Introduction
The natural pathology of spinal cord injury (SCI)
progression usually includes primary and secondary phases of injury
that finally result in severe sensory and motor deficits beyond the
level of the injury site (1).
Primary traumatic mechanical SCI often results in the death of a
number of neurons that can neither be recovered nor regenerated.
The secondary SCI process is characterized by demyelination, neural
apoptosis and post-traumatic inflammatory reactions as a
homeostatic response aimed to clear debris at the lesion site,
while simultaneously preserving organ function (2–4).
However, if the post-traumatic inflammatory reaction is
uncontrolled, it can cause the initial lesion to be enlarged by
means of additional axonal damage, oligodendrocyte death and
demyelination with concomitant increased loss of neurological
function, which is a potentially avoidable event that can be
regulated or reduced by avoiding additional neural death and
defending against functional deficits (5,6). There
is evidence that leukocytes, particularly T lymphocytes and
macrophages that infiltrate the injured spinal cord, are directly
involved in the pathogenesis and extension of SCI (7).
SCI remains a very complex medical and psychological
challenge. Notably, previous studies conducted by authors of the
present study demonstrated that the use of an acellular spinal cord
(ASC) scaffold seeded with bone marrow stromal cells (BMSCs)
conveyed potential benefits to the repair of SCIs by protecting
native tissues and promoting functional recovery in a rat model of
SCI (8,9). However, whether stem cells or scaffolds
are able to ameliorate secondary and extended inflammation to the
lesion site, thereby contributing to SCI repair, has yet to be
explored.
The aim of the present study was to investigate
whether the use of an ASC scaffold seeded with BMSCs was sufficient
to restore the damaged spinal cord and improve functional recovery
in a rat spinal cord hemisected model of SCI through regulation of
apoptosis and the local inflammatory response.
Materials and methods
BMSC proliferation and preparation of
the ASC scaffold
BMSCs were prepared from the femurs and tibias of
6-week-old Sprague-Dawley (SD) rats, as described in a previous
study (8). Briefly, BMSCs were
isolated from the marrow cavity and then filtered through a 70-µm
nylon mesh to remove any remaining clumps of tissue. After
centrifugation at 200 × g for 5 min, the collected cells
were placed in 75-cm2 culture flasks and cultured in
Dulbecco's modified Eagle's medium containing 20% fetal bovine
serum (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA) and
1% penicillin and streptomycin (Invitrogen; Thermo Fisher
Scientific, Inc.). Following incubation for 72 h, non-adherent
cells were removed by washing with phosphate-buffered saline (PBS).
The culture medium was replaced every 4 days. The cells after three
passages were used for subsequent experiments.
The ASC scaffold was prepared as described
previously (9). Briefly, the
thoracic spinal cords were harvested from sacrificed adult SD rats
and the ASC was chemically extracted. The thoracic spinal cord was
treated with a series of detergents consisting of distilled water,
Triton X-100 (Amresco LLC, Solon, OH, USA) and sodium deoxycholate
solution (Amresco LLC). All samples were then freeze-dried for 24 h
and sterilized by irradiation with cobalt-60 gamma rays (3 kGy)
prior to usage.
Spinal cord hemisection model and
graft transplantation
The study protocol was approved by the Ethics
Committee for Animal Research of Ningxia People's Hospital
(Yinchuan, China) and performed in accordance with international
standards for animal welfare. All surgical procedures were
performed under anesthesia produced by the intraperitoneal
injection of 10% chloral hydrate (0.4 ml/100 g; Guangzhou Chemical
Plant, Guangzhou, China). A total of 24 adult male SD rats (body
weight, 200–250 g) were provided by Ningxia Medical University
Animal Center, Yinchuan, China) and maintained in housing with a
constant temperature (25°C, 50±5°C humidity), a 12-h light/dark
cycle and free access to food and water. Rats underwent laminectomy
at the T9-10 level to expose one thoracic spinal cord segment. A
small incision was made in the right dorsal spinal cord and a
22-gauge needle made of ethylene tetrafluoroethylene was used to
remove 2 mm of the right hemicord (9). The rats were then randomly divided into
three experimental groups according to the type of graft: i)
Animals that received implantation of an ASC scaffold with BMSCs
(n=8), ii) animals that received implantation of an ASC scaffold
with no BMSCs (n=8), and iii) animals for which the lesion cavity
was left empty, as a control group (n=8). After surgery, all rats
received daily subcutaneous ampicillin (100 mg/kg) for 3 days to
avoid infection, and their bladders were expressed manually twice
per day until bladder control was regained.
Behavioral analysis
Motor function of the right hind limb was assessed
in an open-field via the Basso, Beattie and Bresnahan (BBB)
locomotor scoring system on a scale from 0 to 21 (10). Briefly, individual rats were placed
on an open field at 2 and 8 weeks after transplantation and
observed for 4 min by two observers blinded to the treatment.
Tissue processing
The animals were anesthetized at the end of each
study using an overdose of chloral hydrate. After transcardial
perfusion, which was performed as previously described (9), the spinal cords were dissected, fixed
in 4% paraformaldehyde solution overnight, cryoprotected in a 30%
sucrose solution in PBS, then embedded in Tissue-Tek Optimal
Cutting Temperature compound mounting media (Sakura Finetek USA,
Inc., Torrance, CA, USA) and cut into 5-µm coronal sections using a
cryostat. Every seventh section was stained with hematoxylin and
eosin (H&E).
Histological and quantitative
analysis
Immunofluorescence was used to assess the presence
of markers of inflammatory cells around the SCI lesion site at 2
weeks after transplantation and the sections were stained with
H&E to observe morphological aspects of the spinal cord from
rats in each experimental group (n=3 per group) at 8 weeks after
transplantation. Briefly, spinal cord sections were washed with PBS
and permeabilized with 0.1% Triton X-100 for 5 min. After washing
in PBS again, the sections were blocked with 10% bovine serum
albumin (Sigma-Aldrich; Merck Millipore, Darmstadt, Germany).
Fluorescein isothiocyanate-labeled primary antibodies against
immunoglobulin M (FITC anti-rat IgM; dilution, 1:100; cat. no. RUO
400801; BioLegend, Inc., San Diego, CA, USA), macrophages
(anti-CD68/FITC; 100 µg; dilution, 1:100; cat. no. BS-0649R; Bioss,
Inc., Woburn, MA, USA), and T lymphocytes (anti-CD5/FITC; dilution,
1:100; cat. no. BS-10218R; Bioss, Inc.) were used to evaluate each
section. Finally, each section was stained with
4,6-diamidino-2-phenylindole (DAPI; Molecular Probes; Thermo Fisher
Scientific, Inc.) to visualize nucleated cells.
Terminal deoxynucleotidyl transferase
dUTP nick end labeling (TUNEL)
A TUNEL kit (Nanjing Keygen Biotech Co., Ltd.,
Nanjing, China) was used to evaluate the extent of apoptosis,
according to the manufacturer's instructions. Tissue sections were
analyzed (n=3 per group) to detect the localization of apoptotic
cells, indicated by green fluorescence, and cell nuclei, indicated
by blue fluorescence using fluorescence microscopy.
Quantitation of positive cells in
spinal cord tissues
Slides prepared as outlined above were examined
using a light microscope at ×40 magnification. Positive cells were
counted in three representative sections from at least four animals
in each group. The results are presented as the mean plus standard
deviation number of positive cells per field.
Statistical analysis
Quantitative data from locomotor behavior scores and
the number of cells positive for markers of inflammation are
expressed as the mean ± standard deviation or mean plus standard
deviation, respectively. P<0.05 was considered to indicate a
statistically significant difference. Statistical analysis was
performed by analysis of variance followed by Bonferroni's post-hoc
test (multiple-comparisons tests) among three groups using SPSS
statistical software (version 13.0; SPSS, Inc., Chicago, IL,
USA).
Results
Isolation of BMSCs and seeding of ASC
scaffolds transplanted into hemisected spinal cords
BMSCs were successfully isolated from the marrow of
femurs and tibias of anesthetized rats. After adherence and passage
three times, the BMSCs were collected ex vivo via chemical
extraction and seeded in ASC scaffolds. The ASC scaffolds with or
without seeded BMSCs were implanted immediately into spinal cord
hemisection cavities to bridge lesion sites after surgery.
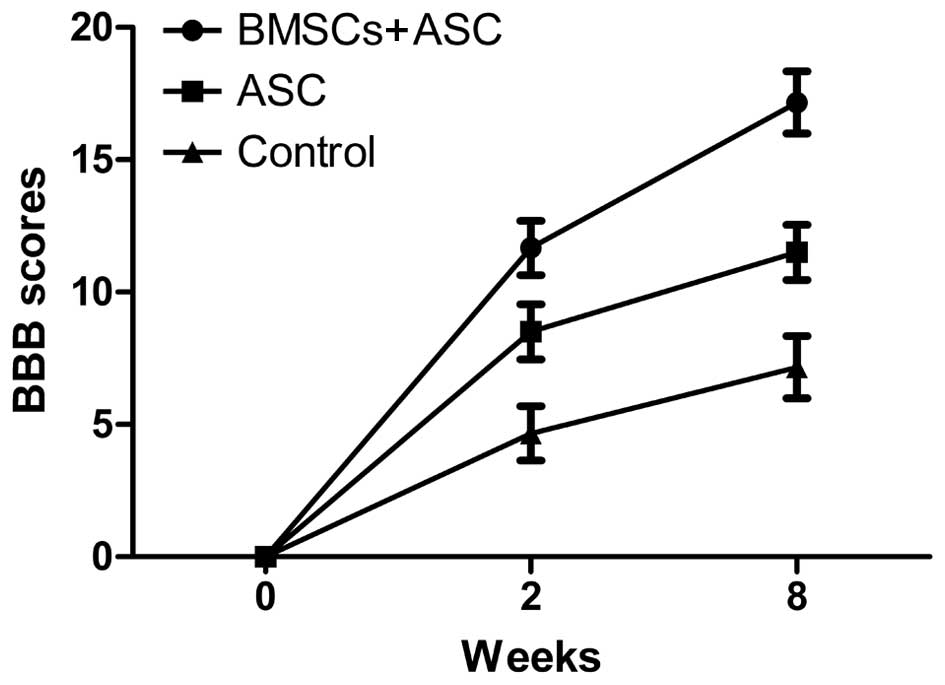
Assessment of locomotor behavior
All animals survived surgery and were subsequently
used for analysis. The BBB scoring behavioral assessment system was
used to assess locomotor recovery. The rats in each experimental
group showed gradual improvement in functional recovery during the
convalescent period. However, at 2 and 8 weeks after SCI, the rats
implanted with ASC scaffolds seeded with BMSCs showed significantly
enhanced BBB scores compared with the ASC group and the control
group (12.45±0.54 and 17.92±0.46 for the BMSCs + ASC group vs.
9.32±0.70 and 12.23±0.80 for the ASC group and 5.23±0.67 and
7.93±0.46 for the control group, respectively). These results
showed that BMSCs seeded in ASC scaffolds improved the recovery of
motor function following surgery (Fig.
1).
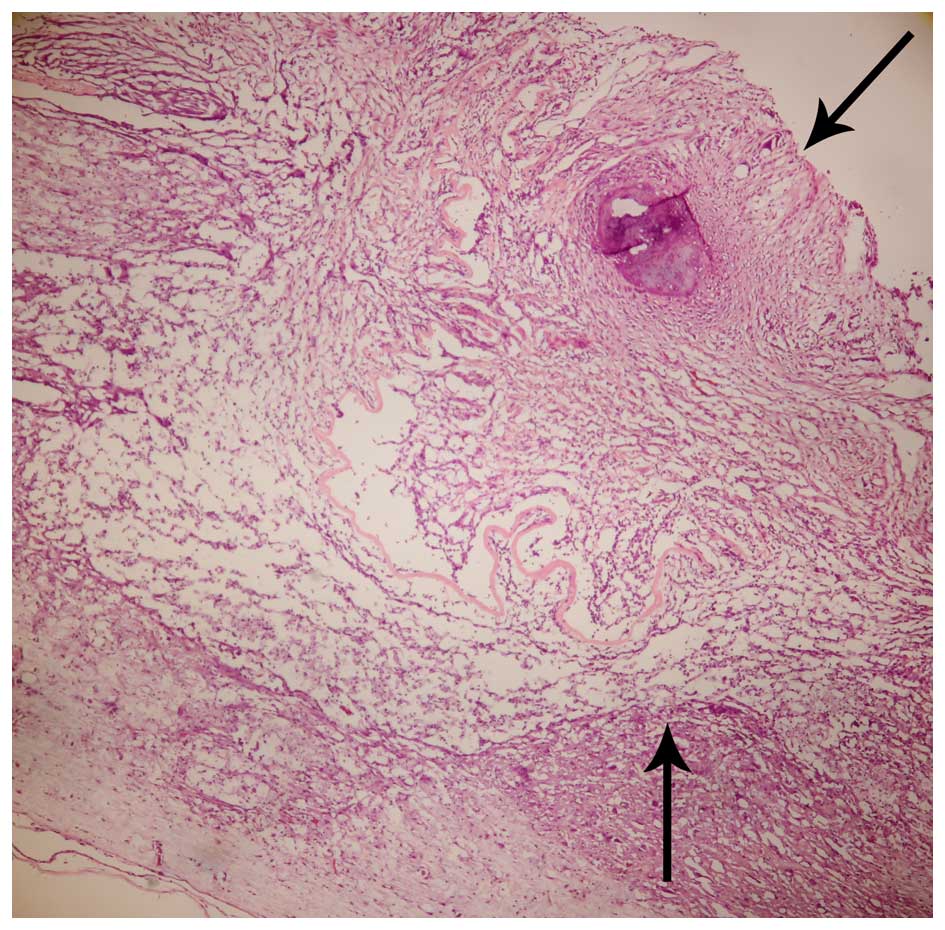
Histological and immunofluorescence
analysis
BMSCs adhering to ASC scaffolds were implanted into
the spinal cord hemisection cavities and were found to adhere well
with native tissue and to form a bridge across the lesion cavity at
8 weeks after transplantation (Fig.
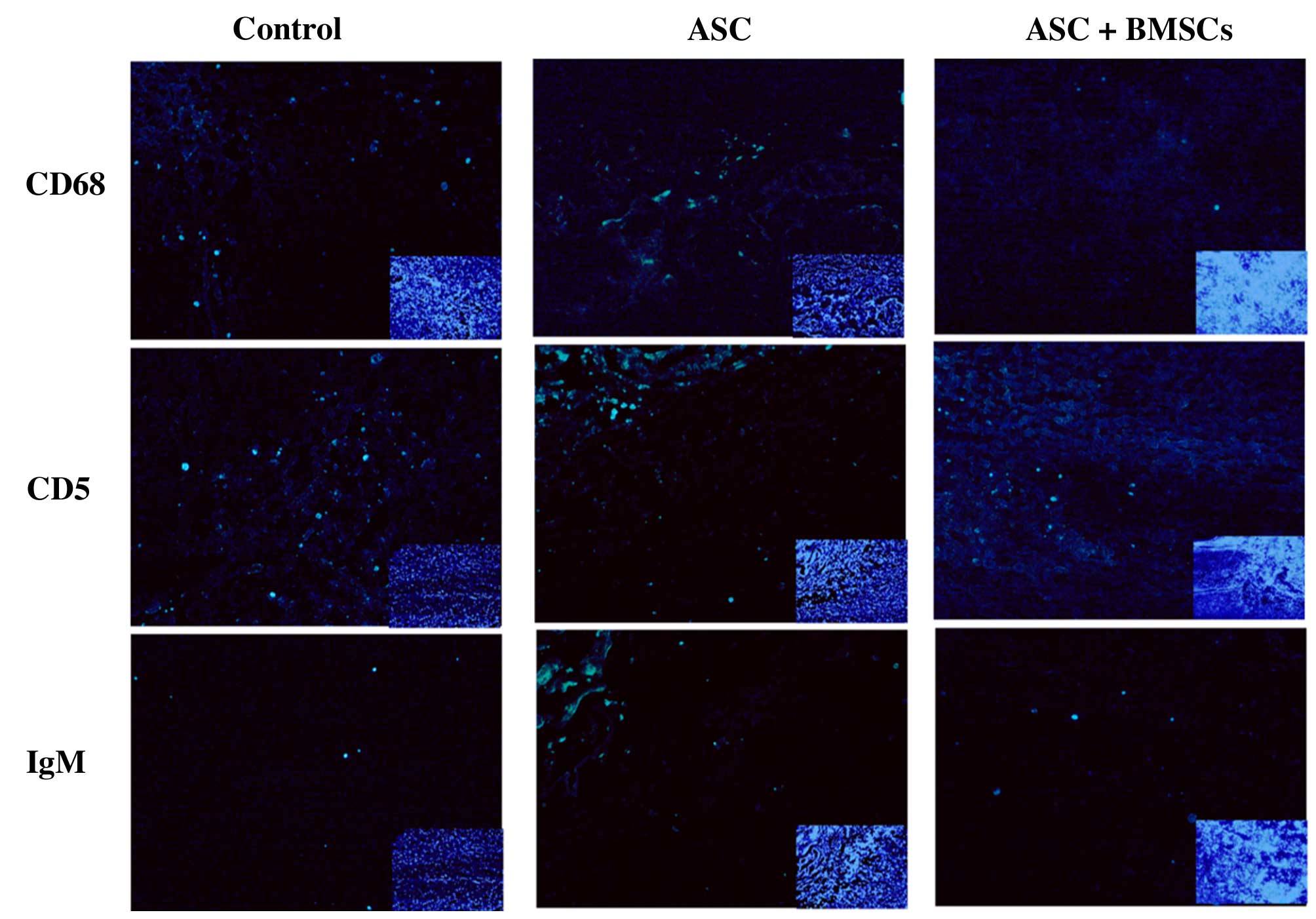
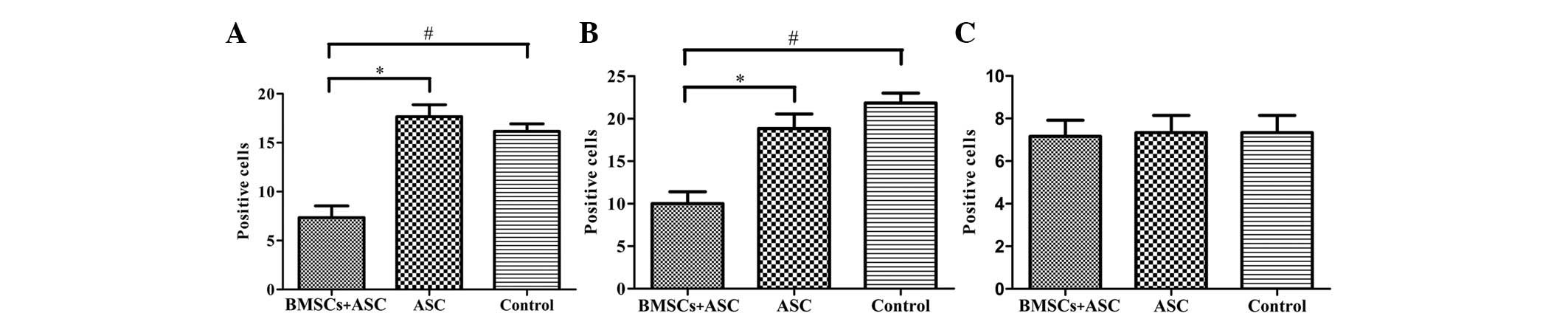
2). The effect of BMSCs seeded in ASC scaffolds on inflammatory
cell regulation following SCI was evaluated by immunofluorescent
staining for markers of inflammatory cells around the lesion sites
at 2 weeks after surgery. CD68 and CD5 markers were used to
evaluate the presence of macrophages and T lymphocytes,
respectively. When BMSCs seeded in ASC scaffolds were implanted,
the numbers of macrophages (microglia) and T lymphocytes
(P<0.05) were significantly decreased around the SCIs when
compared with the other groups at 2 weeks after surgery. However,
the results of IgM staining revealed no significant differences in
IgM-positive expression levels among the three groups (P>0.05;
Figs. 3 and 4). These results indicate that BMSCs played
a role in decreasing the number of inflammatory cells at the
surgical site.
Analysis of cell apoptosis
Tissue specimens were analyzed by TUNEL assay to
detect the localization of apoptotic cells by green fluorescence
and of cell nuclei by blue fluorescence using fluorescence
microscopy. The extent of apoptosis among the BMSCs in the ASC
scaffold group was significantly decreased, as compared with the
other groups (P<0.05; Fig. 5).
However, there was no significant difference between the ASC group
and the control group, indicating that the seeded BMSCs effectively
resisted apoptosis.
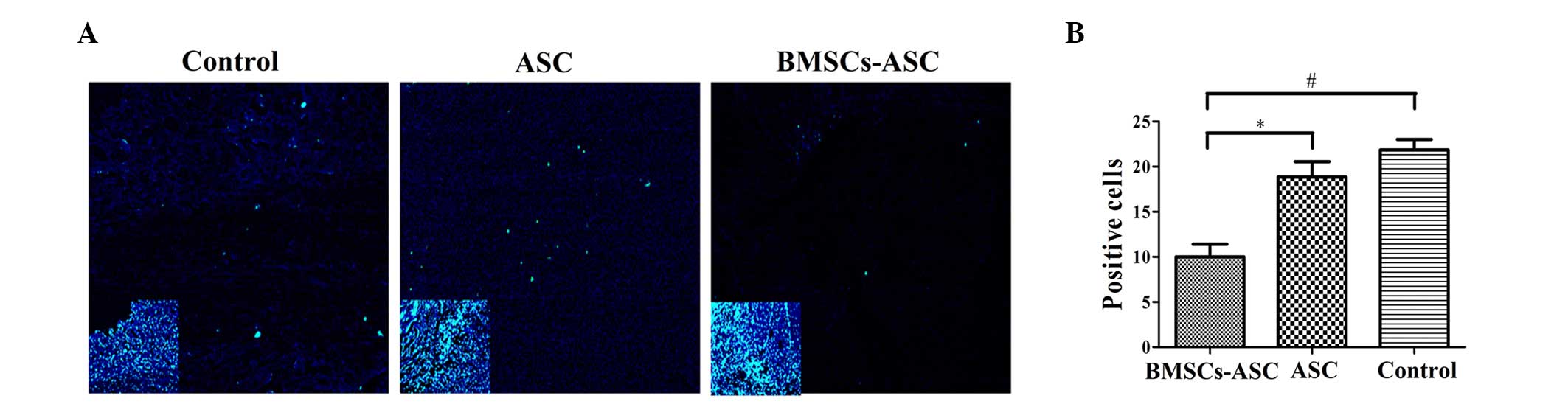 | Figure 5.Distribution of TUNEL-positive cells
around the injury sit (green, TUNEL; blue, DAPI). (A) Less positive
TUNEL staining was visible at the injury sites of the ASC + BMSCs
group. Main image, magnification ×400; inset image, magnification
×40. Insets indicate DAPI staining of contraction figure in main
images. (B) Quantitative comparison of the positive cells shown by
TUNEL among groups. Significantly fewer positively stained cells
were observed in the BMSCs + ASC group at 2 weeks post-spinal cord
injury compared with that in the other two groups (*P<0.05,
#P<0.05). TUNEL, terminal deoxynucleotidyl
transferase dUTP nick end labeling; DAPI,
4′,6-diamidino-2-phenylindole; ASC, acellular spinal cord; BMSCs,
bone marrow stromal cells. |
Discussion
In the current study, ASC scaffolds seeded with
BMSCs were implanted into spinal cord defects in hemisections of SD
rats with SCI, as described in our previous study (8), to investigate the roles of BMSCs in the
control of inflammation around grafts in the repair of SCIs. SCI
causes the blood-spinal cord barrier to break down, which elicits
an inflammatory response and progressive hemorrhagic necrosis and
apoptosis at the lesion epicenter. As a result, catastrophic
destruction of the structure of the spinal cord occurs with
subsequent functional disability below the injury site (11). Accordingly, control of inflammation
holds promise for the improvement of SCI repair (12). The results of the present study
demonstrated that BMSCs seeded in ASC scaffolds are able to
decrease the distribution of blood-derived immune cells around the
SCI to promote functional recovery.
The results also indicated that ASC scaffolds seeded
with BMSCs have good biocompatibility when used to bridge lesions
in the host spinal cord. Moreover, the use of an ASC scaffold alone
or with BMSCs significantly improved the recovery of locomotor
function, in accordance with the results of our previous study
(8). BBB scores also indicated that
rats implanted with ASC scaffolds seeded with BMSCs exhibited
better recovery of locomotor function than those treated with ASC
scaffold alone or the untreated SCI group at 2 and 8 weeks after
SCI. The use of BMSCs seeded in ASC scaffolds is a novel approach
for the control of inflammation and reduction of tissue damage to
promote functional recovery after SCI.
Markers of immune cells were used to evaluate the
inflammatory response of the grafts implanted for SCI repair.
Previous evidence suggests that leukocytes, particularly T
lymphocytes and macrophages, which infiltrate the injured spinal
cord are directly involved in the pathogenesis and extension of
SCIs. Moreover, certain inflammatory processes, such as the
production of cytokines, proteolytic enzymes and oxidative
metabolites, exacerbate injury (13). In addition, inflammatory reactions
can occur for weeks after SCI, with induction of the recruitment of
macrophages and T cells from hours to weeks after injury (14,15). The
results of the present study demonstrated that the extent of
inflammation caused by hematogenous macrophages and T lymphocytes
in the SCI site was decreased by BMSCs seeded in scaffolds,
suggesting that the BMSCs decreased the migration of inflammatory
cells (16,17). A possible explanation of this
phenomenon is that BMSCs have low expression levels of
histocompatibility complex antigens (class II) and release soluble
factors and cytokines to regulate the inflammation reaction in
response to SCI (17,18). However, neither ASC alone or ASC
combined with BMSCs showed a regulatory effect on IgM expression.
On the basis of these observations, BMSCs appear to convey an
advantage in cell-based therapy protocols, as transplantation
improved the condition of the microenvironment and promoted motor
function by reducing the recruitment of inflammatory cells early
after trauma to improve SCI repair.
Apoptosis is a physiological process of programmed
cell death that is essential for normal tissue development. Thus,
resistance to apoptosis following SCI can directly benefit the
repair of neural cells. In the present study, extensive cell
apoptosis was observed by TUNEL assay in the control group and the
group treated with ASC scaffold alone, but apoptosis was
significantly decreased in rats implanted with ASC plus BMSCs.
Hence, BMSCs play an important role by inhibiting the extent of
apoptosis of cells in both the white and grey matter of the spinal
cord. Although the cell types were not identified, it may be
speculated that they are oligodendroglia cells, on the basis of our
previous study (8).
In conclusion, the findings of this study
demonstrated that BMSCs with ASC scaffolds significantly improve
functional recovery through control of apoptosis and inflammation
in the early recovery period following SCI trauma. Therefore, this
model presents a potential therapeutic strategy for repair of SCIs.
However, future studies are required to further elucidate the
molecular mechanisms underlying the reestablishment of spinal cord
function.
Acknowledgements
This study was supported by a grant from the Natural
Science Foundation of Ningxia Province (grant no. NZ14288).
References
|
1
|
Rossi SL and Keirstead HS: Stem cells and
spinal cord regeneration. Curr Opin Biotechnol. 20:552–562. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Enzmann GU, Benton RL, Talbott JF, Cao Q
and Whittemore SR: Functional considerations of stem cell
transplantation therapy for spinal cord repair. J Neurotrauma.
23:479–495. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bartholdi D and Schwab ME:
Methylprednisolone inhibits early inflammatory processes but not
ischemic cell death after experimental spinal cord lesion in the
rat. Brain Res. 672:177–186. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Beck KD, Nguyen HX, Galvan MD, Salazar DL,
Woodruff TM and Anderson AJ: Quantitative analysis of cellular
inflammation after traumatic spinal cord injury: Evidence for a
multiphasic inflammatory response in the acute to chronic
environment. Brain. 133:433–447. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Amar AP and Levy ML: Pathogenesis and
pharmacological strategies for mitigating secondary damage in acute
spinal cord injury. Neurosurgery. 44:1027–1039; discussion
1039–1040. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Esposito E, Mazzon E, Paterniti I,
Impellizzeri D, Bramanti P and Cuzzocrea S: Olprinone attenuates
the acute inflammatory response and apoptosis after spinal cord
trauma in mice. PloS One. 5:e121702010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Esposito E, Bruscoli S, Mazzon E,
Paterniti I, Coppo M, Velardi E, Cuzzocrea S and Riccardi C:
Glucocorticoid-induced leucine zipper (GILZ) over-expression in T
lymphocytes inhibits inflammation and tissue damage in spinal cord
injury. Neurotherapeutics. 9:210–225. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chen J, Zhang Z, Liu J, Zhou R, Zheng X,
Chen T, Wang L, Huang M, Yang C, Li Z, et al: Acellular spinal cord
scaffold seeded with bone marrow stromal cells protects tissue and
promotes functional recovery in spinal cord-injured rats. J
Neurosci Res. 92:307–317. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liu J, Chen J, Liu B, Yang C, Xie D, Zheng
X, Xu S, Chen T, Wang L, Zhang Z, et al: Acellular spinal cord
scaffold seeded with mesenchymal stem cells promotes long-distance
axon regeneration and functional recovery in spinal cord injured
rats. J Neurol Sci. 325:127–136. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Basso DM, Beattie MS and Bresnahan JC: A
sensitive and reliable locomotor rating scale for open field
testing in rats. J Neurotrauma. 12:1–21. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bracchi-Ricard V, Lambertsen KL, Ricard J,
Nathanson L, Karmally S, Johnstone J, Ellman DG, Frydel B, McTigue
DM and Bethea JR: Inhibition of astroglial NF-κB enhances
oligodendrogenesis following spinal cord injury. J
Neuroinflammation. 10:922013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang CY, Chen JK, Wu YT, Tsai MJ, Shyue
SK, Yang CS and Tzeng SF: Reduction in antioxidant enzyme
expression and sustained inflammation enhance tissue damage in the
subacute phase of spinal cord contusive injury. J Biomed Sci.
18:132011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
de Rivero Vaccari JP, Lotocki G, Marcillo
AE, Dietrich WD and Keane RW: A molecular platform in neurons
regulates inflammation after spinal cord injury. J Neurosci.
28:3404–3414. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bareyre FM and Schwab ME: Inflammation,
degeneration and regeneration in the injured spinal cord: Insights
from DNA microarrays. Trends Neurosci. 26:555–563. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bethea JR and Dietrich WD: Targeting the
host inflammatory response in traumatic spinal cord injury. Curr
Opin Neurol. 15:355–360. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lei J, Wang Z, Hui D, Yu W, Zhou D, Xia W,
Chen C, Zhang Q and Xiang AP: Ligation of TLR2 and TLR4 on murine
bone marrow-derived mesenchymal stem cells triggers differential
effects on their immunosuppressive activity. Cell Immunol.
271:147–156. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Watanabe S, Uchida K, Nakajima H, Matsuo
H, Sugita D, Yoshida A, Honjoh K, Johnson WE and Baba H: Early
transplantation of mesenchymal stem cells after spinal cord injury
relieves pain hypersensitivity through suppression of pain-related
signaling cascades and reduced inflammatory cell recruitment. Stem
Cells. 33:1902–1914. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kim HJ, Park JB, Lee JK, Park EY, Park EA,
Riew KD and Rhee SK: Transplanted xenogenic bone marrow stem cells
survive and generate new bone formation in the posterolateral
lumbar spine of non-immunosuppressed rabbits. Eur Spine J.
17:1515–1521. 2008. View Article : Google Scholar : PubMed/NCBI
|