Introduction
Diabetes mellitus, a complex metabolic disease in
lipids, carbohydrates and proteins, is known to be the third
leading cause of mortality worldwide (1). As reported, non-insulin-dependent
diabetes mellitus is known as the most common form of diabetes type
II diabetes (2). According to
statistics, there will be 8 billion individuals suffering from type
II diabetes in 2025. Insulin secretion deficiency, increased levels
of blood glucose, organ damage and nephropathy are various
complications observed in most patients with diabetes (3,4). The
most typical therapeutic regimen for diabetes can only regulate
glycometabolism, insulin level and microcirculation (5), and fails to control diabetic
complications. However, traditional therapies, including insulin
injection and oral anti-hyperglycemic agents, will cause some
serious adverse effects (6), such as
hepatocellular-cholestatic liver injury, diarrhea, hypoglycemia,
weight gain and even gastrointestinal disturbances (6). Therefore, a search for alternative
treatment is required.
Due to their pharmacological functions,
polysaccharides separated from herbs attract much attention.
Polysaccharide obtained from large yellow croaker swim bladder
exhibits curative effects on nephritis via regulating nuclear
factor kappa B (NF-κB)-associated signaling pathways (7). Polysaccharide-enriched Cordyceps
militaris extract displays significant hypoglycemic and
anti-nephritic activities in established type II diabetic rats
(8). Auricularia auricular,
one of the most important artificial cultivation mushrooms, is rich
in hetero-polysaccharides. In various established in vitro
systems, two types of sulfated polysaccharide obtained from
Auricularia auricular show potential anti-oxidant activities
(9). Another study demonstrates that
purified Auricularia auricular polysaccharide (AAP) shows a
synergistic protective effect against radiation-induced injury
associated with its antioxidant and immunomodulatory activities
(10). However, limited information
is available on the potential anti-diabetic and anti-nephritic
properties of its polysaccharide-enriched water extract.
In patients with diabetic, abnormal high glucose
concentration leads to auto-oxidation and disequilibrium of the
production and scavenging of free radicals (11,12).
However, the oxidative system is considered to be a therapeutic
target of nephritis (13). As
reported previously, due to the regulatory property on various
genes of NF-κB, it serves an important role in apoptosis,
inflammation and autoimmune diseases (14). Activated NF-κB influences the
expression of interleukin-2 (IL-2) and tumor necrosis factor-α
(TNF-α) (15,16).
We therefore hypothesized that AAP may possess
therapeutic effects on diabetes and diabetes-associated nephritis.
In diet streptozotocin (STZ)-induced diabetic Sprague-Dawley rats,
the anticipatory effects of AAP were successfully confirmed. In
particular, it was assessed how oxidative factors and inflammatory
mediators were affected during model establishment and drug
administration.
Materials and methods
Preparation of AAP
Auricularia auricular powder (100 g; Jilin
Agricultural University) was extracted twice in hot water at 90°C
for 3 h. The remaining protein in the water extract was removed
using Sevag reagent (V (n - butanol): V (chloroform) = 1:4; 50 ml).
The precipitation was collected and freeze-dried for further
experiments by adding 4 folds of ethanol. The content of the total
polysaccharide separated from Auricularia auricular was
93.9±1.82 mg/g.
Establishment of diabetic rat model
and drug administration
The experimental protocol was approved by the
Laboratory Animal Centre of Jilin University (Changchun, China).
Sprague-Dawley male rats weighing 120–140 g were housed in groups
of two in clear plastic cages and maintained on a 12-h light/dark
cycle (lights, 07:00-19:00 h) at 23±1°C with water and food
available ad libitum.
To establish diabetes, 48 male Sprague-Dawley rats
(age, 4 weeks) were administrated a high-fat high sucrose diet
(HFHSD) containing 68.8% standard chow, 20% sucrose, 10% lard, 0.2%
cholesterol and 1% salt mixture. This was administered for 8 weeks
followed by a 3-day intraperitoneal injection of 30 mg/kg
streptozotocin (STZ; Sigma-Aldrich) (once daily) (17). Then, 4 h after the STZ injection,
rats were fed with 5% glucose solution to prevent hypoglycemia.
Rats with fasting serum glucose levels ranging between 11 and 26
mmol/l were identified as the diabetic model (18). The 48 diet-STZ-induced diabetic male
rats were divided into four groups randomly, and received four
weeks of drug administration, as follows: Diabetic model (DM; n=12)
group, rats treated with normal saline orally; positive control
(n=12) group, rats treated with 100 mg/kg metformin (DH; Beijing
Jingfengzhiyao Co., Ltd., Beijing, China) orally; low dose AAP
treatment group (n=12), rats treated with 100 mg/kg AAP orally; and
high dose AAP treated group (n=12), rats treated with 400 mg/kg AAP
orally.
An additional 12 male Sprague-Dawley rats were fed a
normal diet for 8 weeks and administered 3-day citrate buffer
injections, and these rats served as a control group (CT). They
were then treated with normal saline orally for four weeks. After
the last treatment, food and water intake in all rats were
monitored for 24 h.
Oral glucose tolerance test
(OGTT)
After four-weeks of treatment, an OGTT was
performed. Briefly, after a 12-h fasting, rats received
physiological saline, metformin (100 mg/kg) and AAP (100 and 400
mg/kg), respective to their groups. After 30 min, all rats were
treated with 2 g/kg glucose orally. Blood samples were collected at
0, 30, 60 and 120 min, and the blood glucose levels were determined
using a Glucose Assay kit (Nanjing Biotechnology Co., Ltd.,
Nanjing, China). The area under the blood glucose curve (AUC) was
calculated according to the following equation: AUC = (basal
glycaemia + glycaemia 0.5 h) × 0.25 + (glycaemia 0.5 h + glycaemia
1 h) × 0.25 + (glycaemia 1 h + glycaemia 2 h) × 0.5.
Sample collection and biochemical
analysis
Before sacrifice, blood was sampled from each rat
under anesthesia, and centrifuged at 3,000 × g for 10 min.
The collected serum was frozen at −80°C. Urine was also collected
at 24 h. The levels of blood urea nitrogen (BUN; C013-2), uric acid
(UA; C012-1), superoxide dismutase (SOD; A001-3), glutathione
peroxidase (GSH-Px; A005), malondialdehvde (MDA; A003-1) and
reactive oxygen species (ROS; E004) in serum, and protein
concentration in urine were detected using commercial kits (Nanjing
Biotechnology, Co., Ltd., Nanjing, China).
Serum levels of IL-2 (K00031), interferon-γ (IFN-γ;
K00006), tumor necrosis factor-α (TNF-α; K00163) and NF-κB
(YY43059) were detected using enzyme-linked immunosorbent assay
kits (Calbiotech, Inc., El Cajon, CA. USA).
Statistical analysis
All values were expressed as the mean ± standard
error. One-way analysis of variance was used to detect statistical
significance followed by post-hoc multiple comparisons (Dunn's
test). Analyses were performed using SPSS software, version 16.0
(SPSS, Inc., Chicago, IL, USA). P<0.05 was considered to
indicate a statistically significant difference.
Results
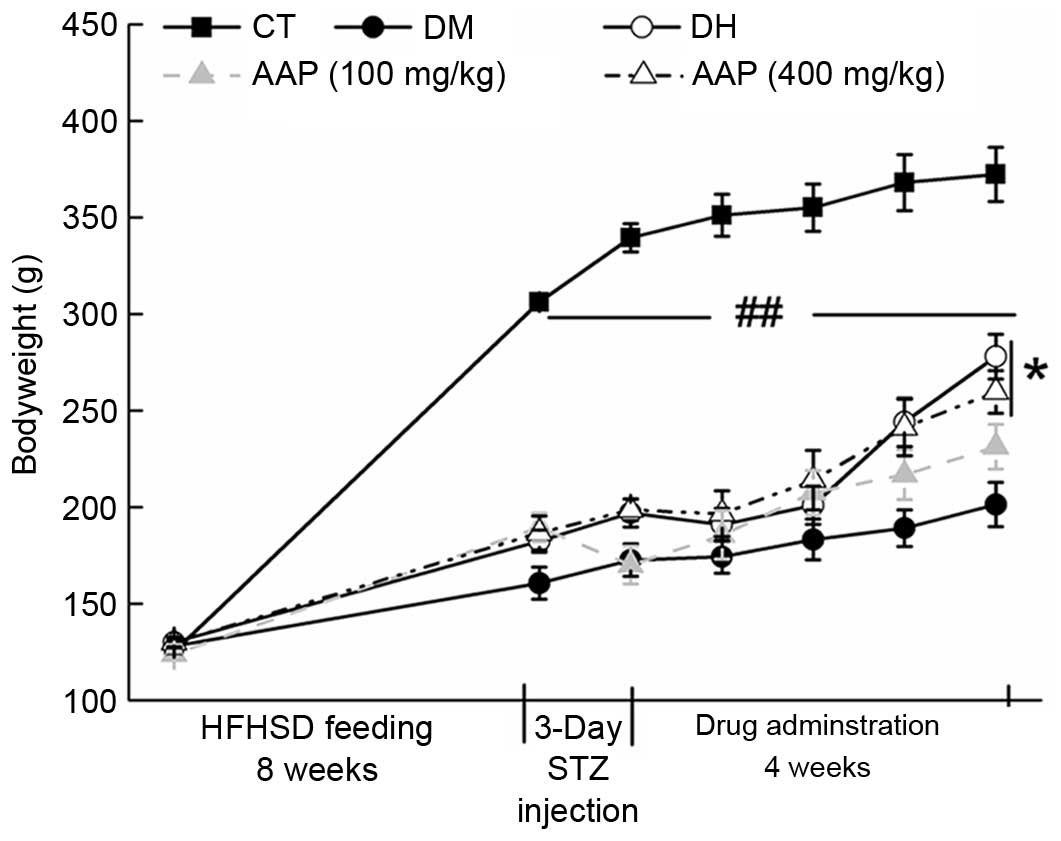
Body weight monitoring
The growth of diet-STZ-induced diabetic rats was
inhibited significantly compared with the CT group (P<0.01;
Fig. 1). Four-week 400 mg/kg AAP
treatment significantly restored the body weight of diabetic rats
(P<0.05; Fig. 1).
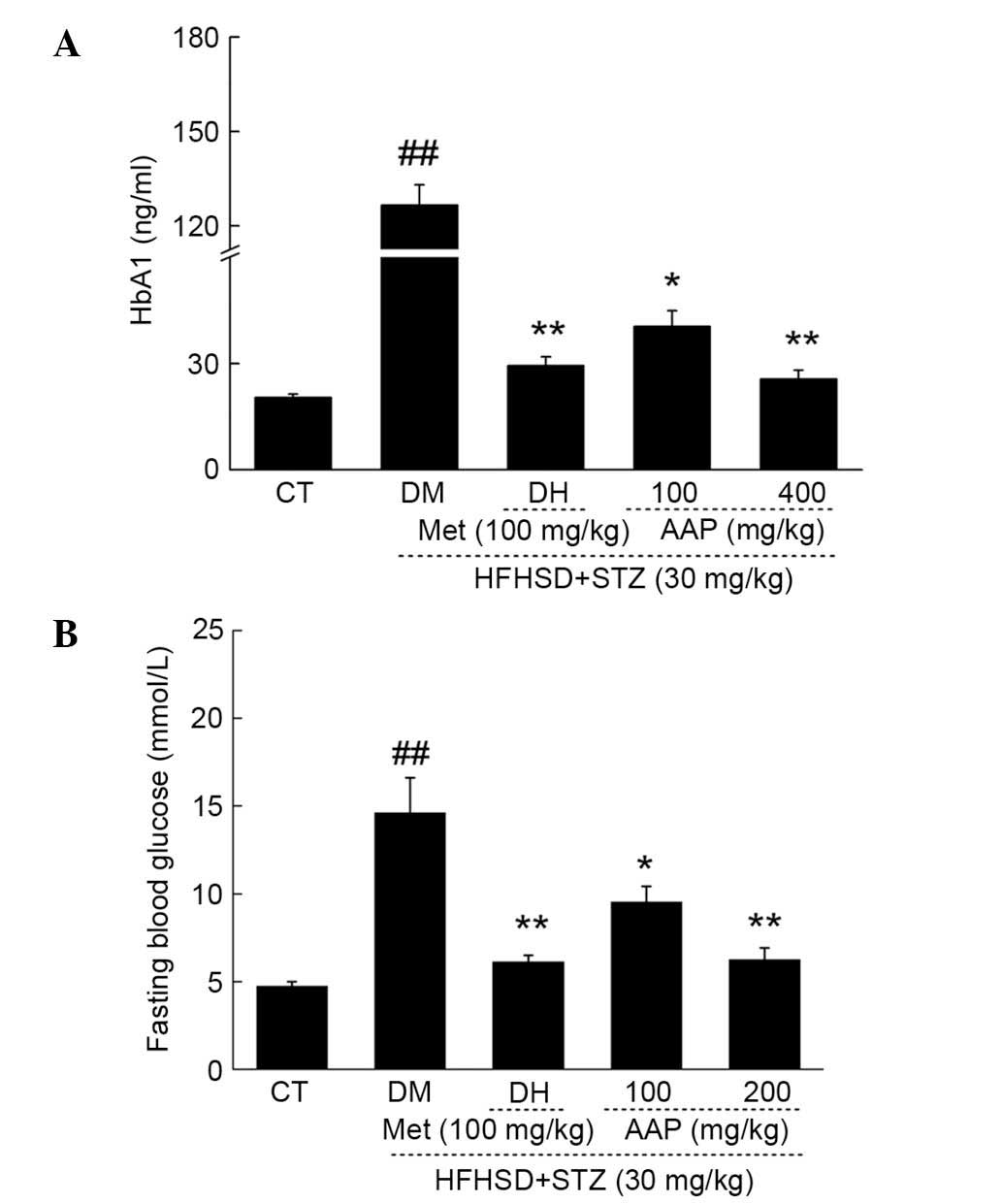
Hypoglycemic effect of AAP in a rat
diabetes model
The fasting blood glucose (FBG) and HbA1 levels in
serum were measured to evaluate the hypoglycemic activity of AAP.
DH and AAP administration significantly reduced the low level of
HbA1 in diet-STZ-induced diabetic rats (P<0.05; Fig. 2A). Particularly, 400 mg/kg AAP
reduced HbA1 serum levels by 80.2% compared with the level in
diabetic rats (P<0.01; Fig. 2A).
In addition, compared with the model group, DH (100 mg/kg) and AAP
(100 and 400 mg/kg) treatment significantly reduced FGB levels by
58.1, 34.7 and 57.2%, respectively (P<0.05; Fig. 2B).
OGTT was performed to avoid the false positive
obtained from FBG. Between 0 and 120 min, significantly higher FBG
levels were detected in diabetic rats compared with rats in the CT
group (P<0.05; Fig. 3A). In
addition, DH and AAP significantly prevented increases in serum
glucose (P<0.05; Fig. 3A). The
glucose response during OGTT was displayed via AUC calculation. A
marked enhancement of AUC in the DM group was observed; in
contrast, AAP treatment at doses of 100 and 400 mg/kg resulted in a
43.3 and 60.2% significant reduction in AUC (P<0.01; Fig. 3B).
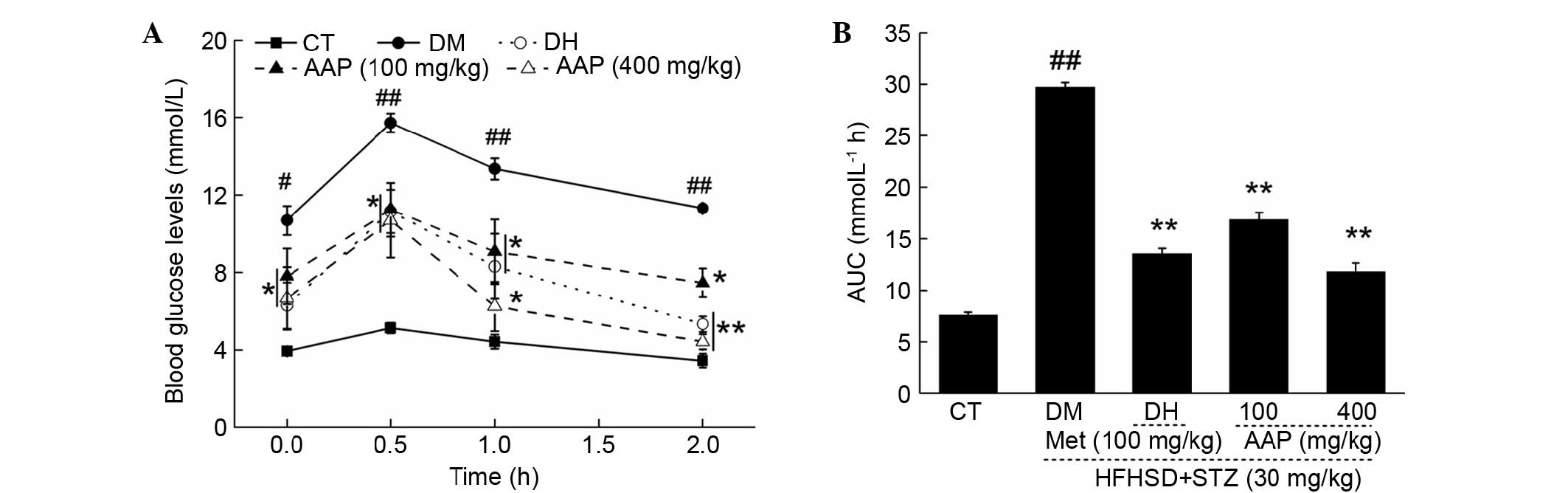 | Figure 3.After four-weeks of AAP treatment,
(A) blood glucose levels and (B) AUC of OGTT in experimental rats
were detected. Data are expressed as the mean + standard error
(n=12). #P<0.05, ##P<0.01 vs. CT;
*P<0.05, **P<0.01 vs. DM rats. AAP, Auricularia
auricular polysaccharide; AUC, area under the curve; CT,
control group; DH, metformin group; DM, diabetic model group;
HFHSD, high-fat high sucrose diet; Met, metformin; STZ,
streptozotocin. |
Anti-diabetic nephropathic effects of
AAP in a rat diabetes model
In diet-STZ-induced diabetic rats, the level of
blood urea nitrogen (BUN) and uric acid (UA) in serum, and protein
concentration in urine, were significantly enhanced (P<0.05),
which are considered as sensitive indexes in kidney injury
(19). Similar to in the DH group,
AAP treatment markedly reduced the increased levels of serum BUN
and UA to 36.2 and 36.7%, respectively (Fig. 4A and B). Interestingly, only 100
mg/kg AAP significantly reduced urine protein level by 25.7% in
diet-STZ-induced diabetic rats compared with the DH group
(P<0.05; Fig. 4C).
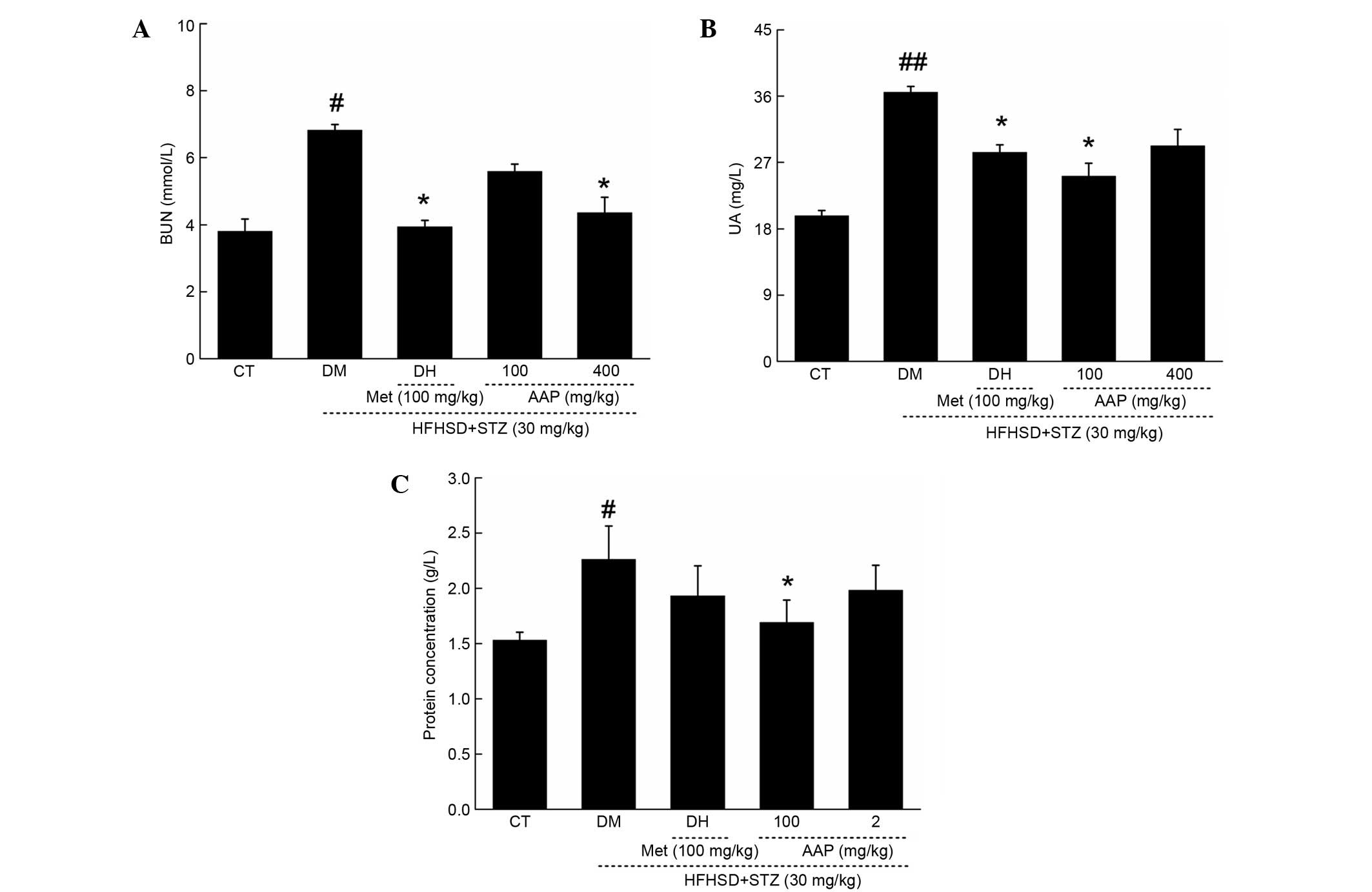 | Figure 4.Diet STZ-induced diabetic rats were
treated with 100 mg/kg met (DH) and AAP (100 and 400 mg/kg) for
four weeks. Serum (A) BUN, (B) UA and (C) urine protein levels in
all rats were detected. Data are expressed as the mean + standard
error (n=12) and analyzed using a one-way followed by Dunn's test.
#P<0.05 and ##P<0.01 vs. CT; *P<0.05
vs. DM rats. AAP, Auricularia auricular polysaccharide; BUN,
blood urea nitrogen; CT, control group; DH, metformin group; DM,
diabetic model group; HFHSD, high-fat high sucrose diet; Met,
metformin; STZ, streptozotocin; UA, uric acid. |
Anti-oxidative effects of AAP in
diet-STZ-induced diabetic rats
As reported, oxidative stress is responsible for
diabetes and its associated complications (20). In diet-STZ-induced-diabetic rats,
significantly decreased levels of SOD and GSH-Px, and significantly
increased levels of MDA and ROS, were observed (P<0.05; Fig. 5). Compared with the DH group, AAP
administration at a dose of 400 mg/kg resulted in a 20.8%
enhancement of SOD activity and a 34.5% increment in GSH-Px level
(P<0.01; Fig. 5A and B). In
addition, compared with the DH group, AAP treatment at a dose of
400 mg/kg significantly reduced MDA levels by 32.3% (P<0.05;
Fig. 5C) and ROS levels by 30.2%
(P<0.01; Fig. 5D) in serum.
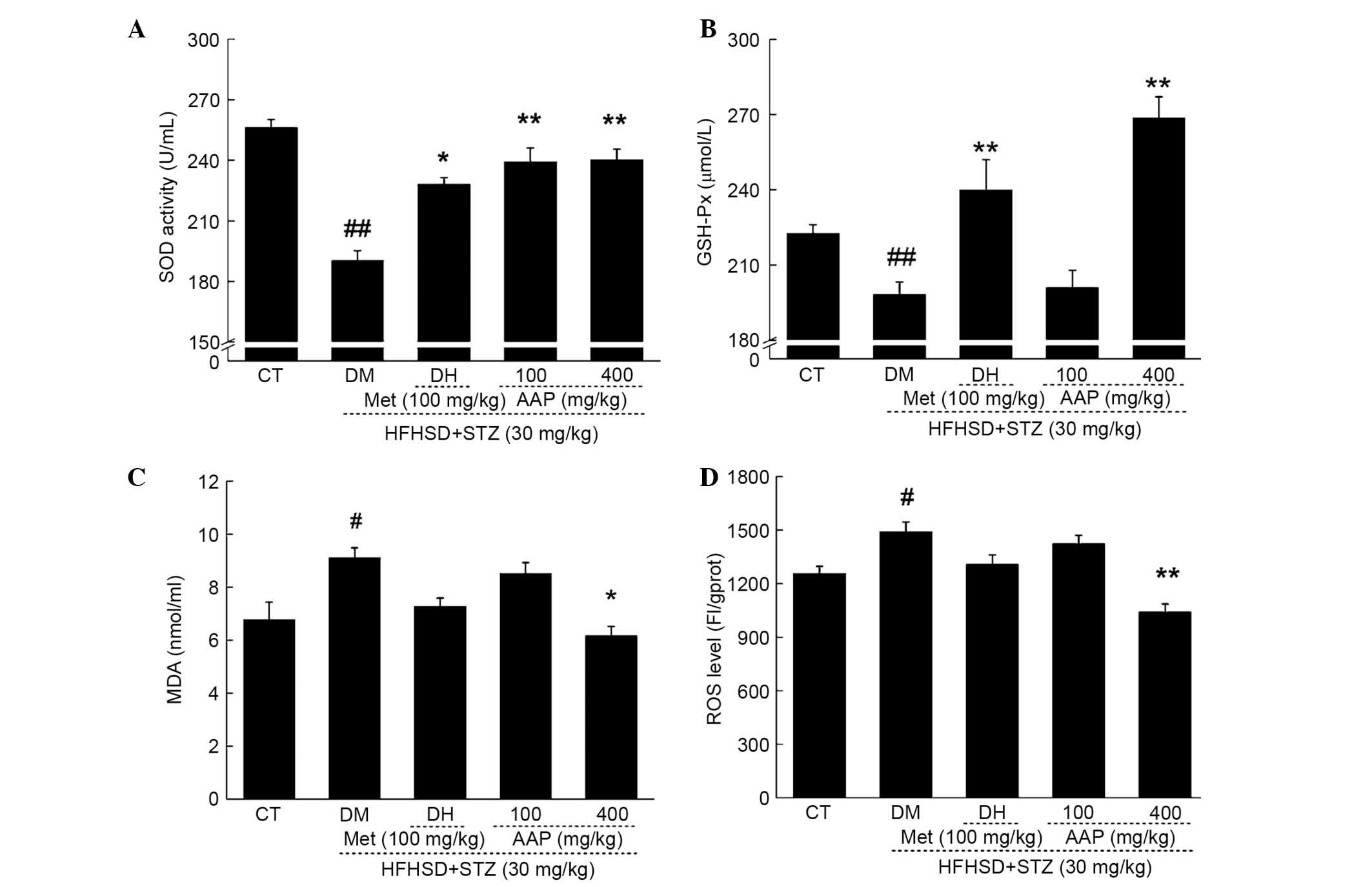 | Figure 5.Diet STZ-induced diabetic rats were
treated with 100 mg/kg met (DH) and AAP (100 and 400 mg/kg) for
four weeks. Serum (A) SOD, (B) GSH-Px, (C) MDA and (D) ROS levels
in all experimental groups were detected. Data are expressed as the
mean + standard error (n=12) and were analyzed using a one-way
analysis of variance followed by Dunn's test.
#P<0.05, ##P<0.01 vs. CT; *P<0.05,
**P<0.01 vs. DM rats. AAP, Auricularia auricular
polysaccharide; CT, control group; DH, metformin group; DM,
diabetic model group; GSH-Px, glutathione peroxidase; HFHSD,
high-fat high sucrose diet; MDA, malondialdehyde; Met, metformin;
ROS, reactive oxygen species; SOD, superoxide dismutasel; STZ,
streptozotocin. |
Regulatory effects of AAP on serum
inflammatory factors
Compared with control rats, HFHSD and STZ injection
significantly reduced serum levels of IFN-γ (47.4%; P<0.01) and
significantly elevated serum levels of TNF-α (18.7%; P<0.05),
IL-2 (36.7%; P<0.01) and NF-κB (36.7%; P<0.01) (Fig. 6). The DH group demonstrated enhanced
IFN-γ levels after four-weeks of treatment compared with the levels
in the DM group (P<0.01; Fig.
6A). Four-week AAP administration at a dose of 400 mg/kg
resulted in a significantly 84.7% increase in IFN-γ levels
(P<0.01), and a 19.7, 20.1 and 31.8% reduction in TNF-α, IL-2
and NF-κB levels in diabetic rats, respectively (Fig. 6B-D).
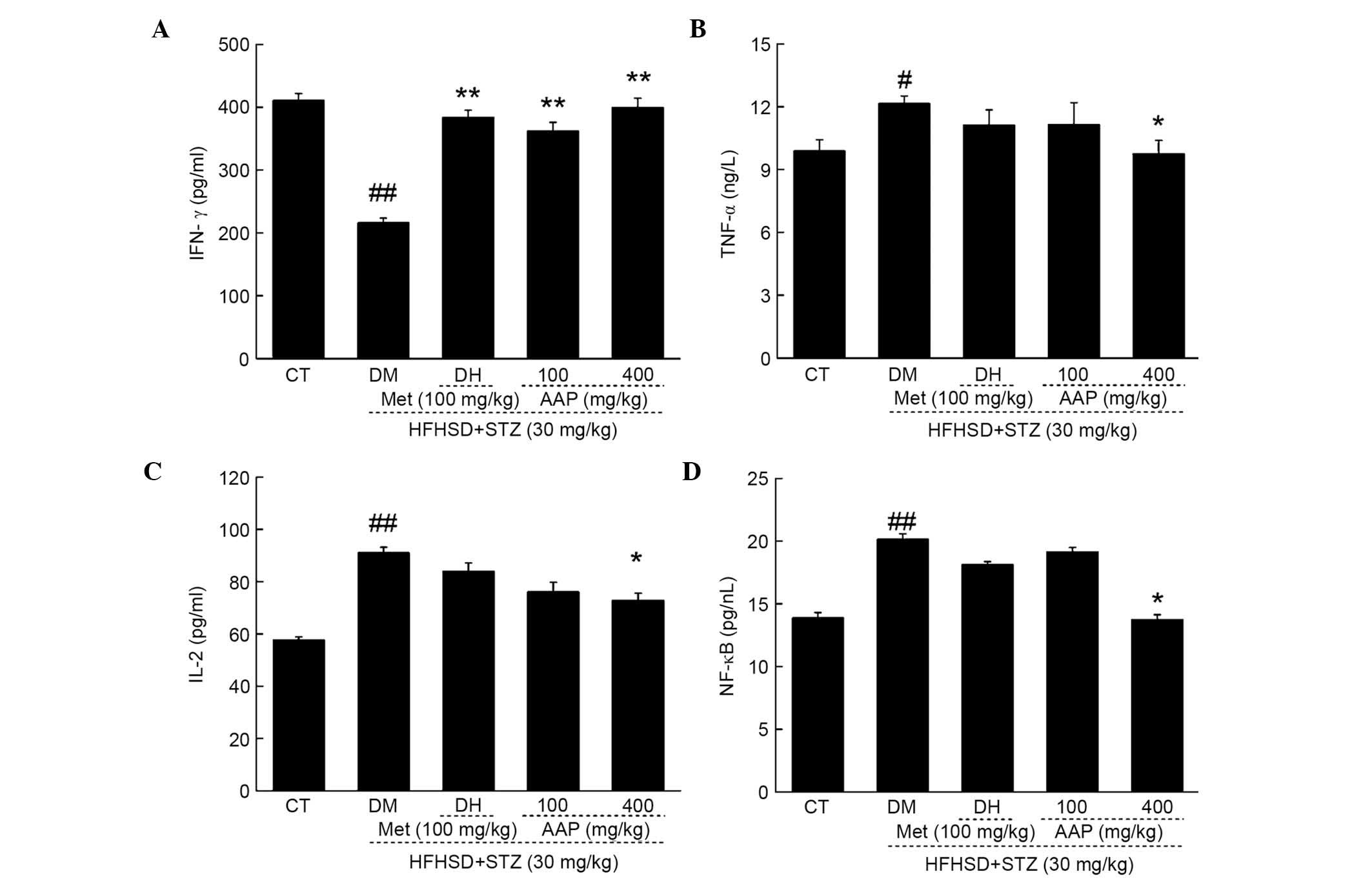 | Figure 6.Diet STZ-induced diabetic rats were
treated with 100 mg/kg met (DH) and AAP (100 and 400 mg/kg) for
four weeks. The levels of (A) IFN-γ, (B) TNF-α, (C) IL-2 and (D)
NF-κB in serum were determined. Data are expressed as the mean +
standard error (n=10) and analyzed using a one-way analysis of
variance followed by Dunn's test. #P<0.05,
##P<0.01 vs. CT; *P<0.05, **P<0.01 vs. DM rats.
AAP, Auricularia auricular polysaccharide; CT, control
group; DH, metformin group; DM, diabetic model group; HFHSD,
high-fat high sucrose diet; IFN-γ, interferon-γ; IL-2,
interleukin-2; Met, metformin; NF-κB, nuclear factor-κB; STZ,
streptozotocin; TNF-α; tumor necrosis factor-α. |
Discussion
Due to various pathological changes, a number of
complications, including retinopathy and nephropathy, occur in
diabetic patients (21). In the
present study, the hypoglycemic and anti-diabetic nephropathic
effects of AAP were confirmed in diet-STZ-induced diabetic
rats.
HbA1 analysis reflects the chronic glycemic control
in patients ranging between 8 and 12 weeks. The reduced serum HbA1
concentration and fasting blood glucose were confirmed by the
hyperglycemic activity of AAP in type II diabetes. Additionally,
OGTT is considered as a second diagnostic indicia to avoid
false-positive results obtained from FBG (22), which was normalized via AAP
administration compared with diabetic rats. However, four-week AAP
treatment resulted in a reduction in serum BUN and UA, and urine
protein levels, which serve as sensitive indexes for kidney injury
(19); these results demonstrated
the effect of renal protection of AAP in diabetic rats.
As previously reported, due to various pathological
characteristics, ROS were accumulated in diabetic patients
(11,12). The increased level of glucose leads
to the auto-oxidative glycosylation of proteins (22). A superabundance of oxygen free
radicals causes oxidative stress and cell damage, which can be
cleared by SOD and/or GSH-Px (23).
SOD and GSH-Px are considered to contribute towards protecting
against cell oxidative damage via clearing increased levels of MDA
and ROS. In diabetes mellitus, the deficiency of anti-oxidant
activity of SOD and GSH-Px may be associated with higher
concentrations of peroxide (24).
Antioxidants are the barriers against free radical attacks
(25). Anti-oxidant compounds have
already been confirmed to show significant properties in inhibiting
pancreatic β-cell destruction caused by alloxan (26). Furthermore, in circulation
inflammatory cells and local resident cells, ROS contributes to
tissue damage, which is associated with the inflammatory response
(27). In anti-glomerular basement
membrane antibody nephritic rats, over-producing ROS was noted
(27). Directly, Cordyceps
militaris extracts displays anti-diabetic, hypolipidemic, and
even anti-nephritic effects via the modulation of oxidative
resistance (13). In combination
with the results of the present study, the hypoglycemic and
anti-diabetic nephropathic activities of AAP may be partially
associated with the modulation of anti-oxidants and free
radicals.
Diet-STZ-induced rat models of hyperglycemia serve
as stable type II diabetic models for pharmaceutical screening
(28). Diabetic nephropathy, a
serious microvascular complication of diabetes (29), is successfully stimulated in the
animal models established in the present study. The pathological
lesions of the kidney are at least partially caused by STZ
injection, which is responsible for oxidative stress inflammation
(30) and renal injury (31). As described previously, four-week AAP
administration strongly suppressed the increased levels of BUN and
UA in serum, and protein concentration in urine. In addition, the
modulation activity of AAP on serum levels of IFN-γ, TNF-α, IL-2
and NF-κB was observed. These pro-inflammatory cytokines may
recruit lymphocytes and natural killer cells to inflammatory sites,
and may cause severe and constant glomerulonephritis deterioration
(32). It is reported in one study
that the association between cytokine-producing frequencies in αβ
and γδT cells are as follows: TNF-α >IFN-γ >IL-2 (33). The beneficial role of the
inflammatory cytokine IFN-γ has been confirmed by previous studies
(34), and a lower IFN-γ secretion
from T cells is found in patients with cystic fibrosis (35). Additionally, IFN-γ and TNF-α are
reported to show suppressive effects on white blood cell precursors
(36). IL-2, known as an important
factor for the immune response, promotes the activation of B cell
proliferation, which is regulated by NF-κB. NF-κB, an important
factor for renal protection (32,37,38), is
activated by pro-inflammatory stimuli; consequently,
pro-inflammatory cytokines are transcribed (39). The results from the present study
demonstrate that AAP-mediated renal protection in type II diabetic
rats is mainly through its modulation on NF-κB.
In conclusion, in diet-STZ-induced diabetic rats,
the anti-diabetic and anti-nephritic properties of AAP are
successfully confirmed. Additionally, the data suggest that the
effects are at least partially associated with their modulation on
the anti-oxidative system and the NF-κB signaling pathway. AAP have
great potential as a novel agent for diabetes and in treating
complications of nephritis.
Acknowledgements
The present study was supported by the Science and
Technology Development Program of Jilin Province in China (grant
no. 20160520036JH), and the “Twelfth Five-Year” Science and
Technology Planning Project of Jilin Province in China (grant no.
2014B033).
References
|
1
|
Wild S, Roglic G, Green A, Sicree R and
King H: Global prevalence of diabetes: Estimates for the year and
projections for 2030. Diabetes Care. 27:1047–1053. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cao Z and Cooper M: Pathogenesis of
diabetic nephropathy. J Diabetes Investig. 2:243–247. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
You Q, Chen F, Wang X, Luo PG and Jiang Y:
Inhibitory effects of muscadine anthocyanins on α-glucosidase and
pancreatic lipase activities. J Agric Food Chem. 59:9506–9511.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Winkler G, Hidvégi T, Vándorfi G and
Jermendy G: Risk-stratified screening for type 2 diabetes in adult
subjects: Results from Hungary. Orv Hetil. 151:691–696. 2010.(In
Hungarian). View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Levterova BA, Dimitrova DD, Levterov GE
and Dragova EA: Instruments for disease-specific quality-of-life
measurement in patients with type 2 diabetes mellitus-a systematic
review. Folia Med (Plovdiv). 55:83–92. 2013.PubMed/NCBI
|
|
6
|
Scheen A: Antidiabetic agents in subjects
with mild dysglycaemia: Prevention or early treatment of type 2
diabetes? Diabetes Metab. 33:3–12. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jiang X, Zhao X, Luo H and Zhu K:
Therapeutic effect of polysaccharide of large yellow croaker swim
bladder on lupus nephritis of mice. Nutrients. 6:1223–1235. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang G, Huang Y, Bian Y, Wong JH, Ng TB
and Wang H: Hypoglycemic activity of the fungi Cordyceps militaris,
Cordyceps sinensis, Tricholoma mongolicum, and Omphalia lapidescens
in streptozotocin-induced diabetic rats. Appl Microbiol Biotechnol.
72:1152–1156. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang H, Wang ZY, Yang L, Yang X, Wang X
and Zhang Z: In vitro antioxidant activities of sulfated
derivatives of polysaccharides extracted from Auricularia
auricular. Int J Mol Sci. 12:3288–3302. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bai H, Wang Z, Cui J, Yun K, Zhang H, Liu
RH, Fan Z and Cheng C: Synergistic radiation protective effect of
purified Auricularia auricular-judae polysaccharide (AAP IV) with
grape seed procyanidins. Molecules. 19:20675–20694. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yao Y, Sang W, Zhou M and Ren G:
Antioxidant and alpha-glucosidase inhibitory activity of colored
grains in China. J Agric Food Chem. 58:770–774. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Adisa RA, Choudhary MI and Olorunsogo OO:
Hypoglycemic activity of Buchholzia coriacea (Capparaceae) seeds in
streptozotocin-induced diabetic rats and mice. Exp Toxicol Pathol.
63:619–625. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Dong Y, Jing T, Meng Q, Liu C, Hu S, Ma Y,
Liu Y, Lu J, Cheng Y, Wang D and Teng L: Studies on the
antidiabetic activities of Cordyceps militaris extract in
diet-streptozotocin-induced diabetic Sprague-Dawley rats. Biomed
Res Int. 2014:1609802014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gilmore TD: Introduction to NF-kappaB:
Players, pathways, perspectives. Oncogene. 25:6680–6684. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Freise C and Querfeld U: The lignan
(+)-episesamin interferes with TNF-α-induced activation of VSMC via
diminished activation of NF-ĸB, ERK1/2 and AKT and decreased
activity of gelatinases. Acta Physiol (Oxf). 213:642–652. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Park JH and Levitt L: Overexpression of
mitogen-activated protein kinase (ERK1) enhances T-cell cytokine
gene expression: Role of AP1, NF-AT and NF-κB. Blood. 82:2470–2477.
1993.PubMed/NCBI
|
|
17
|
Fröde T and Medeiros Y: Animal models to
test drugs with potential antidiabetic activity. J Ethnopharmacol.
115:173–183. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Federiuk I, Casey H, Quinn M, Wood M and
Ward W: Induction of type-1 diabetes mellitus in laboratory rats by
use of alloxan: Route of administration, pitfalls, and insulin
treatment. Comp Med. 54:252–257. 2004.PubMed/NCBI
|
|
19
|
Kumar G, Shetty A and Salimath P:
Modulatory effect of bitter gourd (Momordica charantia LINN.) on
alterations in kidney heparan sulfate in streptozotocin-induced
diabetic rats. J Ethnopharmacol. 115:276–283. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang BS, Lee CP, Chen ZT, Yu HM and Duh
PD: Comparison of the hepatoprotective activity between cultured
Cordyceps militaris and natural Cordyceps sinensis. J Agric Food
Chem. 4:489–495. 2012.
|
|
21
|
International Diabetes Federation, .
Diabetes and the millennium development goals. Diabetes Res Clin
Pract. 100:409–410. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Park JH, Park NS, Lee SM and Park E:
Effect of dongchunghacho rice on blood glucose level, lipid profile
and antioxidant metabolism in streptozotocin-induced diabetic rats.
Food Sci Biotechnol. 20:933–940. 2011. View Article : Google Scholar
|
|
23
|
Whaley-Connell A, McCullough PA and Sowers
JR: The role of oxidative stress in the metabolic syndrome. Rev
Cardiovasc Med. 12:21–29. 2011.PubMed/NCBI
|
|
24
|
Suryawanshi NP, Bhutey AK, Nagdeote AN,
Jadhav AA and Manoorkar GS: Study of lipid peroxide and lipid
profile in diabetes mellitus. Indian J Clin Biochem. 21:126–130.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bergamini CM and Seghieri G: ROS and
kidney disease in the evolution from acute phase to chronic end
stage disease: A commentary on ‘Oxidative signaling in renal
epithelium: Critical role of cPLA2 and p38SAPK’. Free Radic Biol
Med. 41:190–192. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sebai H, Selmi S, Rtibi K, Souli A, Gharbi
N and Sakly M: Lavender (Lavandula stoechas L.) essential oils
attenuate hyperglycemia and protect against oxidative stress in
alloxan-induced diabetic rats. Lipids Health Dis. 12:1892013.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Fu H, Li J, Li QX, Xia L and Shao L:
Protective effect of ligustrazine on accelerated anti-glomerular
basement membrane antibody nephritis in rats is based on its
antioxidant properties. Eur J Pharmacol. 563:197–202. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhang M, Lv XY, Li J, Xu ZG and Chen L:
The Characterization of High-Fat Diet and Multiple Low-Dose
Streptozotocin Induced Type 2 Diabetes Rat Model. Exp Diabetes Res.
2008:7040452008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
van Dijk C and Berl T: Pathogenesis of
diabetic nephropathy. Rev Endocr Metab Disord. 5:237–248. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lei Y-C, Hwang JS, Chan CC, Lee CT and
Cheng TJ: Enhanced oxidative stress and endothelial dysfunction in
streptozotocin-diabetic rats exposed to fine particles. Environ
Res. 99:335–343. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Valentovic MA, Alejandro N, Carpenter A
Betts, Brown PI and Ramos K: Streptozotocin (STZ) diabetes enhances
benzo (alpha) pyrene induced renal injury in Sprague Dawley rats.
Toxicol Lett. 164:214–220. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Li S, Zhang Y and Zhao J: Preparation and
suppressive effect of astragalus polysaccharide in
glomerulonephritis rats. Int Immunopharmacol. 7:23–28. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Raga S, Julia MR, Crespi C, Figuerola J,
Martínez N, Milà J and Matamoros N: Gammadelta T lymphocytes from
cystic fibrosis patients and healthy donors are high TNF-alpha and
IFN-gamma-producers in response to Pseudomonas aeruginosa. Respir
Res. 4:92003. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Johansen HK, Hougen HP, Rygaard J and
Høiby N: Interferon-gamma (IFN-gamma) treatment decreases the
inflammatory response in chronic Pseudomonas aeruginosa pneumonia
in rats. Clin Exp Immunol. 103:212–218. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Moss RB, Bocian RC, Hsu YP, Dong YJ, Kemna
M, Wei T and Gardner P: Reduced IL-10 secretion by CD4+ T
lymphocytes expressing mutant cystic fibrosis transmembrane
conductance regulator (CFTR). Clin Exp Immunol. 106:374–388. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Selleri C, Sato T, Anderson S, Young NS
and Maciejewski JP: Interferon-gamma and tumor necrosis
factor-alpha suppress both early and late stages of hematopoiesis
and induce programmed cell death. J Cell Physiol. 165:538–546.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhang S, Xin H, Li Y, Zhang D, Shi J, Yang
J and Chen X: Skimmin, a coumarin from hydrangea paniculata, slows
down the progression of membranous glomerulonephritis by
anti-inflammatory effects and inhibiting immune complex deposition.
Evid Based Complement Alternat Med. 2013:8192962013.PubMed/NCBI
|
|
38
|
Pan P, Wang YJ, Han L, Liu X, Zhao M and
Yuan YF: Effects of sodium houttuyfonate on expression of NF-κB and
MCP-1 in membranous glomerulonephritis. J Ethnopharmacol.
131:203–209. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Barnes PJ and Karin M: Nuclear
factor-kappaB: A pivotal transcription factor in chronic
inflammatory diseases. N Engl J Med. 336:1066–1071. 1997.
View Article : Google Scholar : PubMed/NCBI
|