTTGGTTAGGAGG |
| IL-1β |
AAGCTCTCCACCTCAATGGACA |
|
|
GTCTGCTCATTCACGAAAABBGAG |
| IL-6 |
TCCAGTTGCCTTCTTGGGAC |
|
|
GTGTAATTAAGCCTCCGACTTG |
| IFN-γ |
TCAAGTGGCATAGATGTCGAAGAA |
|
|
TGGCTCTGCAGGATTTTCATG |
| iNOS |
CTGCAGCACTTGGATCAGGAACCTG |
|
|
GGGAGTAGCCTGTGTGCACCTGGAA |
| COX-2 |
TTGAAGACCAGGAGTACCGC |
|
|
GGTACAGTCCCATGACATCG |
| RANKL |
AAAACGCAGATTTGCAGGAC |
|
|
GGCCACATCCAACCATGAG |
| MMP-2 |
CCAGATCACATACAGGATCATTG |
|
|
CTCCCAGCGTCCAAAGTT |
| MMP-9 |
CTAAAGGCCATTCGAACACC |
|
|
AAAGGCGTGTGCCAGAAG |
| MMP-13 |
AGGTGACTGGCAAACTTGAT |
|
|
CCAGAAGACCAGAAGGTCCA |
| GAPDH |
CCACAGTCCATGCCATCAC |
|
|
TCCACCACCCTGTTGCTGTA |
For the experiment, 1 µg of total RNA and 20 µl of
total reagents using One-step RT-PCR PreMix (iNtRON Biotechnology,
Seongnam, Gyeonggi-do, Korea) was used. The PCR reaction was
performed with a GeneAmp PCR System 9700 (Applied Biosystems;
Thermo Fisher Scientific Inc., Waltham, MA, USA). The PCR products
were separated by electrophoresis on 2% (w/v) agarose gels and
visualized with ethidium bromide. The quantity of mRNA was
normalized to the amount of GAPDH, which was utilized as a
housekeeping gene for each experimental condition. Bands were
quantified by densitometry software (Scion Image version Beta 3b;
Scion Corp., Frederick, MD, USA).
Statistical analysis
Statistical analysis was performed using GraphPad
Prism 5 package (GraphPad Software Inc., San Diego, CA, USA). All
data are expressed as mean ± standard deviation. One-way analysis
of variance followed by Dunnett's post-hoc multiple comparison
tests was used to assess statistical significance. P<0.05 was
considered to indicate a statistically significant difference.
Results
Effect of FSE on macroscopic features
of CIA
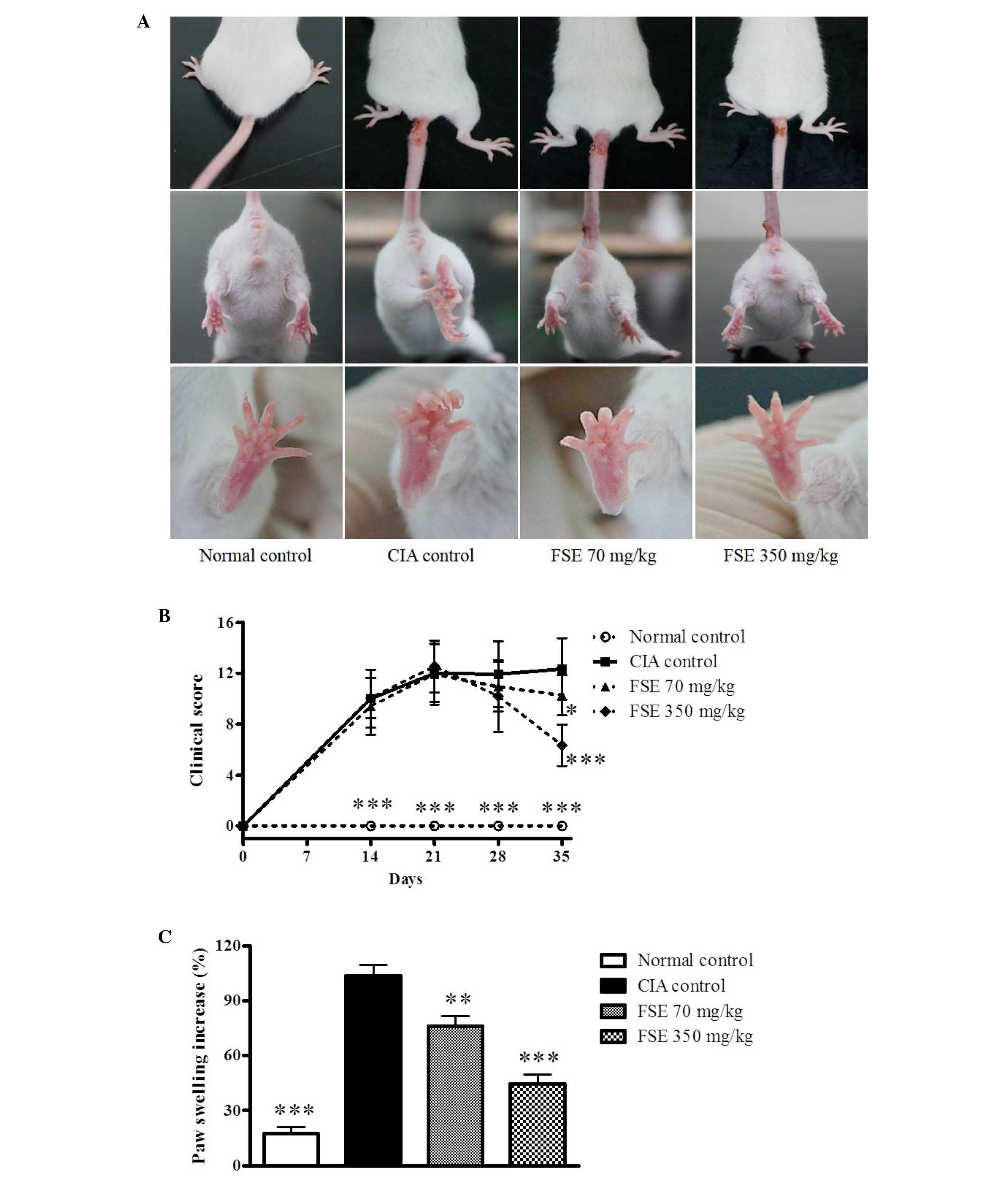
As displayed in Fig.
1A, significant increases in paw edema were recorded in CIA
control mice following 2 intradermal injections of CII
(P<0.001). In addition, macroscopic redness was observed, and a
number of edemas were observed in several of the other joints of
the CIA control mice. A significant reduction in paw edema was
observed with FSE at 70 and 350 mg/kg/day, p.o. compared with the
CIA control group (P<0.01 and P<0.001, respectively). The
initial signs of arthritis development were visible between days 7
and 14 after primary immunization. The clinical scores of the CIA
control group increased gradually after immunization, reaching a
maximum score of 12.07 ± 2.77 by day 35. Treatment with FSE at 70
and 350 mg/kg/day, p.o. resulted in significant attenuation of
arthritis clinical scores (P<0.05 and P<0.001, respectively;
Fig. 1B). The CIA control group
revealed a considerable difference when compared with the normal
group in paw swelling on day 35. FSE reduced paw swelling in a
dose-dependent manner when compared with the CIA control group
(Fig. 1C).
Effect of FSE on histopathological
changes
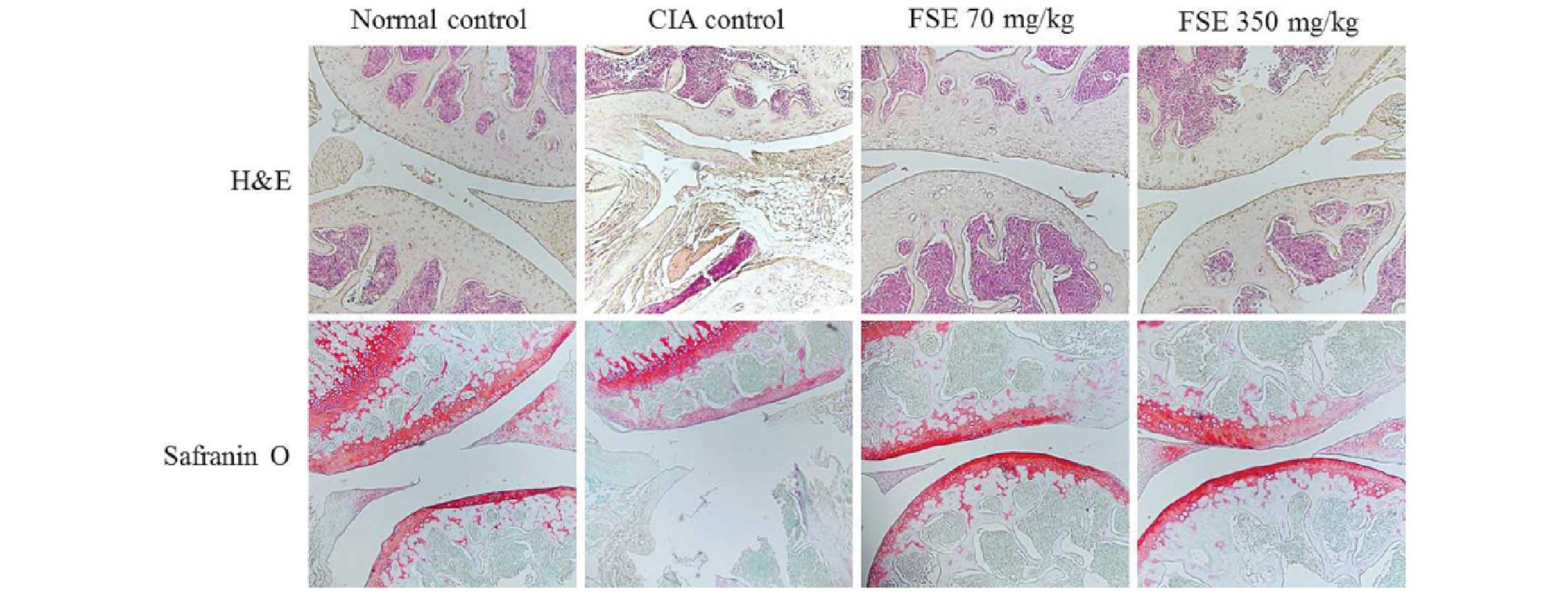
The current study conducted H&E and Safranin
O/Fast Green staining to assess the effect of FSE on histological
changes and cartilage destruction in the ankle joints. As indicated
in Fig. 2, the normal control group
displayed normal healthy articular space and tissues. In contrast,
the CIA control group demonstrated synovial hyperplasia,
destruction of articular cartilage and narrowed articular cavity.
Groups treated with FSE (70 and 350 mg/kg) displayed a marked
reduction in synovial hyperplasia, destruction of articular
cartilage and articular cavity changes (such as increased
protrusion of synovial villi into the articular cavity) when
compared with the CIA control group.
Effect of FSE on spleen and thymus
indexes and level of serum IgG2a
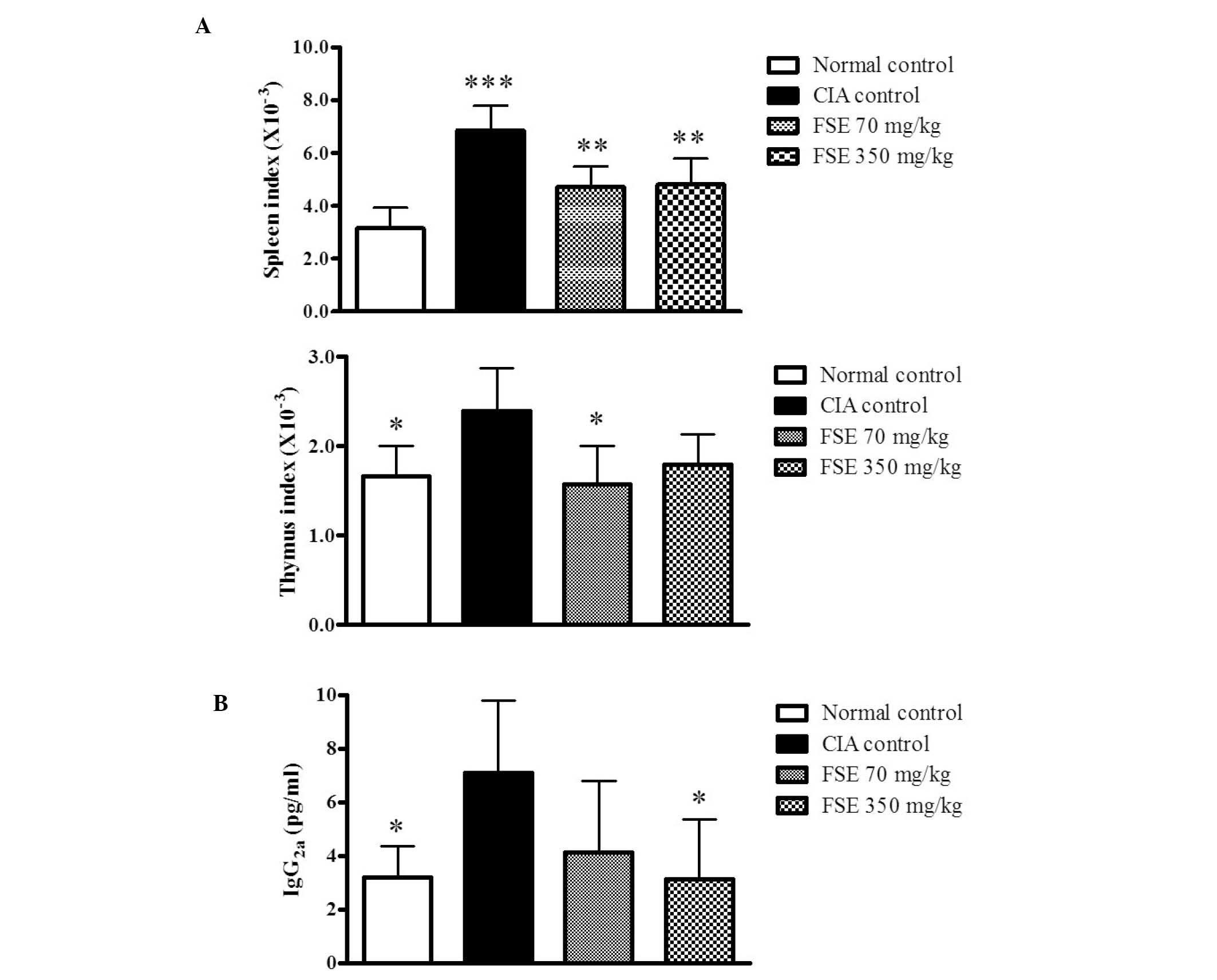
To examine the effects of FSE on the immune response
in CIA mice, the current study investigated spleen and thymus
indexes and levels of serum IgG2a. The CIA control group differed
from the normal control group, displaying significantly high spleen
and thymus indexes. FSE reduced the indexes of spleen and thymus
when compared with those of the CIA control group (P<0.01 and
P<0.05, respectively). However, FSE (350 mg/kg/day p.o.) did not
induce a significantly lower thymus index compared with the CIA
control group (Fig. 3A). The serum
IgG2a level, which is known to be pathogenic in CIA (15) was also evaluated. The CIA control
group had increased serum IgG2a levels relative to those of the
normal control group (P<0.05), whereas the FSE (350 mg/kg/day
p.o.) group displayed suppressed serum IgG2a levels compared with
those of the CIA control group (P<0.05; Fig. 3B).
FSE suppresses production and mRNA
expression levels of proinflammatory cytokines
RA is a systemic autoimmune disease, therefore
circulating proinflammatory cytokine levels were measured in the
serum and mRNA expression levels of these genes were investigated
in joint tissues obtained on day 35. The CIA control group revealed
increases in the levels of TNF-α, IL-1β, IL-6 and IFN-γ when
compared with those of the normal control group, whereas FSE
suppressed these cytokines when compared with those of the CIA
control group (P<0.05, P<0.01 and P<0.001, respectively;
Fig. 4A). Consistent with results in
the serum, significant induction of the TNF-α, IL-1β, IL-6 and
IFN-γ genes was observed in joint tissues of the CIA control group
in comparison with the normal control group (P<0.001). FSE
downregulated the activation of these genes in a dose-dependant
manner compared with the CIA control group (P<0.05 and
P<0.001, respectively; Fig.
4B).
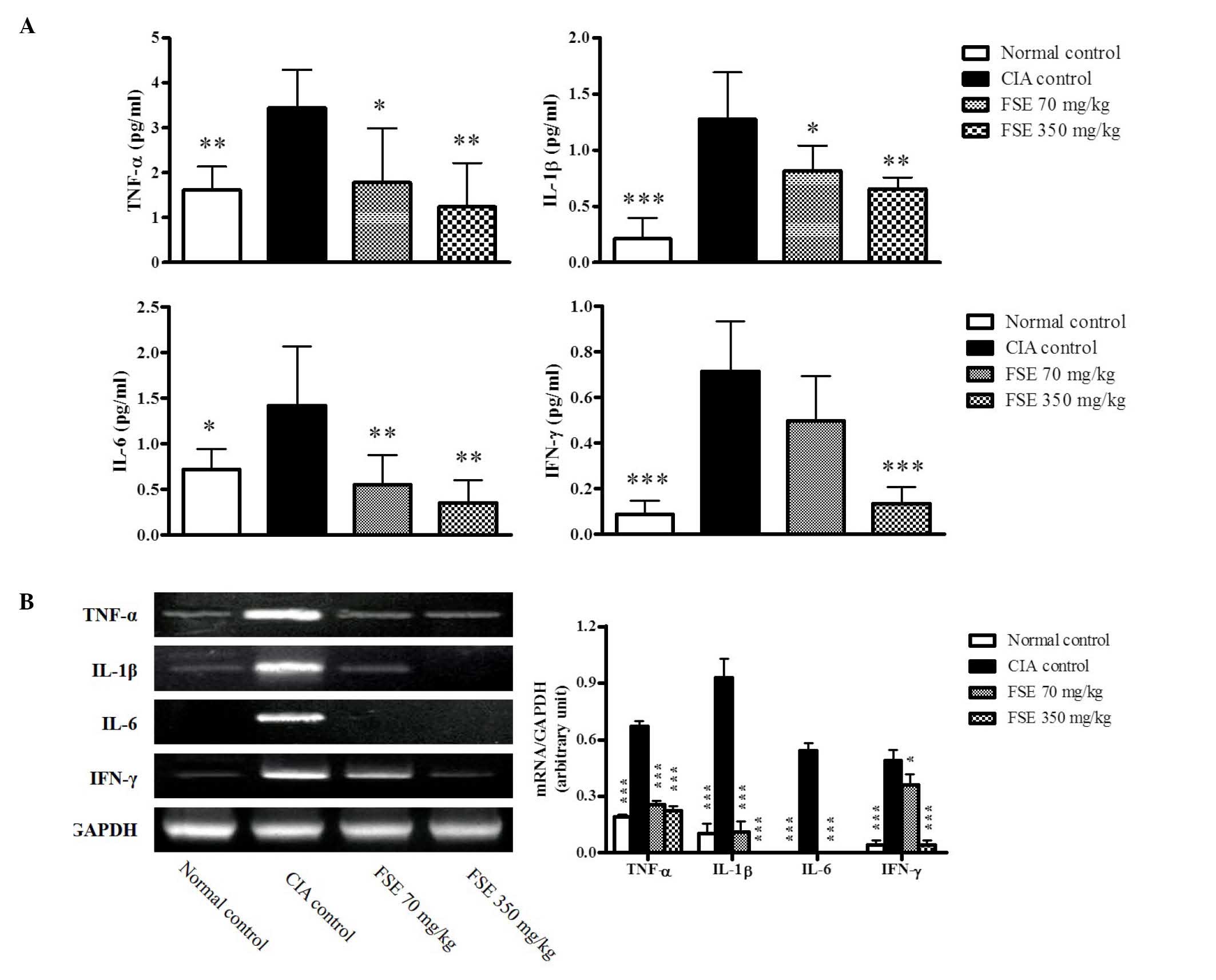 | Figure 4.Effects of FSE on serum levels of
IL-1β, IL-6, TNF-α and INF-γ and mRNA expression levels of IL-1β,
IL-6, TNF-α and INF-γ from joint tissue in a CIA mouse model. (A)
Serum IL-1β, IL-6, TNF-α and INF-γ levels. Data are recorded as
mean ± standard deviation from 5 animals. (B) Representative IL-1β,
IL-6, TNF-α and INF-γ mRNA expression level, quantified by the
densitometric analysis of bands and normalized to those of GAPDH
mRNA. Data are recorded as mean ± standard deviation from 3
experiments on joint tissues. *P<0.05, **P<0.01,
***P<0.001 vs. CIA control group. CIA, type II collagen
(CII)-induced arthritis; FSE, Fructus sophorae extract; IL,
interleukin; TNF, tumor necrosis factor; IFN, interferon; GAPDH,
glyceraldehyde 3-phosphate dehydrogenase. |
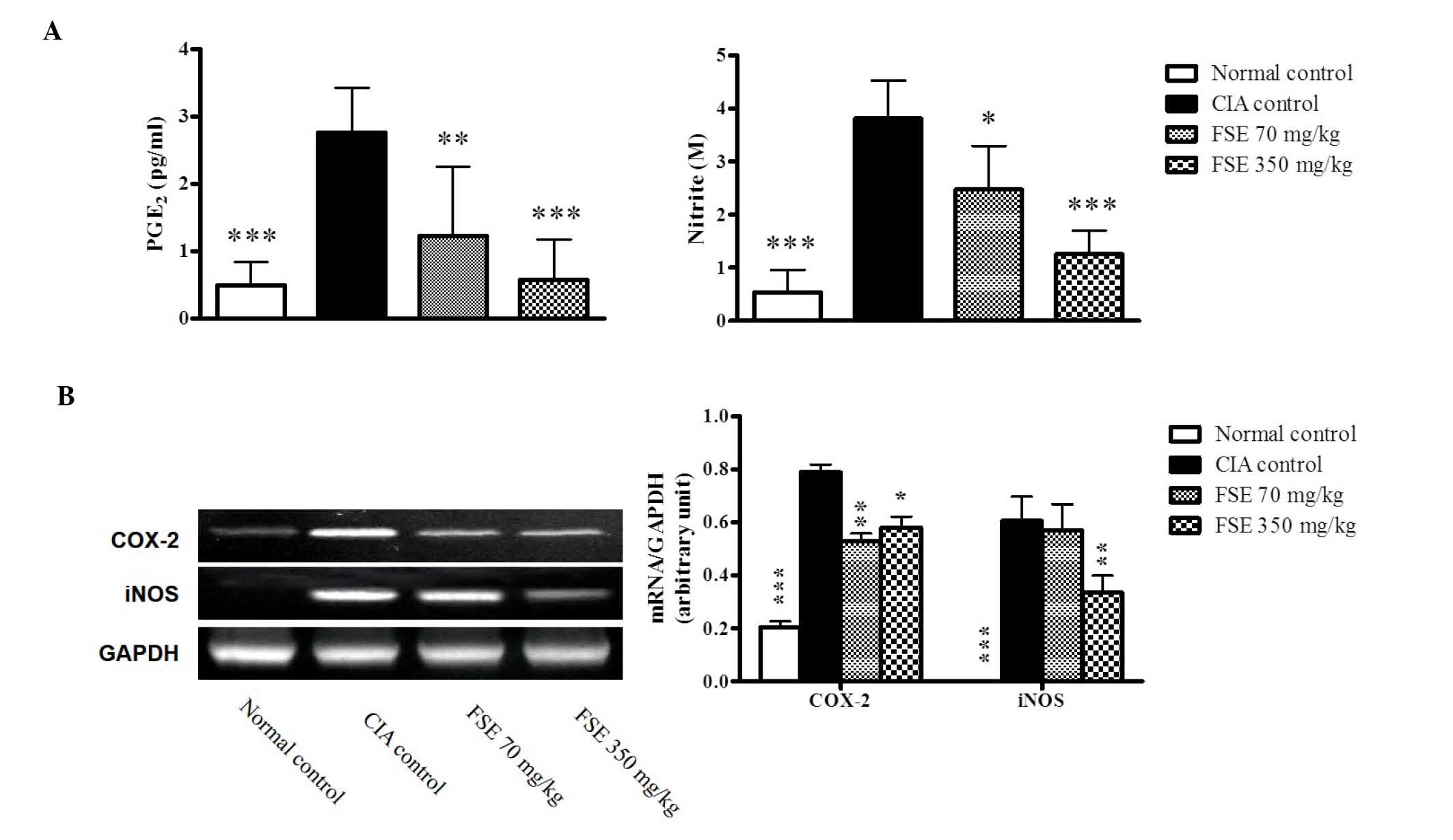
Effect of FSE on PGE2 and NO
production and mRNA expression levels of COX-2 and iNOS
PGE2 and NO, produced by COX-2 and iNOS,
respectively, are important inflammatory mediators involved in the
pathogenesis of RA. Levels of PGE2 and NO, the metabolites of COX-2
and iNOS, were evaluated in the serum and mRNA expression levels of
COX-2 and iNOS were investigated in joint tissue obtained on day
35. A significant increase in PGE2 and NO production was observed
in the CIA control group. FSE significantly reduced the levels of
PGE2 and NO in a dose-dependent manner when compared with those of
the CIA control group (Fig. 5A and
B). Consistent with results in the serum, a marked induction of
the COX-2 and iNOS genes was observed in the joint tissues of the
CIA control group in comparison with the normal control group. FSE
downregulated the expression of COX-2 (both treatments) and iNOS
(350 mg/kg group) when compared with those of the CIA control group
(Fig. 5C).
Effect of FSE on the mRNA expression
levels of RANKL and MMPs
The mRNA expression of RANKL, MMP-2, MMP-9 and
MMP-13 genes was investigated in joint tissue obtained on day 35.
Significant induction of the RANKL, MMP-2, MMP-9 and MMP-13 genes
was observed in the joint tissues of the CIA control group compared
with those of the normal control group (P<0.001). Treatment with
70 or 350 mg/kg FSE significantly downregulated expression of RANKL
(P<0.001) MMP-2, MMP-9 and MMP-13 (P<0.05 or P<0.001)
compared with the CIA control group (Fig. 6). These findings demonstrate that FSE
suppresses synovial inflammation, cartilage damage and bone
destruction.
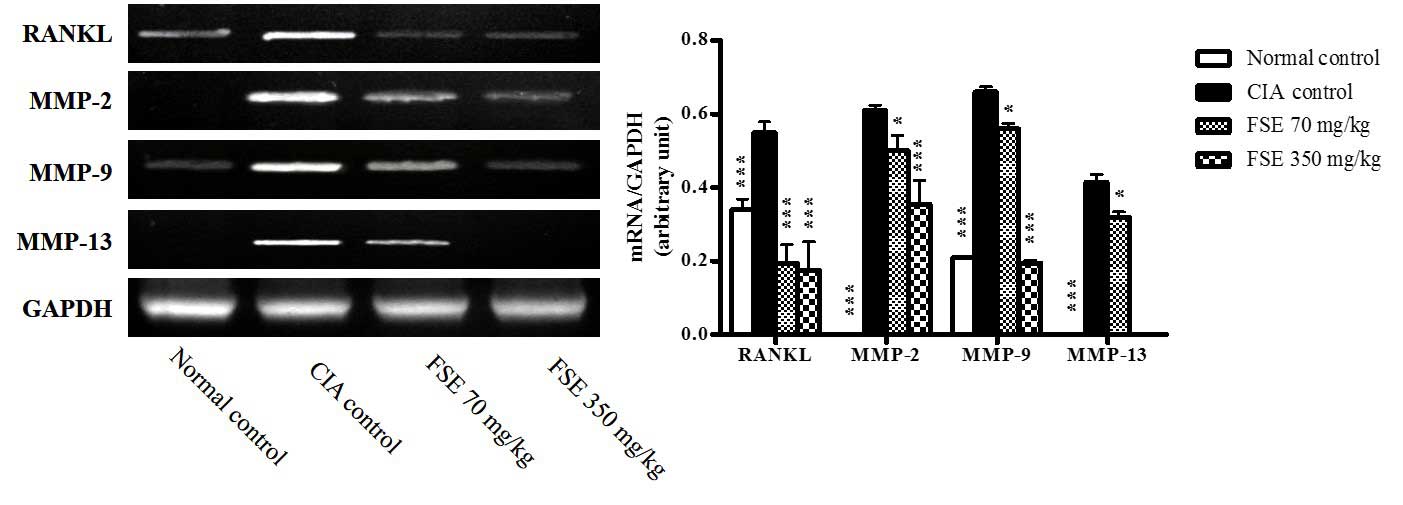 | Figure 6.Effects of FSE on mRNA expression
levels of RANKL, MMP-2, MMP-9, and MMP-13 from joint tissue in CIA
mice model. Representative RANKL, MMP-2, MMP-9, and MMP-13 mRNA
expression level. The quantity of autoradiograph bands was
conducted as described in Fig. 4.
Data are recorded as mean ± standard deviation from 3 experiments
on joint tissues. Statistics were determined as described in
Fig. 1. *P<0.05, ***P<0.001
vs. CIA control group. CIA, type II collagen (CII)-induced
arthritis; FSE, Fructus sophorae extract; RANKL, receptor activator
of nuclear factor-κB ligand; MMP, matrix metalloprotease; GAPDH,
glyceraldehyde 3-phosphate dehydrogenase. |
Discussion
Fructus sophorae, the dried ripe fruit of S.
japonicum (L.), is an herb for clearing heat (reducing fever),
purging Pathogenic Fire and cooling the blood to stop bleeding in
Korean and Chinese medicine, and has traditionally been used for
its hemostatic properties (1). The
current study revealed that some components in Fructus sophorae
have hemostatic properties as well as anti-obesity, anti-tumor,
anti-menopausal and anti-hemorrhoid effects (16–18).
However, a limited amount of research has investigated whether
Fructus sophorae has therapeutic effects on RA.
In the present study, it was determined that FSE had
potentially protective effects in a CII-induced CIA mouse model.
The CIA mouse model is a useful animal model for the assessment of
RA characterized by synovitis, leading to the progressive
destruction of cartilage and bone (14).
The initial clinical indicators of CIA were observed
to be articular weakness, periarticular erythema and edema in the
hind paws. FSE reduced the clinical symptoms, including arthritis
clinical scores and paw swelling of CIA mice, in a dose-dependent
manner (Fig. 1). Histological
observations supported the protective effect of FSE against the
progress of the pathology of CIA, including synovial hyperplasia, a
narrowing joint space and cartilage erosion (Fig. 2). The aforementioned results suggest
that FSE has an inhibitory effect against the clinical pathological
development of CIA.
As RA is an autoimmune disorder, the effect of FSE
on immune organs was also assessed and it was established that FSE
downregulated the spleen and thymus indexes in mice with CIA
(Fig. 3A).
With regard to the pathological changes associated
with CIA, the immune and inflammatory systems demonstrated
increased serum levels of IgG2a, proinflammatory cytokines, T
helper (Th) 1-mediated autoimmune component IFN-γ and other
inflammatory mediators. In addition, this system induces an
increase in gene expression associated with synovial inflammation,
cartilage and bone destruction.
The B cell, which is predominantly mediated by the
IgG2 isotype of anti-CII immunoglobulin, is critical to the
development of CIA (15,19). In the current study, FSE inhibited
serum IgG2a levels in a dose-dependent manner (Fig. 3B). This suggests that FSE may reduce
joint damage by inhibiting the production of IgG2a by controlling
the organs of the immune system, including the spleen and the
thymus in CIA pathology.
Proinflammatory cytokines, in particular TNF-α, IL-1
and IL-6, are expressed in joints and serve an important role in
the development of collagen-induced arthritis (6,7). The
aforementioned cytokines contribute to synovial inflammation by
inducing the production of other cytokines, chemokines and small
proinflammatory mediators (for example PGE2 and NO) (20). It has been determined that these
cytokines also participate in the destruction of bone and cartilage
by inducing expression of RANKL and MMPs in RA (6,21,22).
IFN-γ produced by CD4 Th1 cells is a potent inducer of the
inflammatory response in CIA (15).
In the present study, FSE inhibited serum levels and gene
expression of proinflammatory cytokines TNF-α, IL-1 and IL-6 and
Th1-mediated cytokine IFN-γ (Fig.
4). This suggests that FSE contributes to anti-inflammatory
action by inhibiting serum proinflammatory cytokine release and the
mRNA expression levels of proinflammatory cytokines in ankle joint
tissue.
PGE2 and NO are synthesized in excess from the
synovium of inflamed joints and exacerbate joint damage in RA. PGE2
and COX-2 induce vasodilation, fluid extravasation and pain in
synovial tissue, and are implicated in the development of erosions
of articular cartilage and bone (8).
NO causes T cell dysfunction and contributes to bone loss in
patients with RA (10,23,24).
Evidence from previous arthritis studies reported that the
inhibition of NO and iNOS mRNA levels was effective in the
treatment of RA (25). In the
current study, FSE significantly inhibited serum levels of PGE2 and
NO and gene expression of COX-2 and iNOS in a dose-dependent manner
(Fig. 5). This suggests that FSE
contributed to anti-inflammatory action via inhibition of serum
PGE2 and NO release, in addition to mRNA expression levels of COX-2
and iNOS in ankle joint tissue.
RANKL and MMPs are potent biomarkers associated with
bone and cartilage degradation. RANKL is critical for
osteoclastogenesis, and regulates the differentiation, maturation
and bone resorptive activity of osteoclasts (12). Expression of RANKL is regulated by
pro-inflammatory cytokines TNF-α, IL-1β and IL-6, as well as
inflammatory mediators such as PGE2 (26). MMPs are a family of zinc-dependent
and calcium-dependent proteolytic enzymes secreted by the resident
cells and invading cells of joint tissues (27). MMPs are also induced by cytokines and
growth factors and serve crucial roles in remodeling connective
tissue and degrading the extracellular matrix (28–30). The
gelatinases MMP-2 and MMP-9 and the collagenase MMP-13 are
important in arthritic disease and cleave native fibrillar collagen
and degrade the extracellular matrix molecules of articular
cartilage in RA (31,32). In the current study, FSE inhibited
gene expression of RANKL, MMP-2, MMP-9 and MMP-13 in the inflamed
RA joint tissue (Fig. 6). This
suggests that FSE protects the cartilage and bone damage by
inhibiting the gene activation of RANKL and MMPs in RA joint
tissue.
The present study has demonstrated that FSE has a
protective effect against disease progression in a mouse model of
CIA. This effect is associated with the suppression of factors
associated with the rheumatoid inflammatory response by FSE,
synovial inflammation and cartilage and bone damage. The present
results suggest that FSE may provide potential protection against
inflammation and bone and cartilage damage in RA and, as such,
would be a valuable candidate for further investigation as a novel
anti-arthritic agent.
In conclusion, the aim of the current study was to
determine whether FSE has anti-arthritic effects. The
anti-arthritic protective effects of FSE were identified, and the
effects were observed to be associated with the suppression of
inflammatory actions and protection of cartilage and bone damage in
the mouse model of CIA.
Acknowledgements
The present study was supported by the R&D
program of MOTIE/KIAT (Establishment of Infra Structure for
Anti-aging Industry Support; Seoul, Korea; grant no. N0000697)
References
|
1
|
Gan T, Liu YD, Wang Y and Yang J:
Traditional Chinese Medicine herbs for stopping bleeding from
haemorrhoids. Cochrane Database Syst Rev. 10:CD0067912010.
|
|
2
|
Joo SS, Won TJ, Kang HC and Lee DI:
Isoflavones extracted from Sophorae fructus upregulate IGF-I and
TGF-beta and inhibit osteoclastogenesis in rat bone marrow cells.
Arch Pharm Res. 27:99–105. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Shim JG, Yeom SH, Kim HJ, Choi YW, Lee DI,
Song KY, Kwon SH and Lee MW: Bone loss preventing effect of
Sophorae Fructus on ovariectomized rats. Arch Pharm Res.
28:106–110. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Joo SS, Kang HC, Lee MW, Choi YW and Lee
DI: Inhibition of IL-1beta and IL-6 in osteoblast-like cell by
isoflavones extracted from Sophorae fructus. Arch Pharm Res.
26:1029–1035. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Scott DL, Wolfe F and Huizinga TW:
Rheumatoid arthritis. Lancet. 376:1094–1108. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
McInnes IB and Schett G: Cytokines in the
pathogenesis of rheumatoid arthritis. Nat Rev Immunol. 7:429–442.
2007. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Brennan FM and McInnes IB: Evidence that
cytokines play a role in rheumatoid arthritis. J Clin Invest.
118:3537–3545. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fattahi MJ and Mirshafiey A:
Prostaglandins and rheumatoid arthritis. Arthritis.
2012:2393102012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cillero-Pastor B, Martin MA, Arenas J,
López-Armada MJ and Blanco FJ: Effect of nitric oxide on
mitochondrial activity of human synovial cells. BMC Musculoskelet
Disord. 12:422011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nagy G, Koncz A, Telarico T, Fernandez D,
Ersek B, Buzás E and Perl A: Central role of nitric oxide in the
pathogenesis of rheumatoid arthritis and systemic lupus
erythematosus. Arthritis Res Ther. 12:2102010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Karmakar S, Kay J and Gravallese EM: Bone
damage in rheumatoid arthritis: Mechanistic insights and approaches
to prevention. Rheum Dis Clin North Am. 36:385–404. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Schett G, Hayer S, Zwerina J, Redlich K
and Smolen JS: Mechanisms of Disease: The link between RANKL and
arthritic bone disease. Nat Clin Pract Rheumatol. 1:47–54. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Udagawa N, Kotake S, Kamatani N, Takahashi
N and Suda T: The molecular mechanism of osteoclastogenesis in
rheumatoid arthritis. Arthritis Res. 4:281–289. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Brand DD, Latham KA and Rosloniec EF:
Collagen-induced arthritis. Nat Protoc. 2:1269–1275. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Brand DD, Kang AH and Rosloniec EF:
Immunopathogenesis of collagen arthritis. Springer Semin
Immunopathol. 25:3–18. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chang L, Ren Y, Cao L, Sun Y, Sun Q, Sheng
N, Yuan L, Zhi X and Zhang L: Simultaneous determination and
pharmacokinetic study of six flavonoids from Fructus Sophorae
extract in rat plasma by LC-MS/MS. J Chromatogr B Analyt Technol
Biomed Life Sci. 904:59–64. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ha do T, Trung TN, Phuong TT, Yim N, Chen
QC and Bae K: The selected flavonol glycoside derived from Sophorae
Flos improves glucose uptake and inhibits adipocyte differentiation
via activation AMPK in 3T3-L1 cells. Bioorg Med Chem Lett.
20:6076–6081. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Park KW, Lee JE and Park KM: Diets
containing Sophora japonica L. prevent weight gain in high-fat
diet-induced obese mice. Nutr Res. 29:819–824. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Brand DD, Marion TN, Myers LK, Rosloniec
EF, Watson WC, Stuart JM and Kang AH: Autoantibodies to murine type
II collagen in collagen-induced arthritis: A comparison of
susceptible and nonsusceptible strains. J Immunol. 157:5178–5184.
1996.PubMed/NCBI
|
|
20
|
Feldmann M and Maini RN: Anti-TNF alpha
therapy of rheumatoid arthritis: What have we learned? Annu Rev
Immunol. 19:163–196. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Dayer JM: The pivotal role of
interleukin-1 in the clinical manifestations of rheumatoid
arthritis. Rheumatology (Oxford). 42:(Suppl 2). ii3–ii10. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hashizume M and Mihara M: The roles of
interleukin-6 in the pathogenesis of rheumatoid arthritis.
Arthritis. 2011:7656242011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Nagy G, Clark JM, Buzás EI, Gorman CL and
Cope AP: Nitric oxide, chronic inflammation and autoimmunity.
Immunol Lett. 111:1–5. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mäki-Petäjä KM, Cheriyan J, Booth AD, Hall
FC, Brown J, Wallace SM, Ashby MJ, McEniery CM and Wilkinson IB:
Inducible nitric oxide synthase activity is increased in patients
with rheumatoid arthritis and contributes to endothelial
dysfunction. Int J Cardiol. 129:399–405. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang B, Ma L, Tao X and Lipsky PE:
Triptolide, an active component of the Chinese herbal remedy
Tripterygium wilfordii Hook F, inhibits production of nitric oxide
by decreasing inducible nitric oxide synthase gene transcription.
Arthritis Rheum. 50:2995–3303. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kotake S, Udagawa N, Takahashi N,
Matsuzaki K, Itoh K, Ishiyama S, Saito S, Inoue K, Kamatani N,
Gillespie MT, et al: IL-17 in synovial fluids from patients with
rheumatoid arthritis is a potent stimulator of osteoclastogenesis.
J Clin Invest. 103:1345–1352. 1999. View
Article : Google Scholar : PubMed/NCBI
|
|
27
|
Murphy G, Knäuper V, Atkinson S, Butler G,
English W, Hutton M, Stracke J and Clark I: Matrix
metalloproteinases in arthritic disease. Arthritis Res. 4:(Suppl
3). S39–S49. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
28
|
Nagase H and Woessner JF Jr: Matrix
metalloproteinases. J Biol Chem. 274:21491–21494. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Vu TH and Werb Z: Matrix
metalloproteinases: Effectors of development and normal physiology.
Genes Dev. 14:2123–2133. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chang YH, Lin IL, Tsay GJ, Yang SC, Yang
TP, Ho KT, Hsu TC and Shiau MY: Elevated circulatory MMP-2 and
MMP-9 levels and activities in patients with rheumatoid arthritis
and systemic lupus erythematosus. Clin Biochem. 41:955–959. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Birkedal-Hansen H: Proteolytic remodeling
of extracellular matrix. Curr Opin Cell Biol. 7:728–735. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kim KS, Choi HM, Lee YA, Choi IA, Lee SH,
Hong SJ, Yang HI and Yoo MC: Expression levels and association of
gelatinases MMP-2 and MMP-9 and collagenases MMP-1 and MMP-13 with
VEGF in synovial fluid of patients with arthritis. Rheumatol Int.
31:543–547. 2011. View Article : Google Scholar : PubMed/NCBI
|