Introduction
Alzheimer's disease (AD) is related to age and is a
neurodegenerative disease featuring progressive cognitive disorder
and memory damage. Since diabetes can increase the risk of vascular
dementia and AD, some scholars regard AD as another type of
diabetes (1). Rasgon and Jarvik
(2) found that cognitive disorders
or AD incidence rates were 2- to 3-fold higher in patients with
type 2 diabetes mellitus (T2DM) than those in control group. Thus,
T2DM was found to be a risk factor in cognitive disorders among
seniors. The main pathologic features of AD include senile plaques,
neurofibrillary tangles, neuro-reduction and insulin
neuro-transduction involved in its metabolic pathway (2).
Insulin-like growth factor-1 (IGF-1) is an important
neurotrophic factor and its receptor IGF-1R is prevalently
expressed in the nervous system (3).
IGF-1 can impact the elimination of β-amyloid peptide (Aβ) and the
phosphorylation of microtubule associated protein (tau), which is
related to PI3K/Akt and MAPK/ERK1/2 signal pathways (4). Forkhead transcription factor O (FOXO)
subfamily protein, an important downstream responsive molecule in
IGF-1 signal pathway, is modified and adjusted by the
post-translational modification in the PI3K/Akt pathway (5). Most studies conducted are centered on
AD animal models while few involve in-clinic observation.
Therefore, this study serves as a reference to clinical diagnosis
and treatment by examining whether the IGF-1 expression in
different groups of seniors is related to diabetes and AD, and the
possible action mechanism.
Patients and methods
Subject information
A total of 30 senior patients who were admitted to
and diagnosed by our hospital with diabetes and AD (group A), 30
with AD but no diabetes (group B), 30 with diabetes but no AD
(group C), and 30 healthy seniors (group D) were continuously
selected from June 2013 to January 2016. The selection criteria for
the study were: i) Age, ≥65; and ii) meet WHO's T2DM diagnosis
standard and Chicago AD diagnosis standard, including pre-clinical
phase and dementia phase. The exclusion criteria included: i)
Serious diabetes complications, such as eye-ground retinal
hemorrhage, diabetic kidney disease, diabetic foot, and
cardiovascular and cerebrovascular diseases, such as ischemic
stroke, head injury, history of surgery and cancer; ii)
neuropsychiatric disorders, autoimmune diseases, severe anxiety and
depression; and iii) non-compliant patient.
The present study was approved by the ethics
committee of Yantai Affiliated Hospital of Binzhou Medical
University. Informed consent was obtained from the patients or
their families. There were 17 males and 13 females in group A;
average age of 70.5±6.3; fasting blood sugar averaged 8.2±2.0
mmol/l; and the course of disease averaged 5.6±1.7 years. There
were 16 males and 14 females in group B, and the average age was
71.7±6.5. There were 15 males and 15 females in group C; average
age of 71.6±6.8; fasting blood sugar averaged 8.5±2.2 mmol/l; and
the course of disease averaged 5.8±1.4 years. There were 16 males
and 14 females in group D, and the average age was 71.5±6.3. The
difference in gender and age among groups was not statistically
significant (P>0.05).
Study methods
We utilized the ELISA method to test the levels of
serotonin IGF-1, Aβ and the phosphorylation of immunohistochemistry
staining microtubule associated protein (tau protein). We utilized
the western blot method to test the level of prion protein (PrP),
FOXO subfamily protein, p-PI3K and p-Akt. ELISA kits were purchased
from Sigma (St. Louis, MO, USA) and the procedures in the manual
were followed during testing. The main procedures of the
immunohistochemical (SP) staining method were producing peripheral
mononuclear cells of paraffin through conventional steps, including
dewaxing, gradient alcoholic dehydration, 3%
H2O2 inactivation of peroxidase, antigen
retrieval, closure, adding phosphorylated tau protein (pSer202)
antibody (1:100; Wuhan Boster Biological Engineering Co., Ltd.,
Wuhan, China), negative control plus phosphate-buffered saline
(PBS), at 4°C overnight, 5 times of 3-min PBS washing, adding
biotin-marked antibody (Beijing ZS-Bio Co., Ltd., Beijing, China),
30-min at 37°C incubation, 5 times of 3-min PBS washing, DAB
coloration, hematoxylin staining, conventional dehydration,
transparency made by xylene, neutral balsam mounting, observation
and capturing images under a microscope (Olympus, Tokyo, Japan). We
continuously observed 3 sections, counted 4 non-overlap horizons
randomly <400-fold enlarged horizon, and tested the ratio of the
number of cells with positive response, which was stained pale
brown, in each horizon. The averages were determined by using the
Image-Pro Plus v6.0 image analysis system.
Western blot analysis involved extraction of general
protein by conventional protein extraction kit (CWbio Co., Ltd.,
Beijing, China), BCA protein quantitation kit (Beijing ZS-Bio Co.,
Ltd.) testing, polyacrylamide gel electrophoresis (separation gel,
stacking gel buffer, Beijing ZS-Bio Co., Ltd.), transmembrane (PVDF
film; Millipore Corp., Billerica, MA, USA), closure, antibody
incubation (first antibody is rabbit anti-rat PrP 1:2,000; FOXO
1:2,000; p-PI3K 1:1,000; p-Akt 1:1,000; GAPDH 1:500; second
antibody is HRP-marked goat anti-rabbit, 1:500), developing and
fixing, scanning and analyzing band density by using gel image
analysis software, expressing relative content of target protein by
using the ratio of target band and relevant GAPDH band signal
intensity. The test was repeated 3 times and the average was
calculated.
Statistical analysis
SPSS 19.0 software (SPSS, Inc., Chicago, IL, USA)
was utilized to analyze the data. Mean ± standard deviation was
used to represent the quantitative data and comparisons among
various groups were analyzed by one-way ANOVA, while the
qualitative data were expressed by the number of cases, and
inter-group comparison was tested by χ2. P<0.05 was
considered to indicate a statistically significant difference.
Results
Comparison of the level of serum IGF-1
and Aβ
The levels of IGF-1 and Aβ in group A were
significantly higher than that in group B, which was significantly
higher than that in groups C and D (P<0.05). The differences
between groups C and D were not statistically significant
(P>0.05; Table I).
 | Table I.Comparison of the level of IGF-1 and
Aβ. |
Table I.
Comparison of the level of IGF-1 and
Aβ.
| Group | IGF-1, ng/ml | Aβ, pg/ml |
|---|
| A | 160.3±42.1 | 268.7±78.5 |
| B | 113.4±34.6 | 184.7±62.3 |
| C | 94.7±25.8 | 105.6±41.4 |
| D | 82.6±20.3 | 112.5±50.6 |
| F-value | 10.325 | 22.624 |
| P-value | <0.001 | <0.001 |
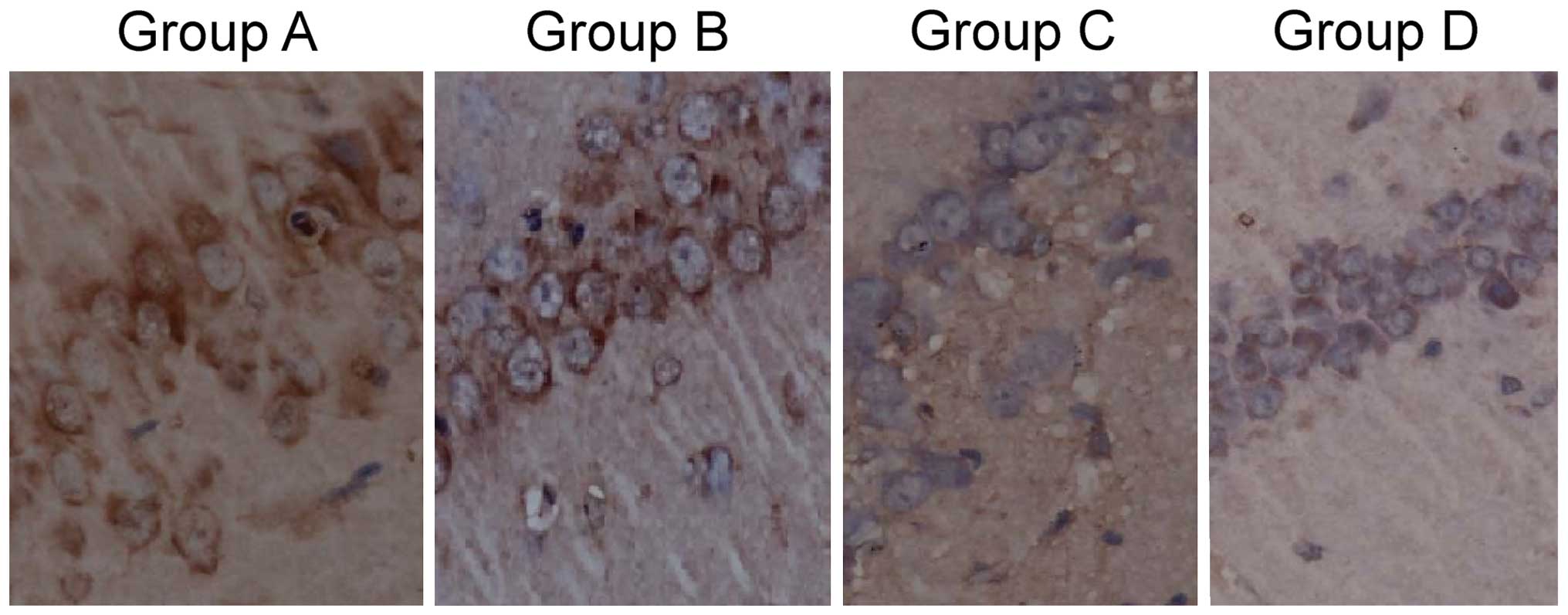
Comparison of tau protein
phosphorylation level
The tau protein positive rate of group A was
75.6±20.3%, which was significantly higher than that of group B,
46.9±15.6%, which was significantly higher than that of groups C
and D, 12.3±5.5 and 10.8±4.7%, respectively. The results were
statistically significant (F=15.634, P<0.001). The difference
between groups C and D were not statistically significant
(P>0.05; Fig. 1).
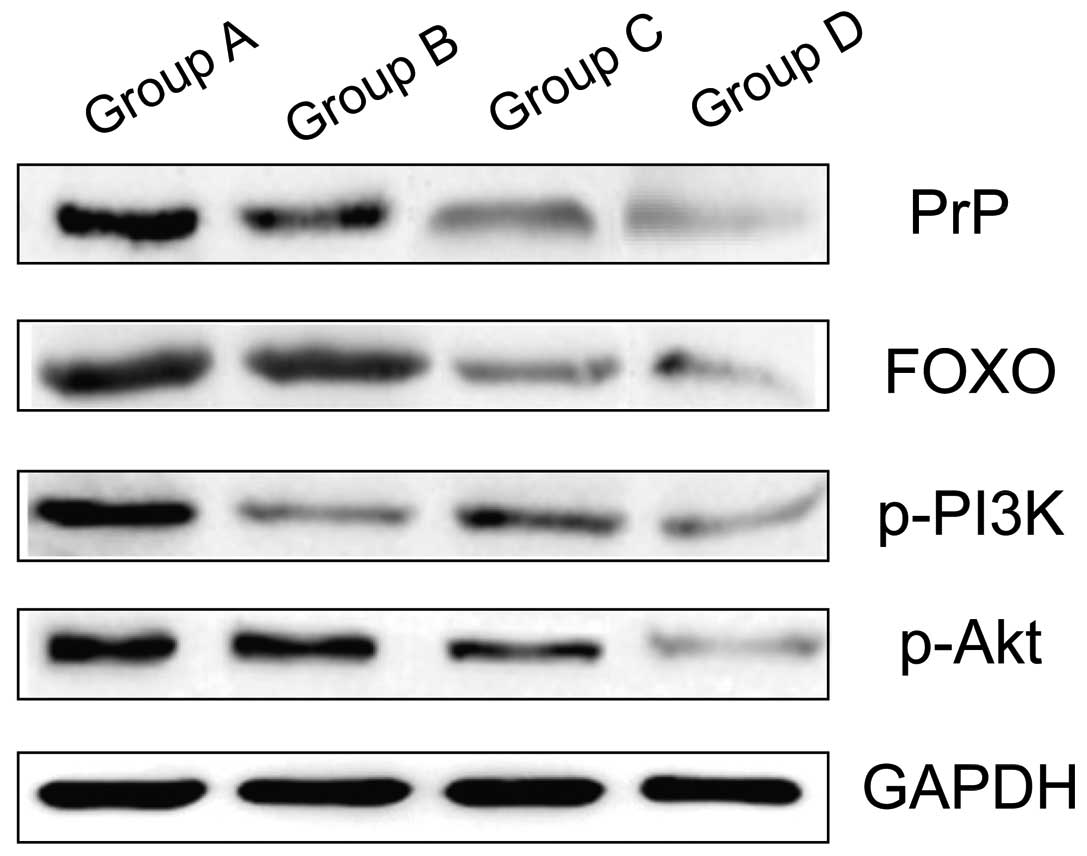
Comparison of the level of PrP, FOX
protein, p-PI3K and p-Akt
The levels of PrP, FOX protein, p-PI3K and p-Akt of
group A were significantly higher that of group B, which were
significantly higher than that of groups C and D, and the results
were statistically significant (P<0.05). The difference between
groups C and D were not statistically significant (P>0.05;
Table II and Fig. 2).
 | Table II.Comparison of the level of PrP, FOX
protein, p-PI3K and p-Akt. |
Table II.
Comparison of the level of PrP, FOX
protein, p-PI3K and p-Akt.
| Group | PrP | FOXO | p-PI3K | p-Akt |
|---|
| A | 0.63±0.07 | 0.74±0.08 | 0.82±0.06 | 0.76±0.05 |
| B | 0.44±0.05 | 0.48±0.06 | 0.53±0.07 | 0.50±0.06 |
| C | 0.18±0.03 | 0.20±0.04 | 0.21±0.05 | 0.19±0.04 |
| D | 0.15±0.03 | 0.17±0.03 | 0.16±0.04 | 0.15±0.03 |
| F-value |
9.637 |
8.457 | 13.265 | 11.527 |
| P-value | <0.001 | <0.001 | <0.001 | <0.001 |
Discussion
AD and Creutzfelt-Jakob disease (CJD) are
neurodegenerative diseases that are caused by conformational change
which result from the abnormal protein folding. In these diseases,
normal and solvable protein transfers to unsolvable protein
aggregation. In AD, this results in Aβ protein deposition and in
CJD, this results in proteinase K resistant prion protein (PrPSc)
deposition (6). It has been found
that PrPs play an important role in the process where Aβ triggers
AD (7). PrP, a highly conserved
protein in animals, is highly expressed in the nervous system. In
normal conditions, PrP is glycoprotein coded by the housekeeping
gene and located on the surface of cell membranes. The change in
natural conformation featuring rich α-spiral to the wrong
conformation featuring rich β-folding (that is PrPC transferring to
PrPSc) will lead to lethal neurodegenerative diseases such as CJD
in mammals. The incidence of AD is accompanied by abnormal protein
folding, conformational change, the formation of protein
aggregation, and neuronal cell degeneration. Protein aggregation
plays an important role in the death of cells (8). Currently, there are two opinions on the
impact of PrP in the incidence of AD: One is that the PrP, as the
membrane-anchored protein, functions as signal molecular receptor
and triggers a pathway in the cell leading to toxicity by combining
with Aβ oligomer outside the cell (9). The second opinion is that the PrP can
impact Aβ formation, and is neuro-protective (10). In fact, the two functions can coexist
and function in the body due to the PrP structural features and
multi-functions resulted from the complexity of glycosylation.
IGF-1, made up of 70 amino acids and also known as
sulphited factor and growth regulator C is a member of the insulin
family. It has the function of stimulating the production,
differentiation, migration, survival and metabolism. In recent
years, IGF-1 has been found closely related to the central nervous
system, especially to AD (11).
IGF-1 functions through the IGF-1R, which is prevalent in the body.
IGF-1R belongs to receptor tyrosine kinase family and is
phosphorylated by IGF-1, which can activate insulin receptor
substrate serial and other signal transduction pathways, including
mitotic active protein kinase pathway, and PI3K/PKB pathway
(12). IGF-1 plays a significant
role in Aβ metabolism and tau protein phosphorylation. Adlerz et
al (13) found that by
stimulating α-secretase, IGF-1 decreased the formation of Aβ. Zhang
et al (14) utilized PC12
cells and found that by stimulating α-secretase, IGF-1 decreased
the formation of Aβ and that IGF-1 treatment can significantly
decrease β-secretase (BACE-1) mRNA and protein levels and further
decrease the formation of Aβ by using quantitative polymerase chain
reaction (PCR) and western blot method for analysis of the results.
This process was related to PI3K/Akt and MAPK/ERK1/2 signal
pathways and the disorders of this signal system are involved in
the formation of AD pathology. Wang et al (15) conducted IGF-1 intervention by using
Aβ25–35 protein to induce injured PC12 cells to establish a cell
model for over-phosphorylated tau proteins and found that IGF-1 can
repress apoptosis of Aβ25-35-induced PC12 cells and tau protein
phosphorylation, the action mechanism of which is activated through
the signal transduction pathway.
FoxO family, a transcription factor, is a critical
factor in INS/IGF-1 signal pathway. The upstream is adjusted by
interconnected pathway of PI3K-PKB and the downstream adjusted
target genes are mostly related to cell cycle, apoptosis, aging and
metabolism (16).
FoxO phosphorylation/dephosphorylating status is
closely related to the transcription adjustment function. FoxO
transcription activeness is subjected to adjustment of the complex
signal pathway. There are mainly two types of FoxO transcription
activeness adjusted by phosphorylation: one is PI3 relying on
pathway for phosphorylation adjustment and the other is non-PI3
relying on pathway for phosphorylation adjustment. PI3K/Akt/FoxO is
a verified signal pathway (17).
Insulin, IGF-1 and other growth factors combine with tyrosine
kinase receptor to activate PI3K. Then Akt, including protein
kinase of Akt family and relevant serum and glucocorticoid, induces
the activation of SGK. RT-PCR and northern blot display that FoxO
transcription factor is highly expressed in tissues and organs of
adults, including in the heart and brain (18). In recent years, the study on FoxO
action mechanism has gradually transferred from tumor cells to
neuronal cells and it was found that when PI3K/Akt is activated, it
can phosphorylate and deacetylate under the control of Sirt1, which
enhanced the survival and production of neuronal cells (19). When the PI3K/Akt pathway is
repressed, it can dephosphorylate and acetylate under the control
of P300, inducing neuronal cell apoptosis (20). Dick and Bading (21) reported that the neuronal cells in CA1
area of hippocampus in particular is extremely prone to damage,
which is related to the highly expressed FoxO transcription factors
in CA1 area (21).
It is concluded from the present study that the
level of IGF-1 and Aβ, tau protein positive rate, and the level of
PrP, FOXO protein, p-PI3K and p-Akt of group A is significantly
higher than that of group B, which is higher than that of groups C
and D, and the results are statistically significant. The
difference between groups C and D is not statistically significant.
In summary, IGF-1 is highly expressed in senior patients with
diabetes and dementia and it can adjust the expression of PrP and
FOXO through p-PI3K/Akt pathway and further impact the formation of
Aβ and tau protein, leading to dementia.
References
|
1
|
Verdelho A, Madureira S, Moleiro C, Ferro
JM, Santos CO, Erkinjuntti T, Pantoni L, Fazekas F, Visser M,
Waldemar G, et al: LADIS study: White matter changes and diabetes
predict cognitive decline in the elderly: The LADIS study.
Neurology. 75:160–167. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rasgon N and Jarvik L: Insulin resistance,
affective disorders, and Alzheimer's disease: Review and
hypothesis. J Gerontol A Biol Sci Med Sci. 59:178–183; discussion
184–192. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Freude S, Schilbach K and Schubert M: The
role of IGF-1 receptor and insulin receptor signaling for the
pathogenesis of Alzheimer's disease: From model organisms to human
disease. Curr Alzheimer Res. 6:213–223. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
White MF: Regulating insulin signaling and
beta-cell function through IRS proteins. Can J Physiol Pharmacol.
84:725–737. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jeong HJ, Jeong HW, Song SS, Kang JW, Seo
JH, Lee YH, Lee KS and Kim DW: Upregulation of peroxiredeoxin III
in the hippocampus of acute immobilization stress model rats and
the Foxo3a-dependent expression in PC12 cells. Cell Mol Neurobiol.
31:1041–1046. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhao WQ, De Felice FG, Fernandez S, Chen
H, Lambert MP, Quon MJ, Krafft GA and Klein WL: Amyloid beta
oligomers induce impairment of neuronal insulin receptors. FASEB J.
22:246–260. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Roychaudhuri R, Yang M, Hoshi MM and
Teplow DB: Amyloid beta-protein assembly and Alzheimer disease. J
Biol Chem. 284:4749–4753. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Haass C and Selkoe DJ: Soluble protein
oligomers in neurodegeneration: Lessons from the Alzheimer's
amyloid beta-peptide. Nat Rev Mol Cell Biol. 8:101–112. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bate C and Williams A: Amyloid-β-induced
synapse damage is mediated via cross-linkage of cellular prion
proteins. J Biol Chem. 286:37955–37963. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cramer PE, Cirrito JR, Wesson DW, Lee CY,
Karlo JC, Zinn AE, Casali BT, Restivo JL, Goebel WD, James MJ, et
al: ApoE-directed therapeutics rapidly clear β-amyloid and reverse
deficits in AD mouse models. Science. 335:1503–1506. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Deochand C, Tong M, Agarwal AR, Cadenas E
and de la Monte SM: Tobacco smoke exposure impairs brain
insulin/IGF signaling: Potential co-factor role in
neurodegeneration. J Alzheimers Dis. 50:373–386. 2015. View Article : Google Scholar
|
|
12
|
Moloney AM, Griffin RJ, Timmons S,
O'Connor R, Ravid R and O'Neill C: Defects in IGF-1 receptor,
insulin receptor and IRS-1/2 in Alzheimer's disease indicate
possible resistance to IGF-1 and insulin signalling. Neurobiol
Aging. 31:224–243. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Adlerz L, Holback S, Multhaup G and
Iverfeldt K: IGF-1-induced processing of the amyloid precursor
protein family is mediated by different signaling pathways. J Biol
Chem. 282:10203–10209. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang H, Gao Y, Dai Z, Meng T, Tu S and
Yan Y: IGF-1 reduces BACE-1 expression in PC12 cells via activation
of PI3-K/Akt and MAPK/ERK1/2 signaling pathways. Neurochem Res.
36:49–57. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang PJ, Zhang Y, Song RR and Shen DF:
Study of the protection and mechanism of IGF-1 on tau protein
hyperphosphorylation in PC12 cells induced by Abeta(1–40). Sichuan
Da Xue Xue Bao Yi Xue Ban. 41:960–964. 2010.(In Chinese).
PubMed/NCBI
|
|
16
|
Puig O, Marr MT, Ruhf ML and Tjian R:
Control of cell number by Drosophila FOXO: Downstream and feedback
regulation of the insulin receptor pathway. Genes Dev.
17:2006–2020. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kim SJ, Winter K, Nian C, Tsuneoka M, Koda
Y and McIntosh CH: Glucose-dependent insulinotropic polypeptide
(GIP) stimulation of pancreatic beta-cell survival is dependent
upon phosphatidylinositol 3-kinase (PI3K)/protein kinase B (PKB)
signaling, inactivation of the forkhead transcription factor Foxo1,
and down-regulation of bax expression. J Biol Chem.
280:22297–22307. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Accili D and Arden KC: FoxOs at the
crossroads of cellular metabolism, differentiation, and
transformation. Cell. 117:421–426. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhao X, Gan L, Pan H, Kan D, Majeski M,
Adam SA and Unterman TG: Multiple elements regulate
nuclear/cytoplasmic shuttling of FOXO1: Characterization of
phosphorylation- and 14-3-3-dependent and -independent mechanisms.
Biochem J. 378:839–849. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Brunet A, Sweeney LB, Sturgill JF, Chua
KF, Greer PL, Lin Y, Tran H, Ross SE, Mostoslavsky R, Cohen HY, et
al: Stress-dependent regulation of FOXO transcription factors by
the SIRT1 deacetylase. Science. 303:2011–2015. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Dick O and Bading H: Synaptic activity and
nuclear calcium signaling protect hippocampal neurons from death
signal-associated nuclear translocation of FoxO3a induced by
extrasynaptic N-methyl-D-aspartate receptors. J Biol Chem.
285:19354–19361. 2010. View Article : Google Scholar : PubMed/NCBI
|