Introduction
Osteoarthritis (OA), which is an age-related
condition, is the leading cause of pain, disability and shortening
of adult working life (1). Incidence
of OA increases with age, with 25% of individuals aged >50 years
old exhibiting OA of the knee. Worldwide, ~10% of men, 18% of
women, and 60–65% of individuals aged over 60 years have
symptomatic OA, and 80% of these patients suffer from limitations
in motion (2,3). Clinical symptoms of OA include
stiffness, pain, limited motion, crepitus, swelling and deformity
(4,5). Despite promising preclinical data
covering various molecule classes, the etiology of OA remains
poorly elucidated (4). Several
biochemical and biomechanical factors have been reported and are
considered to be potential lines of investigation for the
pathogenesis of OA (5,6).
Hypoxia-inducible factor 1α (HIF-1α) is considered
to be a key regulator of cellular adaptation to hypoxic conditions
and catabolic stress through the activation of hypoxic response
elements (7–9). O2-dependent binding of the
von Hippel-Lindau tumor suppressor protein ensures it is able to
associate with HIF-1α for proteasomal degradation and
ubiquitination. The activity of the HIF-1α transactivation domains
also depends on O2 regulation through a previously
undefined mechanism. Articular cartilage is known to
physiologically lack blood vessels, which results in a
significantly decreased oxygen level within the tissue; thus, the
resident cells require well-adapted mechanisms to ensure survival.
Previous studies have demonstrated that HIF-1α is associated with
the regulation of anaerobic energy generation, glucose transport
and matrix synthesis by articular chondrocytes (10–12).
Furthermore, proinflammatory mediators and mechanical load have
also been suggested to increase HIF-1α activity in articular
chondrocytes (13–15). These factors are known to be
associated with the pathogenesis of OA. Therefore, it is a
reasonable assumption that osteoarthritis chondrocytes depend on
HIF-1α to survive and function properly.
HIF-1α transcription factor also has an important
role in maintaining proper cellular functions under hypoxic
conditions (16,17). Grimmer et al (18) have revealed that HIF-1α is associated
with the upregulation of microsomal prostaglandin E synthase-1 and
has an important role in the metabolism of OA cartilage.
Although articular cartilage and/or synovial fluid
levels of several cytokines have previously been investigated in
patients with knee OA (4–6); to the best of our knowledge, there have
been no detailed studies on articular cartilage and synovial fluid
levels of HIF-1α in various clinical stages of primary knee OA. The
association between HIF-1α levels in articular cartilage and
disease severity has not been previously reported in the
literature. The purpose of the present study was to investigate the
expression levels of HIF-1α in the articular cartilage and synovial
fluid of patients with primary knee OA, and evaluate the potential
correlation with the osteoarthritic disease process via Mankin
scoring and radiographic grading of knee OA, and to further
elucidate the pathways associated with the progression of OA.
Materials and methods
Patients and sample preparation
The present study was approved by the Ethical
Committee of Xiangya Hospital Central South University (Changsha,
China), and was conducted in accordance with the Declaration of
Helsinki. A total of 36 patients with primary knee OA (11 males and
25 females; mean age 67.4 years), diagnosed according to the
criteria of the American College of Rheumatology, and 10 normal
healthy individuals (4 males and 6 females; mean age 58.9 years)
were enrolled. Data were reviewed to exclude any forms of secondary
OA and inflammatory-associated joint diseases, such as rheumatoid
arthritis (RA). Table I shows the
patient characteristics.
 | Table I.Patient characteristics. |
Table I.
Patient characteristics.
| Variable | Osteoarthritis | Normal |
|---|
| Number of
patients | 36 | 10 |
| Age range
(years) | 54–85 | 54–63 |
| Mean age
(years) | 67.4 |
58.9 |
| Sex |
|
|
|
Male | 11 | 4 |
|
Female | 25 | 6 |
Disease severity was evaluated with radiographs of
the affected knee according to the Kellgren and Lawrence (KL)
classification (19). A total of 36
osteoarthritic cartilage and synovial tissue samples were harvested
from 36 patients who underwent a total knee replacement due to
primary knee OA. Normal cartilage and synovial tissue was collected
from 10 human knees at the time of autopsy. Osteoarthritic changes
were classified histomorphologically, using modified Mankin scoring
(20) as follows: Normal, 0–1; mild
lesions, 2–5; moderate lesions, 6–9; and severe lesions, 10–14. The
following samples were included in the present study: 10 samples
(Mankin score 0–1), 8 samples (Mankin score 2–5), 13 samples
(Mankin score 6–9) and 15 samples (Mankin score 10–14).
Immunohistochemistry
Specimens were immediately fixed with 4%
paraformaldehyde in PBS, decalcified with 0.2 M
ethylenediaminetetraacetid acid, embedded in paraffin wax and cut
into 5-µM-thick sections. Primary monoclonal antibodies against
HIF-1α (1:10,000; cat. no. NB100-105; Novus Biologicals, LLC,
Littleton, CO, USA) were incubated with the specimens overnight at
4°C for immunohistochemical analyses. A catalyzed signal
amplification kit (DakoCytomation, Carpinteria, CA, USA) based on a
streptavidin-biotin peroxidase reaction was employed for the
visualization of HIF-1α, with diaminobenzidine as a chromogen.
Control sections were incubated with nonimmune goat antisera
(Zhongshan Golden Bridge Biotechnology Co., Ltd., Beijing, China).
Sections were examined under a microscope at 100x magnification to
evaluate the expression of HIF-1α. Relative HIF-1α expression
levels in articular cartilage were quantified and visualized as
gray values. MIAS-4400 ImageJ (National Institutes of Health,
Bethesda, MA, USA) software was used to perform semiquantitative
assessment of the mean gray values of HIF-1α expression. All the
sections were evaluated by a pathologist who was blinded to the
clinical data. All densities were normalized to PBS and repeated in
triplicate. A total of three sections per sample were measured and
the mean was calculated to reduce the error arising from the
variations in section thickness. The final data consisted of a mean
of three independent measurements representing the mean levels of
HIF-1α in the articular cartilage samples.
Enzyme-linked immunosorbent assay
(ELISA)
During total knee replacement surgery, synovial
fluid was aspirated from the affected knee, and immediately
centrifuged at 1,000 × g for 25 min at 4°C to remove joint
debris and cells. According to the manufacturers' instructions, the
expression of HIF-1α in synovial fluid was detected using a
commercial ELISA kit (Uscn Life Science, Inc., Wuhan, China). The
assays had inter-assay coefficients of variation that were <6%
and intra-assay coefficients of variation of <5%. Two
independent experiments were performed.
Statistical analysis
SPSS for Windows (version 17.0; SPSS, Inc., Waltham,
MA, USA) was used for statistical analyses. Student's t-test was
used to compare the means of two independent groups and one way
analysis of variance was used to compare the means of >2
independent groups, followed by Student-Newman-Keul (SNK) test when
comparing among the groups. Spearman's correlation was used to
evaluate the correlation between synovial fluid levels of HIF-1α
and the severity of OA. Pearson's correlation and linear regression
analysis were employed to determine the correlation between
synovial fluid HIF-1α expression levels and the gray values of
HIF-1α in articular cartilage. Spearman's correlation and linear
regression analysis were applied to evaluate the correlation
between the gray values of HIF-1α in articular cartilage and the
Mankin score of OA. All data were expressed as the mean ± standard
deviation. P<0.05 was considered to indicate a statistically
significant difference.
Results
HIF-1α expression levels in articular
cartilage
A total of 46 knees were evaluated from 10 controls
and 36 patients. HIF-1α expression levels were detected in the
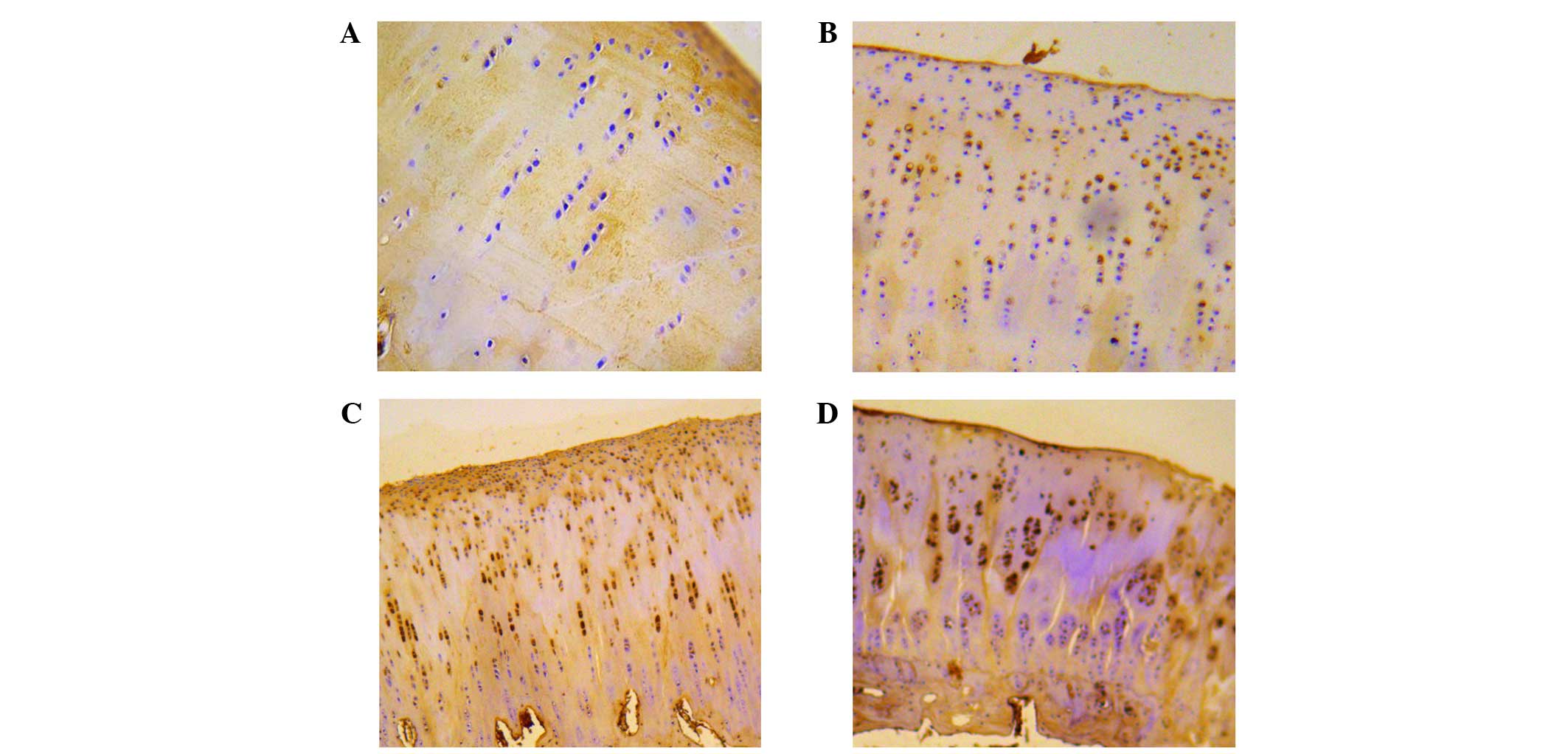
tissues of all the four groups, which were categorized according to
the severity of the lesions in the cartilage (Fig. 1). The average gray value of HIF-1α
expression was 205.49±4.95 in normal cartilage, 185.34±9.09 in
cartilage with mild lesions, 171.26±3.40 in cartilage with moderate
lesions and 155.48±10.41 in cartilage with severe lesions,
respectively (Table II).
Significant differences in the average gray values of HIF-1α
expression were detected among the groups (P<0.05). The average
gray value of HIF-1α expression in each group was demonstrated to
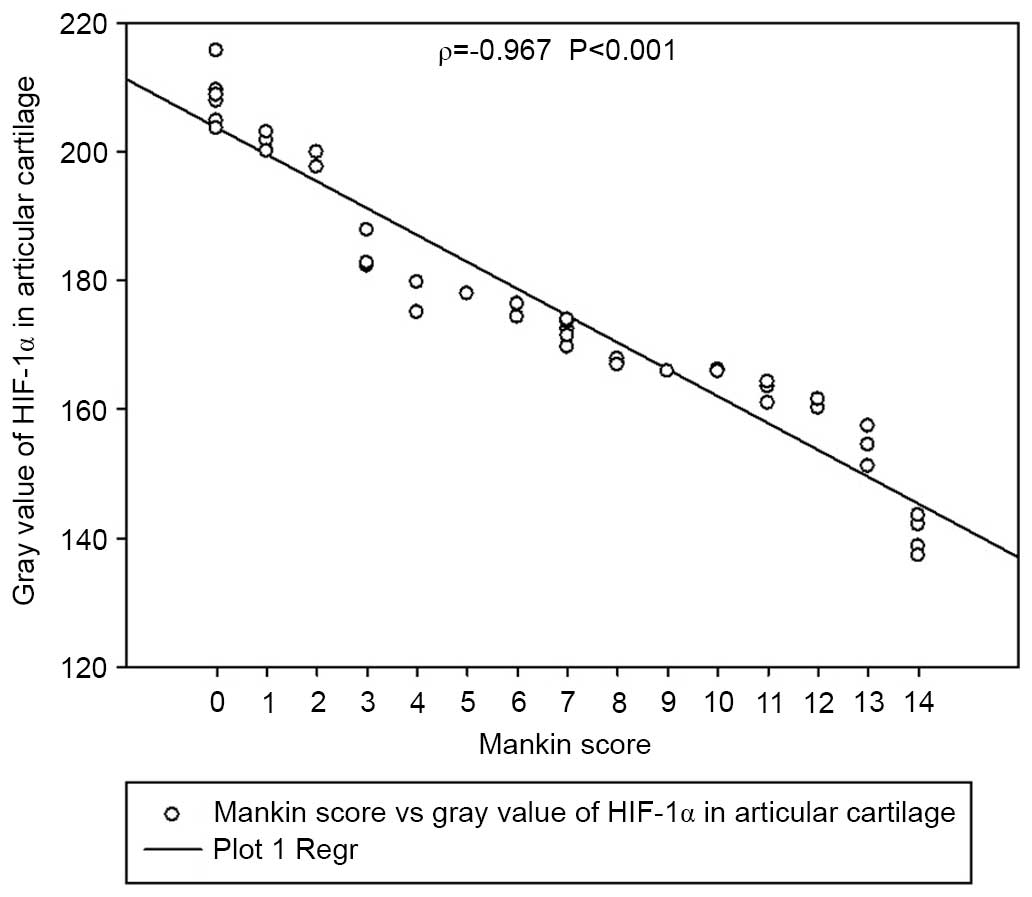
be correlated with disease severity according to the modified
Mankin score (Spearman's ρ=−0.967, P<0.001) (Fig. 2).
 | Table II.Mean gray value of HIF-1α
expression. |
Table II.
Mean gray value of HIF-1α
expression.
| Group | No. of samples | Average gray value
of HIF-1α expression |
|---|
| Normal | 10 | 205.49±4.95 |
| Mild lesions | 8 | 185.34±9.09 |
| Moderate
lesions | 13 | 171.26±3.40 |
| Severe lesions | 15 | 155.48±10.41 |
HIF-1α expression levels in synovial
fluid
The concentrations of HIF-1α in the synovial fluid
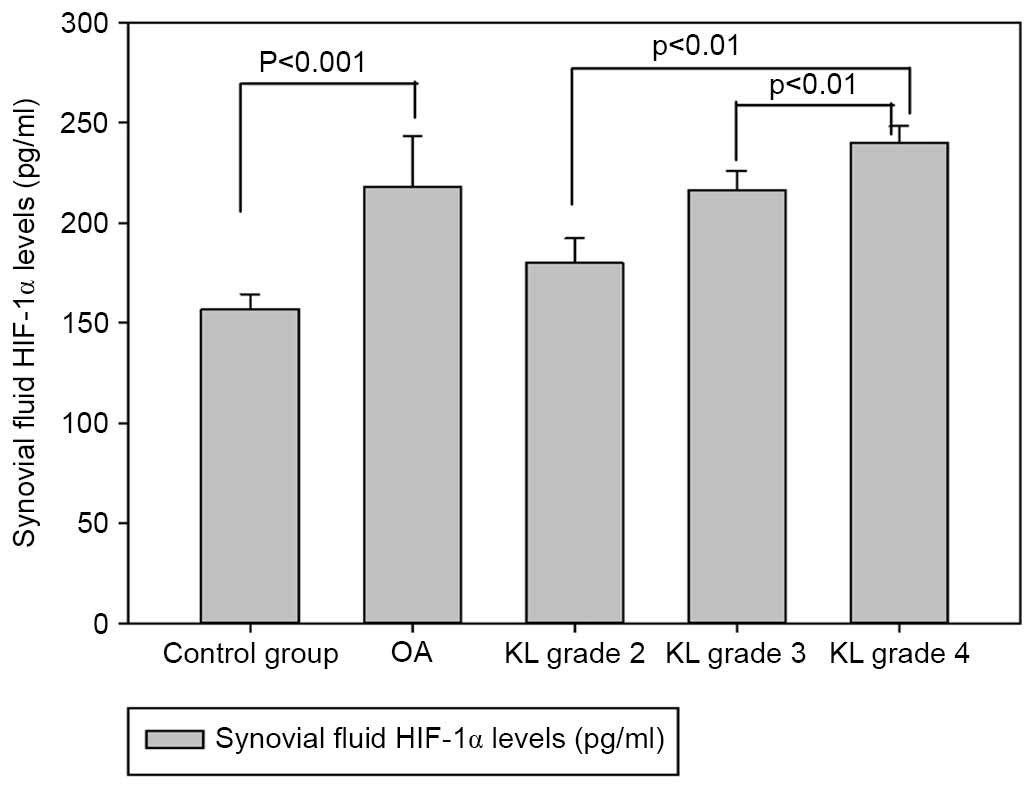
of patients with knee OA are demonstrated in Fig. 3. OA patients exhibited higher HIF-1α
concentrations compared with the healthy controls (218.17±25.12 vs.
156.66±7.74 pg/ml, P<0.001). Synovial fluid concentrations of
HIF-1α were compared and analyzed in relation to the radiological
KL grading values of OA. The concentrations of HIF-1α in the
synovial fluid of KL grade 2 were 179.91±12.49 pg/ml [95%
confidence interval (CI), 169.47–190.36], those from KL grade 3
were 216.37±9.51 pg/ml (95% CI, 210.62–222.12), whereas those from
KL grade 4 were 240.14±8.16 pg/ml (95% CI, 235.62–244.66). The data
indicated that synovial fluid levels of HIF-1α in cartilage graded
as KL grade 4 were significantly increased compared with those of
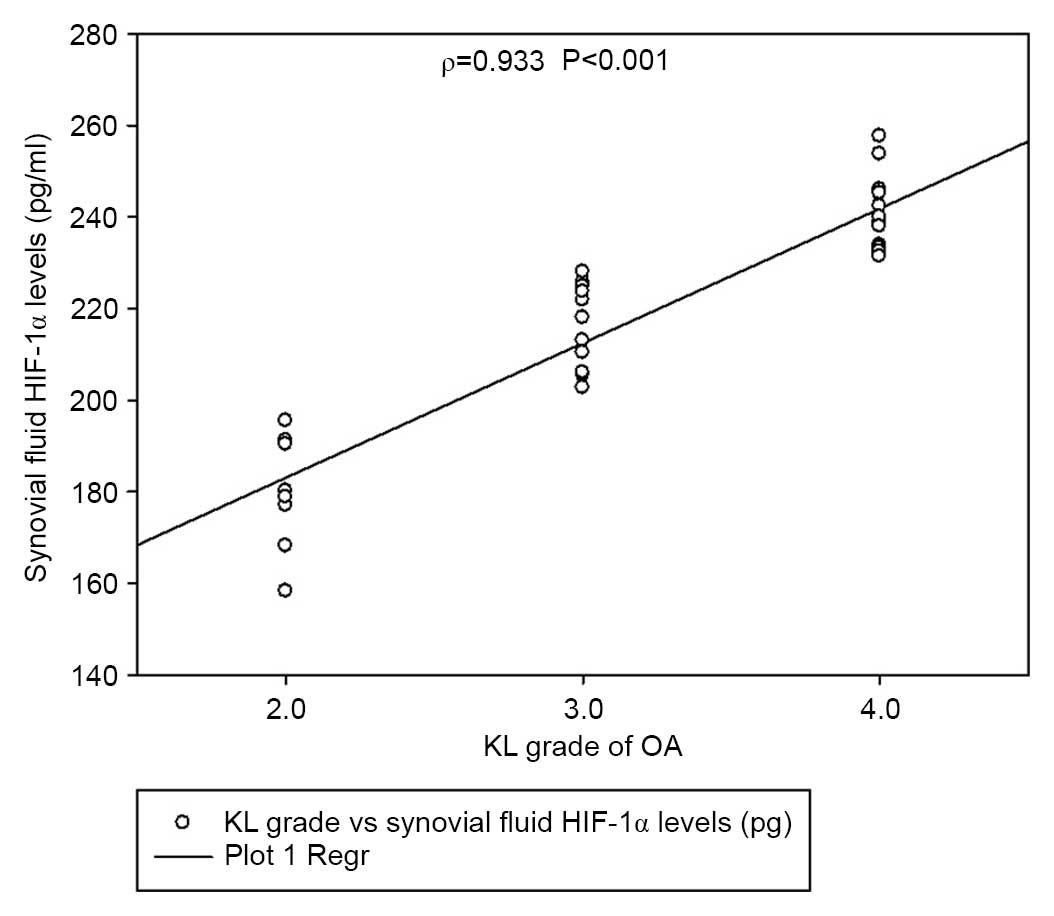
KL grade 2 and 3 (P<0.01). The levels of HIF-1α also correlated
with the severity of disease (Spearman's ρ=0.933, P<0.001;
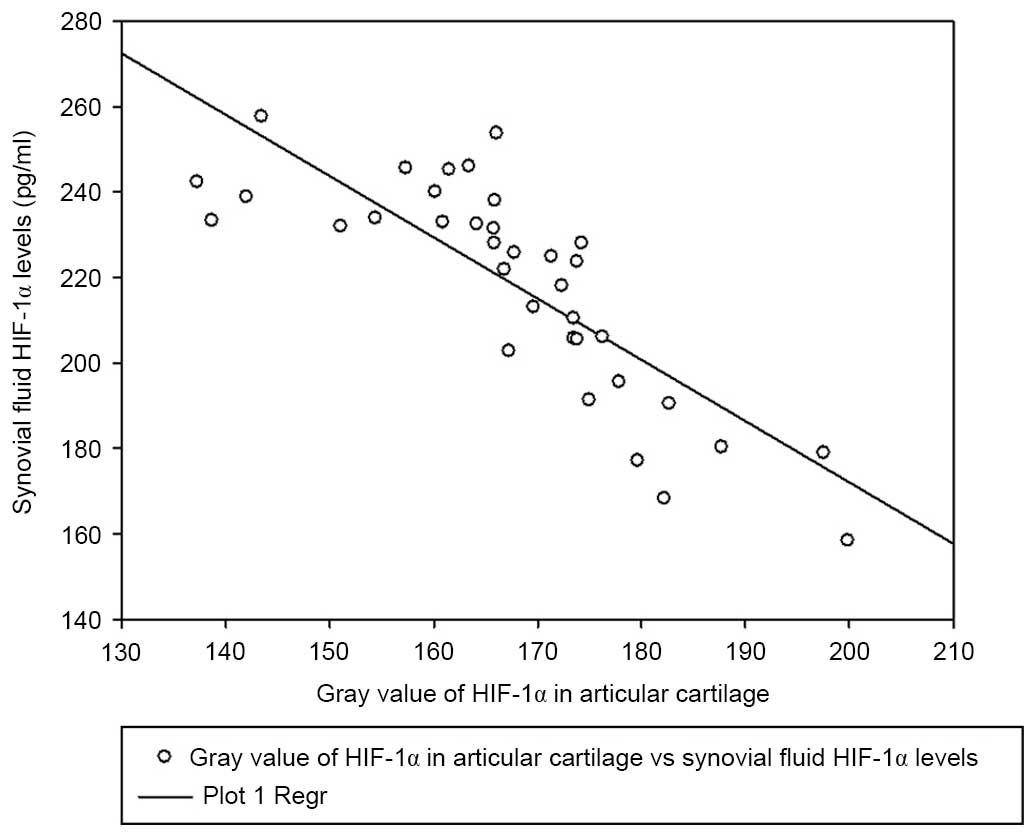
Fig. 4). Notably, synovial fluid
HIF-1α concentrations exhibited a significant correlation with
articular cartilage HIF-1α expression levels (Pearson's ρ=−0.815;
P<0.001; Fig. 5).
Discussion
To the best of our knowledge, the present study is
the first to evaluate the levels of HIF-1α in articular cartilage
and synovial fluid and its correlation with the severity of knee OA
disease. The present findings demonstrated a marked increase in
HIF-1α levels in the articular cartilage and synovial fluid of
patients with knee OA compared with the controls. Previous studies
have indicated that human and bovine articular chondrocytes and
murine epiphyseal chondrocytes express HIF-1α (21–23).
HIF-1α in the synovial fluid is thought to have originated from
local tissues, such as the synovial membrane and articular
cartilage. Numerous studies have demonstrated that HIF-1α is a key
factor that influences articular chondrocyte behavior during
cartilage homeostasis and osteoarthritis (22–24).
HIF-1α is a highly conserved transcription factor that has
important functions in the control of energy generation, matrix
synthesis and cell survival by articular and growth-plate
chondrocytes (25,26). Previous studies have revealed that
the stabilization of HIF-1a may be a potential tool for increasing
cell vitality, matrix synthesis and cartilage integrity in patients
with osteoarthritis (24–26). Notably, synovial fluid HIF-1α
concentrations exhibited a correlation with articular cartilage
HIF-1α expression levels in the present study. Therefore, detecting
the levels of HIF-1α in synovial fluid may be used as a marker to
predict the degree of cartilaginous damage and disease
severity.
HIF-1α is degraded by an O2-dependent
mechanism. Under normoxic conditions, HIF-1α is hydroxylated to
allow binding of the von Hippel-Lindau tumor suppressor protein to
HIF-1α, and this binding triggers subsequent enzymatic degradation.
Under hypoxic conditions (<5% O2), HIF-1α is stable
and can be detected (8). HIF-1α
localizes into the nucleus to associate with HIF-1β to form a
heterodimer and activate hypoxia-inducible target genes (13). Hypoxia has previously been
demonstrated to increase matrix synthesis of epiphyseal
chondrocytes (27), and to induce
vascular endothelial growth factor (VEGF) expression in normal
chondrocytes via HIF-1α activity (28,29).
VEGF is a key angiogenesis factors, which is able to thicken the
synovial membrane, deposit new extracellular matrix and increase
the proliferation of synovial fibroblasts. All those factors are
essential for the development of OA. The importance of angiogenesis
in OA has been further elucidated by several authors in recent
publications that demonstrated that new blood vessels are not only
formed in synovium but also in other tissues (30,31). The
articular cartilage of patients with OA is invaded at the
osteochondral junction by blood vessels from the subchondral bone,
which subsequently induces an increase in blood vessel density in
the non-calcified cartilage (30).
HIF-1α also is a potent transactivator of numerous
genes, including various MMPs (MMP2, 3, 9 and 13) (15) and heat shock protein (28), which are known to act as cellular
chaperones for proteins that are misfolded under conditions of
cellular stress. Therefore, we hypothesize that HIF-1α has an
important role in O2-dependent signaling pathways in the
articular chondrocytes, as supported by previous research (32). Therefore, elevated HIF-1α levels may
be associated with the development of osteoarthritis.
The role of oxygen as an important modulator of gene
expression is well-recognized and extensive evidence has indicated
that HIF is the primary gene and regulatory factor that responds to
key variations in O2 levels (7,8). Genes
regulated at the transcription level by HIF-1α are associated with
numerous cellular functional events, including angiogenesis,
vascular reactivity and remodeling (9), vasomotor control, glucose and energy
metabolism (26), erythropoiesis,
iron homeostasis, pH regulation, cell proliferation and viability,
nucleotide metabolism, matrix metabolism, and metal transport
(29,30). Furthermore, HIF-1α is essential for
chondrogenesis, as it is associated with chondrocyte growth arrest,
survival, maturation, and apoptosis (33–35).
HIF-1α also regulates the configuration and maintenance of
articular cartilage via the induction of anabolic factors and the
suppression of key catabolic factors (36). The migration and invasion of
fibroblast-like synoviocytes (FLSs) have also been demonstrated to
be critical for the pathogenesis of OA (37,38). Li
et al (15) showed that
HIF-1α levels contribute to the migration and invasion of FLSs by
upregulating the expression of MMP2 and MMP9 via the activation of
the NF-κB/HIF-1α signalling pathway. These findings indicated that
levels of HIF-1α in both synovial fluid and cartilage may play an
important role in the pathogenesis of OA.
There are several potential limitations to the
present study. Firstly, the sample size was not large enough to
achieve definitive conclusions. Secondly, only those patients who
attended Xiangya Hospital for treatment of knee OA were
investigated. Thirdly, the cross-sectional design of the study
precluded addressing whether the analyzed level of HIF-1α predicted
alterations in the severity of knee OA.
In conclusion, patients with knee OA exhibited
elevated levels of HIF-1α compared with healthy controls in
synovial fluid. Furthermore, the expression of HIF-1α in articular
cartilage and synovial fluid was significantly correlated with the
severity disease in knee OA. Further studies are required to
elucidate the contribution of HIF-1α to the pathogenesis of OA.
Acknowledgments
This study was supported by grants from the National
Natural Science Foundation of China (grant no. 81371934), the
Open-End Fund for the Valuable and Precision Instruments of Central
South University (grant no. CSUZC2014046) and Hunan Provincial
Innovation Foundation For Postgraduates (grant no. CX2014B111).
References
|
1
|
Gallo J, Goodman SB, Konttinen YT, Wimmer
MA and Holinka M: Osteolysis around total knee arthroplasty: A
review of pathogenetic mechanisms. Acta Biomaterialia. 9:8046–8058.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
van den Berg WB: Osteoarthritis yearn
review: Pathomechanisms. Osteoarthritis Cartilage. 19:338–341.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Saito T and Kawaguchi H: HIF-2α as a
possible therapeutic target of osteoarthritis. Osteoarthritis
Cartilag. 18:1552–1556. 2010. View Article : Google Scholar
|
|
4
|
Lotz M: Osteoarthritis yearn review:
Biology. Osteoarthritis Cartilage. 20:192–196. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tonge DP, Pearson MJ and Jones SW: The
hallmarks of osteoarthritis and the potential to develop
personalised disease-modifying pharmacological therapeutics.
Osteoarthritis Cartilage. 22:609–621. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Alcaraz MJ, Megías J, García-Arnandis I,
Clérigues V and Guillén MI: New molecular targets for the treatment
of osteoarthritis. Biochem Pharmacol. 80:13–21. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Araldi E and Schipani E: Hypoxia, HIFs and
bone development. Bone. 47:190–196. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Coimbra IB, Jimenez SA, Hawkins DF,
Piera-Velazquez S and Stokes DG: Hypoxia inducible factor-1 alpha
expression in human normal and osteoarthritic chondrocytes.
Osteoarthritis Cartilage. 12:336–345. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cramer T, Schipani E, Johnson RS, Swoboda
B and Pfander D: Expression of VEGF isoforms by epiphyseal
chondrocytes during low-oxygen tension is HIF-1alpha dependent.
Osteoarthritis Cartilage. 12:433–439. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Duval E, Baugé C, Andriamanalijaona R,
Bénateau H, Leclercq S, Dutoit S, Poulain L, Galéra P and
Boumédiene K: Molecular mechanism of hypoxia-induced chondrogenesis
and its application in in vivo cartilage tissue engineering.
Biomaterialsm. 33:6042–6051. 2012. View Article : Google Scholar
|
|
11
|
Gelse K, Mühle C, Knaup K, Swoboda B,
Wiesener M, Henning F, Olk A and Schneider H: Chondrogenic
differentiation of growth factor-stimulated precursor cells in
cartilage repair tissue is associated with increased HIF-1alpha
activity. Osteoarthritis Cartilage. 16:1457–1465. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hong YH, Park CW, Kim HS, Won KC, Kim YW
and Lee CK: Effects of hypoxia/ischemia on catabolic mediators of
cartilage in a human chondrocyte, SW1353. Biochem Biophys Res
Commun. 431:478–483. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hatta TK, Kishimoto KN, Okuno H and Itoi
E: Lubricin expression is suppressed by hypoxia through HIF-1
mediated pathway. Osteoarthritis Cartilage. 20:S137–S138. 2012.
View Article : Google Scholar
|
|
14
|
Henrotin Y, Kurz B and Aigner T: Oxygen
and reactive oxygen species in cartilage degradation: Friends or
foes? Osteoarthritis Cartilage. 13:643–654. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li G, Zhang Y, Qian Y, Zhang H, Guo S,
Sunagawa M, Hisamitsu T and Liu Y: Interleukin-17A promotes
rheumatoid arthritis synoviocytes migration and invasion under
hypoxia by increasing MMP2 and MMP9 expression through NF-κB/HIF-1α
pathway. Mol Immunol. 53:227–236. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Koh MY and Powis G: Passing the baton: the
HIF switch. Trends Biochem Sci. 37:364–372. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang C, Yang F, Cornelia R, Tang W,
Swisher S and Kim H: Hypoxia-inducible factor-1 is a positive
regulator of Sox9 activity in femoral head osteonecrosis. Bone.
48:507–513. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Grimmer C, Balbus N, Lang U, Aigner T,
Cramer T, Müller L, Swoboda B and Pfander D: Regulation of Type II
collagen synthesis during osteoarthritis by prolyl-4-hydroxylases:
Possible influence of low oxygen levels. Am J Pathol. 169:491–502.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kellgren JH and Lawrence JS: Radiological
assessment of osteo-arthrosis. Ann Rheum Dis. 16:494–502. 1957.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Van der Sluijs JA, Geesink RG, Van der
Linden AJ, Bulstra SK, Kuyer R and Drukker J: The reliability of
the Mankin scores for osteoarthritis. J Orthop Res. 10:58–61. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang RX, Ren K and Dubner R:
Osteoarthritis pain mechanisms: Basic studies in animal models.
Osteoarthritis Cartilage. 21:1308–1315. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Meretoja VV, Dahlin RL, Wright S, Kasper
FK and Mikos SG: The effect of hypoxia on the chondrogenic
differentiation of co-cultured articular chondrocytes and
mesenchymal stem cells in scaffolds. Biomaterials. 34:4266–4273.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Nomura T, Aoyama M, Waguri-Nagaya Y, Goto
Y, Suzuki M, Miyazawa K, Asai K and Goto S: Tumor necrosis factor
stimulates osteoclastogenesis from human bone marrow cells under
hypoxic conditions. Exp Cell Res. 321:167–177. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lin JL, Wang MJ, Lee DC, Liang CC and Lin
SK: Hypoxia-inducible factor-1alpha regulates matrix
metalloproteinase-1 activity in human bone marrow-derived
mesenchymal stem cells. FEBS Lett. 582:2615–2619. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Makris EA, Hu JC and Athanasiou KA:
Hypoxia-induced collagen crosslinking as a mechanism for enhancing
mechanical properties of engineered articular cartilage.
Osteoarthritis Cartilage. 21:634–641. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ren BF, Deng LF, Wang J, Zhu YP, Wei L and
Zhou Q: Hypoxia regulation of facilitated glucose transporter-1 and
glucose transporter-3 in mouse chondrocytes mediated by HIF-1alpha.
Joint Bone Spine. 75:176–181. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Murata M, Yudoh K and Masuko K: The
potential role of vascular endothelial growth factor (VEGF) in
cartilage: How the angiogenic factor could be involved in the
pathogenesis of osteoarthritis? Osteoarthritis Cartilage.
16:279–286. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhu G, Tang Y, Liang X, Zheng M, Yang J,
Zhou H, Li L and Qin T: Role of hypoxia-inducible factor-1 alpha in
the regulation of plasminogen activator activity in rat knee joint
chondrocytes. Osteoarthritis Cartilage. 17:1494–1502. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhu M, Feng Q and Bian LM: Differential
effect of hypoxia on human mesenchymal stem cell chondrogenesis and
hypertrophy in hyaluronic acid hydrogels. Acta Biomater.
10:1333–1340. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chim SM, Tickner J, Chow ST, Kuek V, Guo
BS, Zhang G, Rosen V, Erber W and Xu JK: Angiogenic factors in bone
local environment. Cytokine Growth Factor Rev. 24:297–310. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Matsuki TY, Arai Y, Tsuchida S, Terauchi
R, Inoue H, Nakagawa S, Inoue A, Mazda O and Kubo T: The roles of
heat shock protein 70 on chondrocyte. Osteoarthritis Cartilage.
21:S1272013. View Article : Google Scholar
|
|
32
|
Pfander D, Swobodal B and Cramer T: The
role of HIF-1alpha in maintaining cartilage homeostasis and during
the pathogenesis of osteoarthritis. Arthritis Res Ther. 8:1042006.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Neve A, Cantatore FP, Corrado A, Gaudio A,
Ruggieri S and Ribatti D: In vitro and in vivo angiogenic activity
of osteoarthritic and osteoporotic osteoblasts is modulated by VEGF
and vitamin D3 treatment. Regul Pept. 184:81–84. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Pufe T, Lemke A, Kurz B, Petersen W,
Tillmann B, Grodzinsky AJ and Mentlerin R: Mechanical overload
induces VEGF in cartilage discs via hypoxia-inducible factor. Am J
Pathol. 164:185–192. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Okada KY, Hosaka Y, Kobayashi H, Sugita S,
Chang S, Mori Y, Akiyama H, Tanaka S, Kawaguchi H and Saito T:
HIF-1α regulates configuration and maintenance of articular
cartilage through induction of anabolic factors and suppression of
catabolic factors. Osteoarthritis Cartilage. 22:S164–S165. 2014.
View Article : Google Scholar
|
|
36
|
Sakamoto J, Origuchi T, Okita M, Nakano J,
Kato K, Yoshimura T, Izumi S, Komori T, Nakamura H, Ida H, et al:
Immobilization-induced cartilage degeneration mediated through
expression of hypoxia-inducible factor-1alpha, vascular endothelial
growth factor, and chondromodulin-I. Connect Tissue Res. 50:37–45.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhang C, Wei X, Chen C, Cao K, Li Y, Jiao
Q, Ding J, Zhou J, Fleming BC, Chen Q, et al: Indian hedgehog in
synovial fluid is a novel marker for early cartilage lesions in
human knee joint. Int J Mol Sci. 15:7250–7265. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Walsh DA, Bonnet CS, Turner EL, Wilson D,
Situ M and Mcwilliams SF: Angiogenesis in the synovium and at the
osteochondral junction in osteoarthritis. Osteoarthritis Cartilage.
15:743–751. 2007. View Article : Google Scholar : PubMed/NCBI
|