Introduction
Angiogenesis is a critical physiological process
responsible for forming new blood vessels from existing blood
vessels (1). Endothelial cells (ECs)
evidently participate in this process with several mechanisms,
including proliferation, migration and assembly (2). These mechanisms are regulated by a
number of angiogenic factors, including angiogenic growth factors
and their receptors, transcription factors, matrix degradation
molecules, cell adhesion molecules, tubule formation and
morphogenesis factors, and molecules for blood vessel maturation
(2). Normal physiological
angiogenesis is rarely observed in adults except in the ovary and
endometrium during females' reproductive life (3). In numerous pathological conditions,
angiogenesis is also required to facilitate tissue repair by
transferring more nutrients and oxygen to the ischemia sites, which
is beneficial to treat several types of illnesses, including
myocardial ischemia, osteonecrosis, bone fracture and ischemic
chronic wounds such as a diabetic limb (4–6).
‘Niu-Xi’ is a widely used traditional Chinese
medicine (TCM). Niu-Xi is traditionally believed to have the
effects of removing ‘blood stasis’, antifertility,
anti-inflammation, stimulating menstruation and curing orthopaedic
diseases, including bone injury (7),
although these effects have not been established clinically. As
listed in the Chinese pharmacopoeia 2010, there are two different
types of medicinal plants used as ‘Niu-Xi’ that are grown in
different provinces in China. One is called Radix Achyranthis
Bidentatae (RAB; named ‘Huai-Niu-Xi’ in Chinese), and the other one
is called Radix Cyathulae (RC; called ‘Chuan-Niu-Xi’ in Chinese).
RAB is the dried root of Achyranthese bidentata Bl, which is
grown in Africa, Asia and the He-Nan province in China. Moreover,
RC is the dried root of Cyathula officinalis Kuan which is
harvested in the Si-Chuan province in China (7). These two TCM remedies are believed to
possess similar therapeutic effects on the above mentioned
illnesses, and can be used interchangeably in different TCM
formulae in numerous cases (7). In
addition to these effects, use of RAB has been attempted for
releasing hypertension dizziness, while RC is typically used with
the aim of treating rheumatism, placenta retention and to relieve
stranguria (7). The major components
in these two radixes are phytoecdysones and triterpenoid saponins.
The majority of them are derivatives of oleanolic acid, and have
anti-inflammatory, anti-oxidant and anti-osteoporosis biological
effects that have been reported (8,9).
However, their chemical markers are different. As suggested by the
Chinese pharmacopoeia, β-ecdysterone is one of the major bioactive
markers in the RAB extract, and cyasterone is found as an authentic
standard in the RC extract (7,10,11).
Although there is no direct concept on angiogenesis
in the principles of TCM, RAB and RC are traditionally applied with
the aim of stimulating menstruation and curing bone diseases in
which angiogenesis is critical (3–5). The aim
of the present study was to explore whether the whole extracts of
RAB and RC both possess potential pro-angiogenic effects, which may
indicate the pharmacological mechanism underlying their
hypothesized traditional therapeutic effects. In the present study,
the pro-angiogenic effects of the RAB and RC whole extracts were
measured in vitro in human umbilical vein endothelial cell
(HUVEC) models, where cell proliferation and migration, and tube
formation were evaluated, Furthermore, an in vivo transgenic
zebrafish model was also used. The underlying mechanisms were also
detected by quantitative polymerase chain reaction (qPCR) for
measuring angiogenesis-related gene regulation in zebrafish.
Materials and methods
Chemicals
High performance liquid chromatography (HPLC) grade
acetonitrile and ethanol were purchased from Fisher Scientific
(Thermo Fisher Scientific, Inc., Leicester, UK). The iScript
one-step RT-PCR kit and SYBR Green reagents used were purchased
from Bio-Rad Laboratories, Inc. (Hercules, CA, USA). HUVECs were
provided by the American Type Culture Collection (Manassas, VA,
USA). The authentic standards (purity, >95%) of ginsenoside Ro,
chikusetsusaponin IVA, β-ecdysterone and cyasterone were from
Shanghai Tauto Biotech Co., Ltd. (Shanghai, China). Trypsin, fetal
bovine serum (FBS), penicillin and streptomycin were supplied by
Gibco (Thermo Fisher Scientific, Inc., Grand Island, NY, USA).
Matrigel™ Matrix Growth Factor Reduced was bought from BD
Biosciences (Franklin Lakes, NJ, USA). F12 medium, heparin,
endothelial cell growth supplement (ECGS) and all other unspecified
chemicals were supplied by Sigma-Aldrich (Merck KGaA, Darmstadt,
Germany).
Plant material and extraction
The dried raw herbs of RAB (harvested in Henan,
China) and RC (harvested in Sichuan, China) were purchased from
Guangzhou Zhixin Pharmaceutical Co., Ltd. (Guangzhou, China). Their
voucher specimens (no. 2013-3413 for RAB and no. 2013-3414 for RC)
were kept in the museum of the Institute of Chinese Medicine of the
Chinese University of Hong Kong (Hong Kong, China) and were
authenticated by thin layer chromatography (HPTLC) according to the
Chinese Pharmacopoeia 2010. The presence of oleanolic acid and
cyasterone were determined in RAB and RC, using HPTLC silica gel
F254 plates (Merck KGaA).
The raw materials (60 g) of RAB and RC were
extracted by 1-h heat water reflux extraction with 2 L of water.
Following filtration, the supernatant was collected, the residue
extracted by heat ethanol reflux extraction with 2 L of 95% ethanol
for 1 h, then filtered again to collect the ethanol. The ethanol
was removed by a vacuum rotary evaporator under reduced pressure,
and then mixed with the supernatant. Following overnight
precipitation, the supernatant was subjected to freeze-drying in
order to collect the whole extract. The dried extract was then
stored in the desiccator before use.
Chemical profiles
RAB and RC whole extracts were completely dissolved
in water to a concentration of 5 mg/ml and filtered with a 0.22-µm
filter. Next, they were subjected to chemical analysis by an
Agilent 1290 Infinity HPLC system (Agilent Technologies, Inc.,
Santa Clara, CA, USA). HPLC was equipped with an online degasser, a
binary-pump, an autosampler and a diode array detector, and an
Alltima HPLC C18 column (250×4.6 mm, 5 µm; Fisher Scientific)
protected by a C18 guard column was used for HPLC analysis
according to previous reports, with minor modifications (10,11).
Moreover, the column was maintained at 40°C. The mobile phase for
the RAB extract consisted of (A) 0.1% acetate acid and (B)
acetonitrile with the following gradient: 10–20% B from 0 to 10
min; 20–40% B from 20 to 30 min; 40–60% B from 30 to 40 min; 60–90%
B from 40 to 60 min and 90% B from 60 to 70 min. The mobile phase
for the RC extract consisted of (A) 0.1% acetate acid and (B)
acetonitrile in the following gradient: 10–20% B from 0 to 10 min;
20–28% B from 10 to 20 min; 28% B from 20 to 40 min; 28–60% B from
40 to 50 min; 60–90% B from 50 to 60 min and 90% B from 60 to 70
min. Moreover, the flow rate was set to 0.7 ml/min and the
injection volume was 10 µl.
Mass spectrometry was performed using an Agilent
6530 Accurate-Mass Quadrupole Time-of-Flight mass spectrometer
(Agilent Technologies, Inc.) equipped with a Jet Stream
electrospray ionization (ESI; Agilent Technologies, Inc., Santa
Clara, CA, USA) source. Parameters for the ESI source were set as
follows: Positive ion mode; gas temperature, 350°C; drying gas, 11
l/min; nebulizer, 55 psi; and capillary, 4,200 V. Moreover, the
mass range was set to 100–1,700. Data were qualitatively analyzed
using MassHunter Workstation software, version B.05.00 (Agilent
Technologies, Inc.).
Cell viability
Cell viability was measured using an MTT assay.
DMEM/F12 medium was mixed with heparin (100 mg/l), ECGS (30 mg/l)
and 0.5% antibiotics (penicillin-streptomycin-100X, 50 mg⁄ml;
Gibco; Thermo Fisher Scientific, Inc.). Next, the mixed DMEM/F12
medium was supplemented with 10% FBS as culture medium or 0.5% FBS
as test medium. In brief, HUVECs (1×104 cells per well)
were seeded onto the 96-well plates with culture medium overnight,
and the medium was then replenished with test medium for another 12
h. RAB and RC whole extract were redissolved into the stock
solution (800 µg/ml) with test medium, filtered by a 0.22-µm
filter, and diluted with test medium to the working concentrations
(0–800 µg/ml) for a 24-h incubation. Following drug treatment, the
medium was replaced with MTT (0.5 mg/ml in test medium) for another
3-h incubation at 37°C, and replenished with dimethyl sulfoxide
(100 µl) in order to dissolve the formed formazan. The absorbance
was detected at 540 nm using a microplate reader.
Wound healing
A wound healing assay (scratch assay) was performed
as described previously (12).
HUVECs (1.5×105 cells per well) were seeded in culture
medium onto 24-well plates overnight. Two crosses were scratched on
the cells with a p200 pipette tip, then medium was removed and the
cells were washed with pre-warmed PBS. Different concentrations
(0–200 µg/ml) of RAB and RC whole extracts in test medium were
prepared as mentioned above and were added for an 8-h treatment.
Images were then captured before and after drug treatment at a ×40
magnification by an inverted microscope (Eclipse TS100; Nikon
Corporation, Tokyo, Japan). Data were analyzed using TScratch
software (13). Four replicates were
performed for each individual experiment, and four experiments were
performed.
Tube formation
RAB and RC whole extracts were redissolved into the
stock solution (800 µg/ml) with culture medium and filtered. HUVECs
(1×104 cells per well) were mixed with various
concentrations (0–200 µg/ml) of RAB and RC whole extracts in
culture medium, and seeded onto matrigel-pre-coated 96-well plates
for a 2-h incubation. Next, the formed tube networks were monitored
at a ×40 magnification using an inverted microscope. The total
length of tubes in each image was measured using Image-Pro Plus 6.0
software (Media Cybernetics, Inc., Rockville, MD, USA). Duplicates
or triplicates were performed for each experiment, and three
experiments were performed in total.
Sprout number count in zebrafish
The zebrafish TG (fli1:EGFP) y1/+(AB)
line was transgenic with ECs expressing enhanced green fluorescent
protein, which was supplied by the Zebrafish International Resource
Centre of the University of Oregon (Eugene, OR, USA). Zebrafish
experiments were approved by the Department of Health, Hong Kong
SAR according to the guidelines in the Care and Use of Animals.
Zebrafish were sacrificed by TRIzol (Qiagen Sciences, Inc.,
Gaithersburg, MD, USA Briefly, zebrafish embryos at 1–4 cell stage
were collected for disinfection with methyl-blue solution (2 µg/ml)
as reported previously (12).
Disinfected embryos were washed twice with embryo medium (0.06 g/l
Instant Ocean Sea Salt, Blacksburg, VA, USA) and RAB and RC whole
extracts were redissolved into the stock solution (800 µg/ml) with
embryo medium, filtered by a 0.22-µm filter. Next, they were
diluted with embryo medium to the working concentrations. Embryos
were incubated with different concentrations (0–200 µg/ml) of RAB
and RC whole extracts. Moreover, embryos receiving embryo medium
alone served as a control. After 72-h post-fertilization, the
morphology of the sub-intestinal vessel (SIV) region in zebrafish
larvae was monitored using an IX71S8F-2 inverted fluorescent
microscope (Olympus Corporation, Tokyo, Japan). The mean sprout
number in the SIV region was calculated by dividing the sum of the
sprout number by the total number of embryos in each group, which
was used for indicating the pro-angiogenic effect.
qPCR analysis of mRNA expression in
zebrafish
After counting sprout numbers, zebrafish were stored
in TRIzol (Qiagen Sciences, Inc.). The total RNA was extracted from
whole embryos using an RNeasy Mini kit (Qiagen Sciences, Inc.)
according to manufacturer's instructions. qPCR was performed with
Qiagen iScript one-step RT-PCR kit and SYBR Green using a CFX96™
Real-Time System (Bio-Rad Laboratories, Inc.). HotStarTaq DNA
Polymerase (Qiagen Sciences, Inc.). The cycling conditions were set
as follows: 50°C for 10 min, 95°C for 5 min, then 50 cycles of 95°C
for 10 sec and 60°C for 30 sec. The RT-PCR primers for migration
genes were synthesized by Tech Dragon Limited (Hong Kong, China)
and are listed in Table I according
to our previous study (12). β-actin
served as a housekeeping gene. All detections were performed in
triplicate, and gene expression analysis was performed to calculate
their normalized expression using the 2−ΔΔCq method
(14) with the CFX Manager™ software
(Bio-Rad Laboratories, Inc.).
 | Table I.Cell migration-related genes (obtained
from Liu et al, 2013). |
Table I.
Cell migration-related genes (obtained
from Liu et al, 2013).
| Primer | Sequences |
|---|
| Matrix
metallopeptidase 2 |
F-AGCTTTGACGATGACCGCAAATGG |
|
|
R-TCAGAATGCTCTAAACCCAGGGCA |
| Matrix
metallopeptidase 9 |
F-AACCACCGCAGACTATGACAAGGA |
|
|
R-GTGCTTCATTGCTGTTCCCGTCAA |
| TIMP
metallopeptidase inhibitor 2 |
F-ATAAGCATGCGCTGAGGAAGAGGA |
|
|
R-AGCTGCAACAATCCAACTCCATGC |
| Plasminogen | F-
CATGCAAAGGCCTTGATGGGAACT |
|
|
R-TTGATCTCCACAACTAGGCACGCT |
| Cadherin-associated
protein β (β-catenin) | F-
ATGAGGGCATGCAGATACCTTCCA |
|
|
R-TTGACCACGGCATGTTTGAGCATC |
| β-actin | F-
TCCCCTTGTTCACAATAACC |
|
|
R-TCTGTTGGCTTTGGGATTC |
Statistical analysis
Data are presented as the mean ± standard error of
the mean. Statistical analysis was performed by Student t-test or
one-way analysis of variance followed by Dunnett's post-test and
analyzed using GraphPad Prism software, version 4 (GraphPad
Software, Inc., La Jolla, CA, USA). P<0.05 was used to indicate
a statistically significant difference.
Results
Chemical profiles
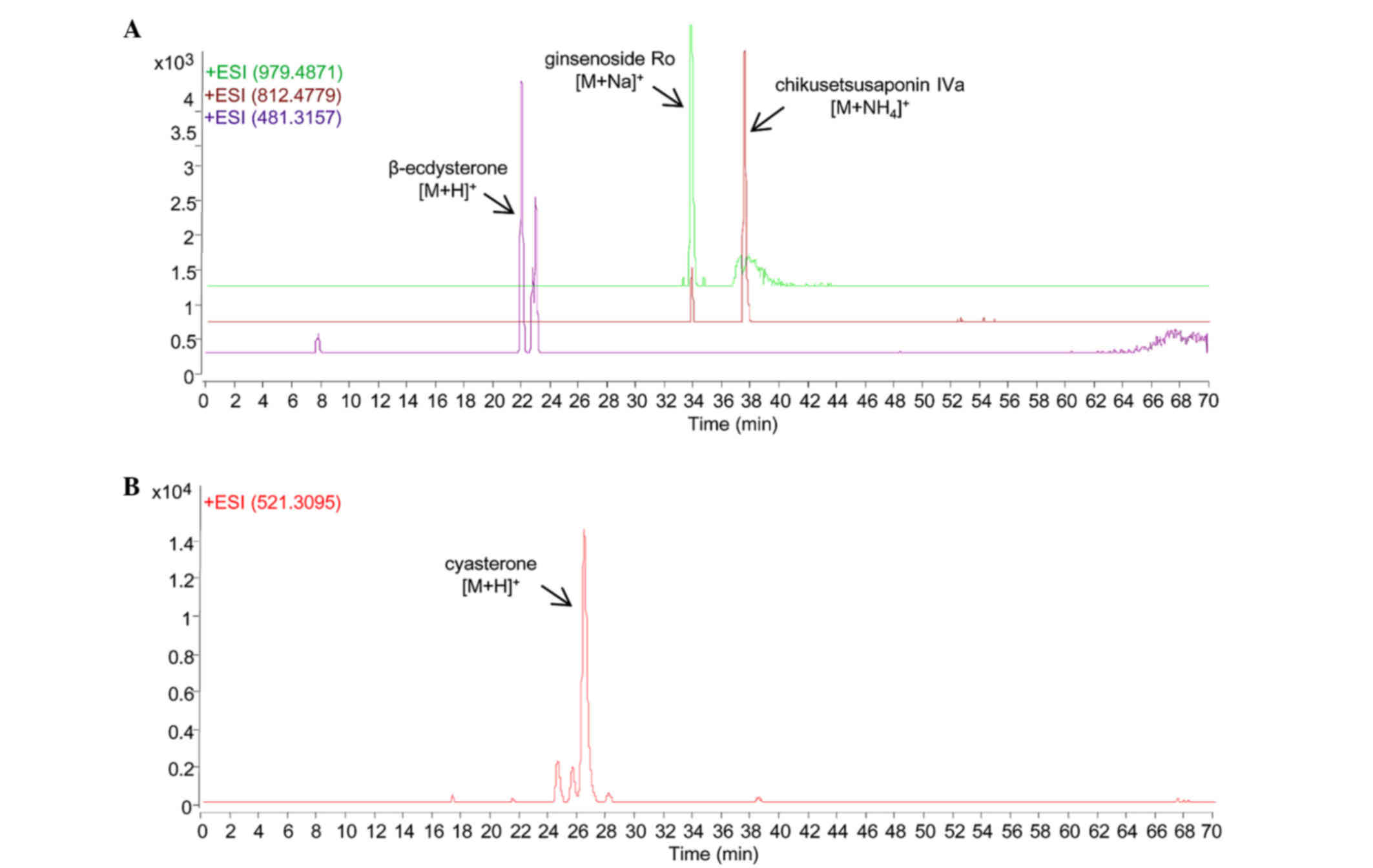
As shown in Fig. 1,
the chemical markers in the RAB extract were identified with their
respective molecular weights and retention times when compared to
their authentic standards, respectively (Fig. 1A). β-ecdysterone, which is the
chemical marker indicated in the Chinese pharmacopoeia for RAB, was
found with [M+H]+ m/z at 481.3157 (error,
17 ppm). The other two chemical markers, namely ginsenoside Ro and
chikusetsusaponin IVA, were detected with [M+Na]+
m/z at 979.4871 (error, 0.8 ppm) and
[M+NH4]+m/z at 812.4779 (error,
2.2 ppm), respectively.
Cyasterone is the chemical marker indicated in the
Chinese pharmacopoeia for RC, and it was identified in the RC
extract with [M+H]+m/z at 521.3095 (error,
3.6 ppm) when compared with its authentic standard.
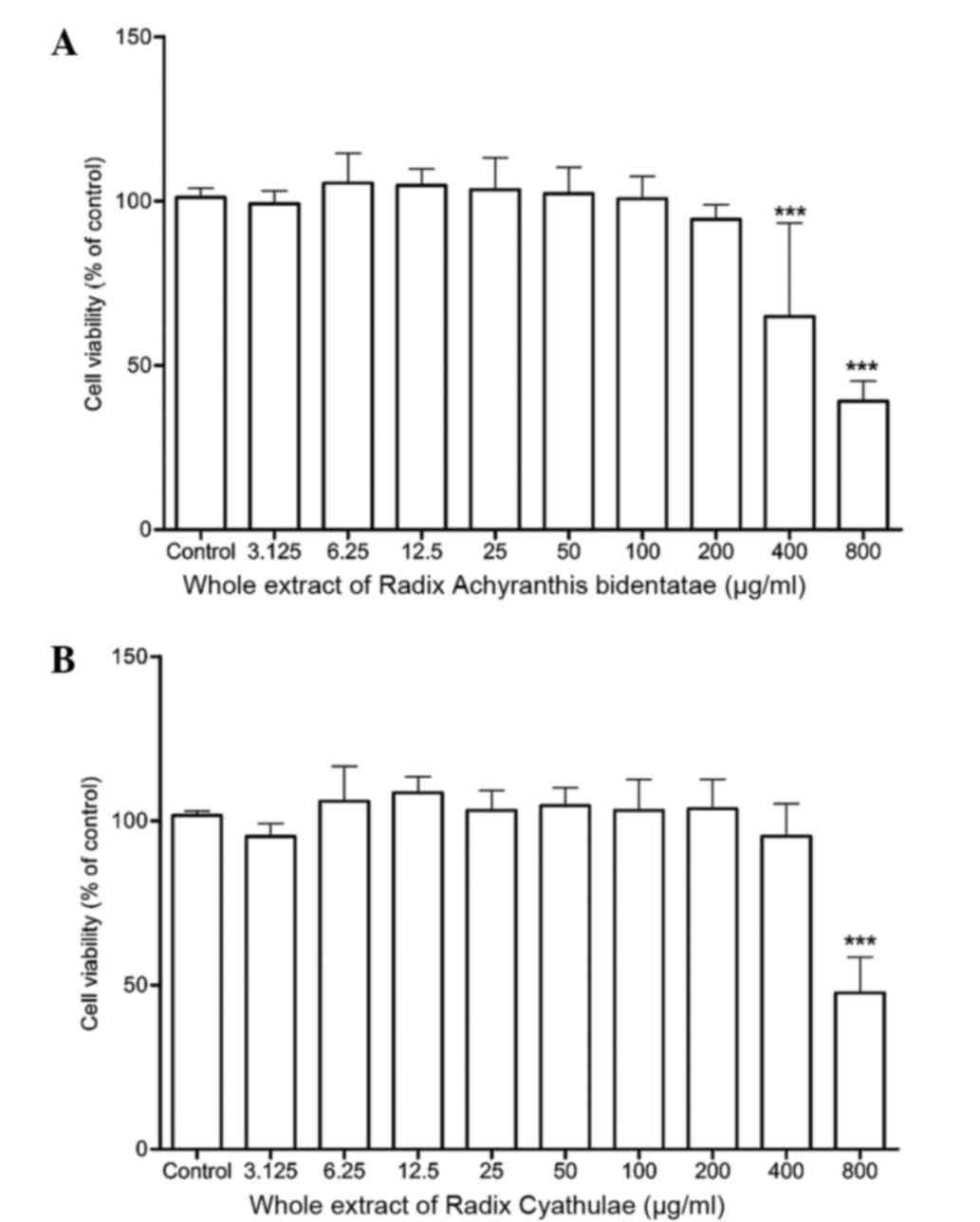
Cell viability
As detected by the MTT assay, 24-h treatment of RAB
whole extract did not significantly alter the cell viability from
3.125 to 200 µg/ml but displayed cytotoxicity at 400 and 800 µg/ml
when compared to the control (P<0.001) (Fig. 2A). Meanwhile, the RC whole extract
also did not significantly increase the cell viability from 3.125
to 400 µg/ml but only demonstrated a significant cytotoxicity at
800 µg/ml (Fig. 2B).
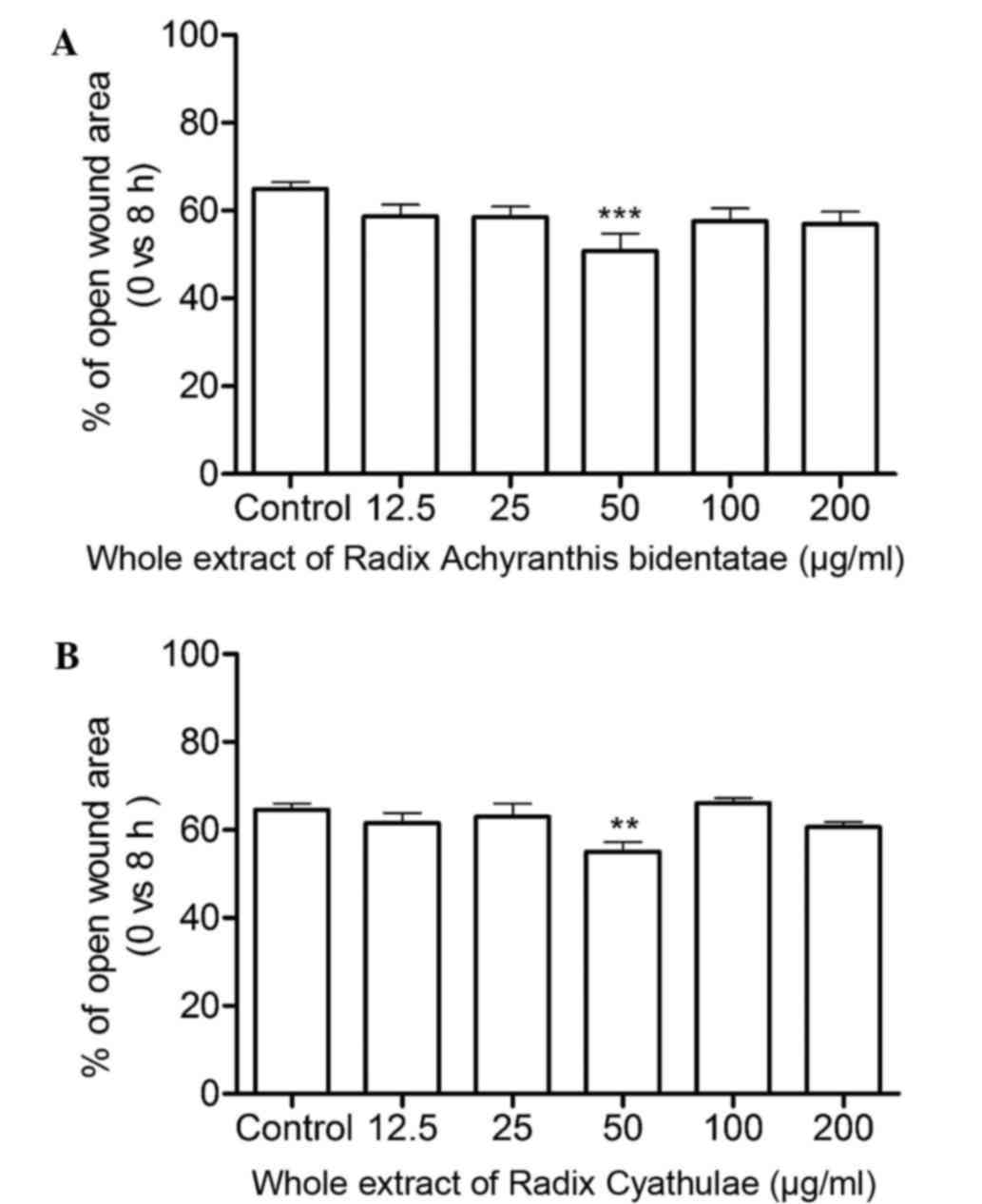
Wound healing
As shown in Fig. 3A,
the RAB whole extract significantly increased cell migration at 50
µg/ml after an 8-h treatment (P<0.001). Moreover, the open wound
area at 50 µg/ml was smaller than that of the control group by
~21.7%. In Fig. 3B, the RC whole
extract evidently enhanced cell migration with a smaller open wound
area by ~14.9%.
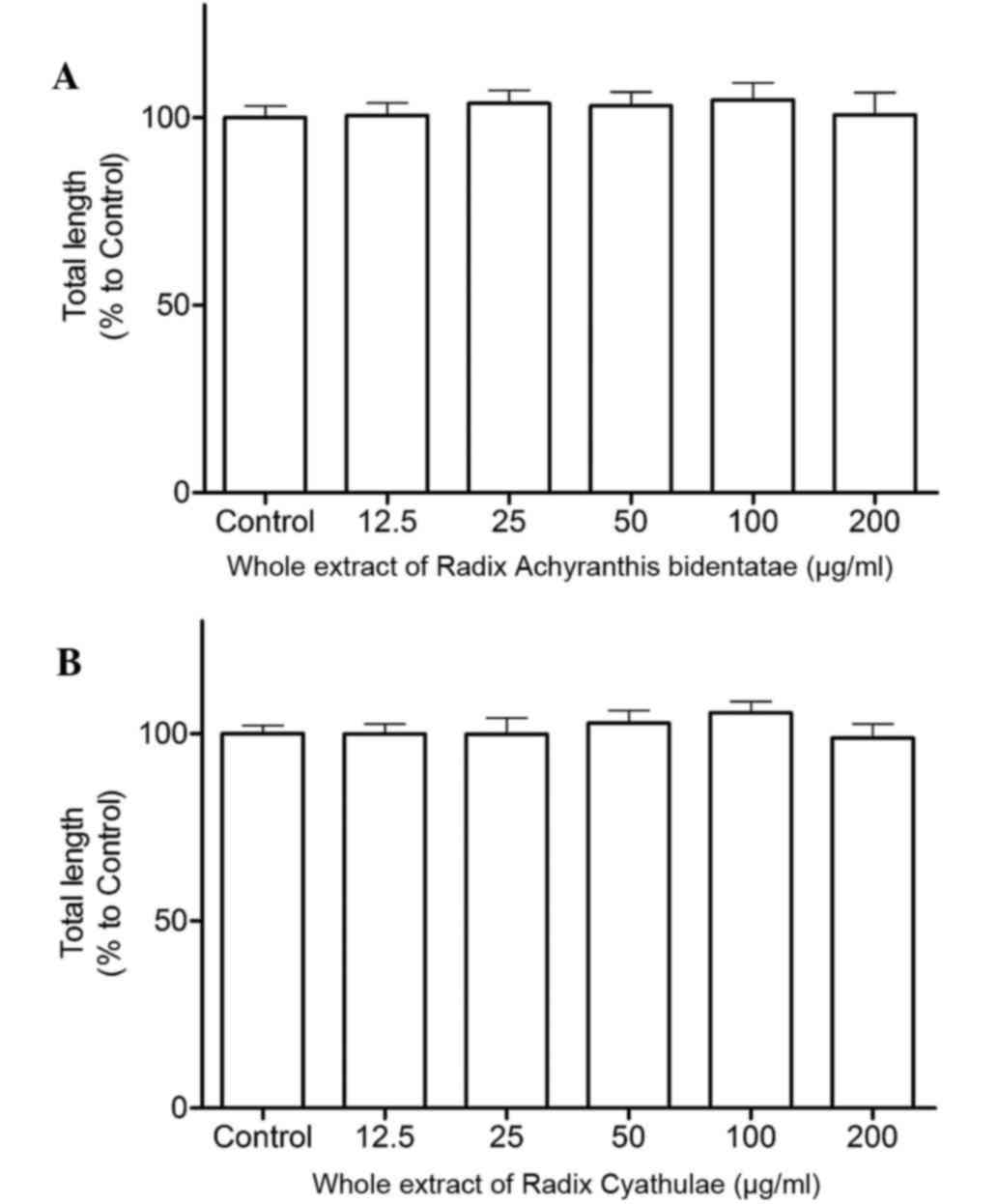
Tube formation
Following a 2-h treatment, RAB and RC whole extracts
at 100 µg/ml both individually increased tube formation in HUVECs
with 5% enhancement, but the increase was not significant compared
to the control (Fig. 4).
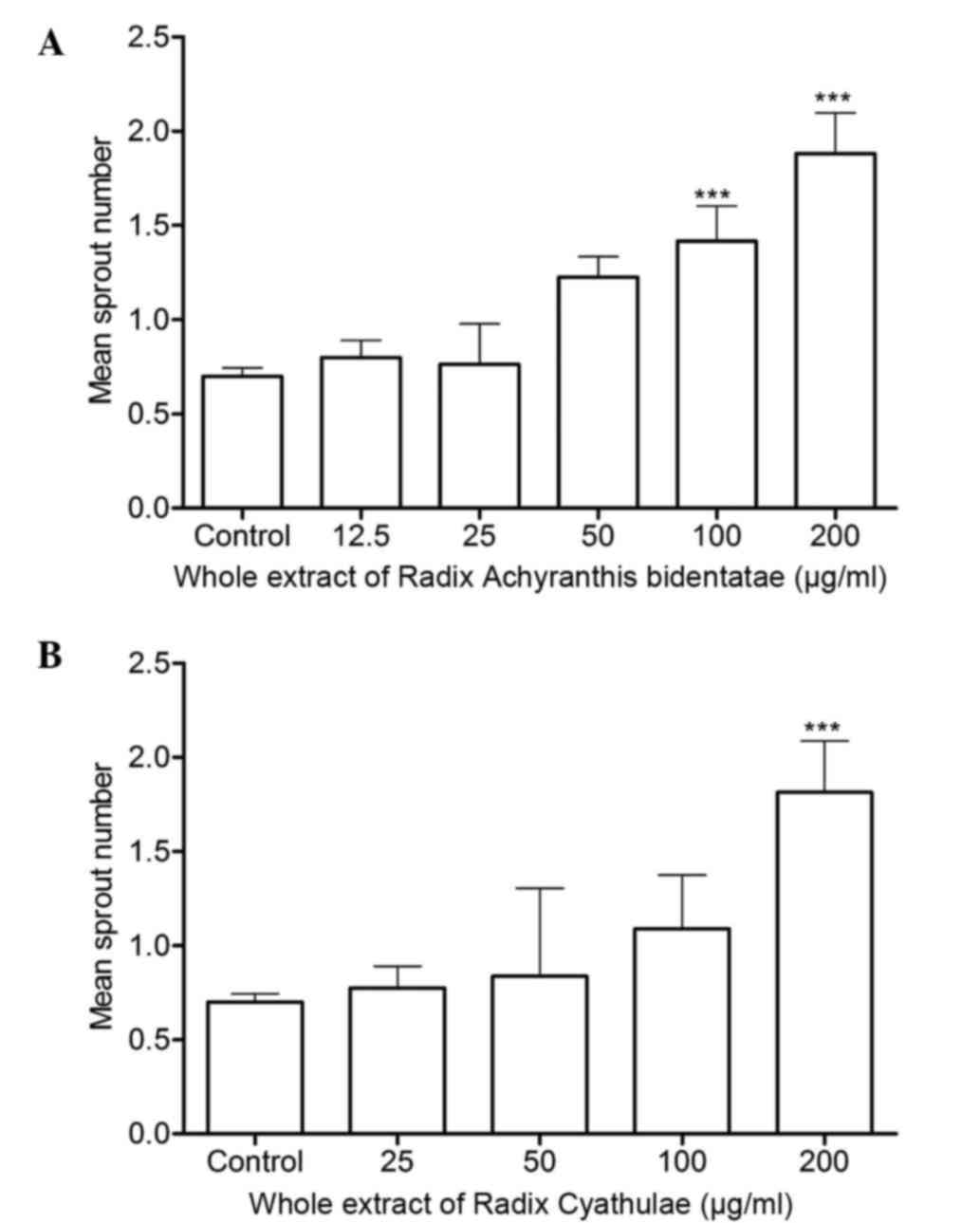
Sprout number count in the zebrafish
SIV region
When compared to the control group, RAB whole
extract at 100 and 200 µg/ml significantly increased the sprout
numbers in the SIV region of zebrafish (P<0.001) (Fig. 5A). Meanwhile, RC whole extract at 200
µg/ml markedly enhanced SIV sprout numbers (Fig. 5B). These indicate that RAB and RC
whole extracts can both promote angiogenesis in zebrafish.
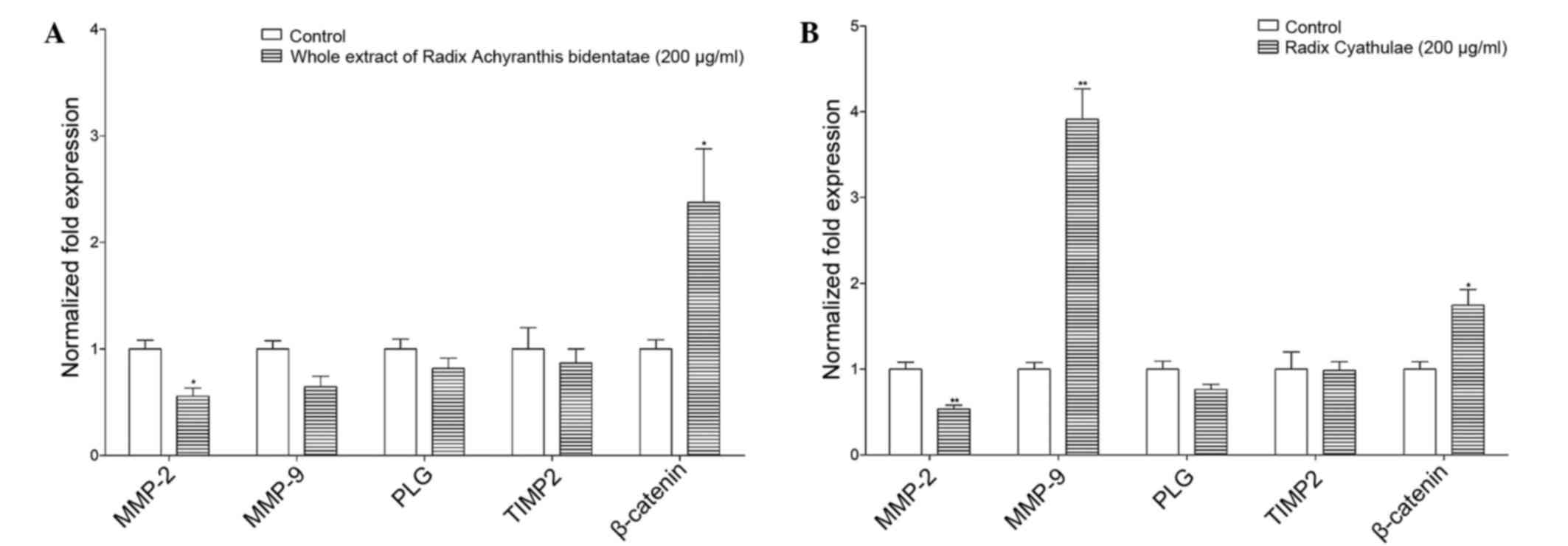
mRNA expression in zebrafish
In Fig. 6, RAB and RC
whole extracts regulated migration-related genes. In detail, RAB
whole extract (200 µg/ml) increased the gene expression of
β-catenin (2.4-fold) but decreased the expression of matrix
metallopeptidase 2 (MMP2) by 45%. Moreover, the expression of MMP9,
plasminogen (PLG) or tissue inhibitor of metalloproteinase 2
(TIMP2) was not significantly reduced by the RAB whole extract.
In addition, the RC whole extract (200 µg/ml)
significantly enhanced the gene expression of β-catenin (1.7-fold;
P<0.05), but reduced the expression of MMP2 by 47%. However, the
RC whole extract did not significantly regulate the expression of
PLG and TIMP2.
Discussion
Angiogenesis is important in the therapeutics used
for menstruation, wound healing and bone diseases. There are
several regulatory sites by exogenous compounds for stimulating
angiogenesis. For example, TCM can activate multiple pathways for
promoting angiogenesis (15–18). However, in the present study, the
whole extracts of RAB and RC promoted angiogenesis mainly via
stimulating migration of ECs in HUVECs in vitro, but not
through cell proliferation or tube formation. Subsequently, several
cell migration-related genes appeared to play roles in
pro-angiogenesis of these two extracts in zebrafish in vivo.
The results in human ECs are quite comparable to those in
zebrafish.
Cell migration is one of the critical processes in
angiogenesis. It highly integrates multistep processes, including
cell polarization, lamellipodia formation and focal adhesions'
assembly and disassembly (19).
Among these steps, proteolysis and adhesion of the extracellular
matrix appear to be the key steps (20). As revealed by qPCR in zebrafish, the
gene expression of matrix degradation-related factor-like MMP9 and
cell-cell adhesion factors such as β-catenin were upregulated. In
Fig. 6, RAB and RC whole extracts
regulated some of these genes, which are important in migration. In
detail, the RAB extract significantly enhanced the gene expression
of β-catenin but decreased that of MMP2, and very mildly reduced
those of MMP9, TIMP2 and PLG. This indicates that enhancing
cell-cell adhesion activity is the major process in the
pro-angiogenic effect of the RAB extract. Similarly, the RC extract
enhanced the gene expression of MMP9 and β-catenin, revealing that
matrix degradation and cell-cell adhesion were simultaneously
triggered by the RC extract for stimulating angiogenesis. As the
same molecular regulator activated by both extracts, β-catenin is a
critical member of the canonical Wnt signaling pathway that is
responsible for vascular sprouting, particularly for cell-to-cell
adhesion (21). Furthermore, it can
trigger signal transduction for vascular remodeling and
differentiation (22). In addition,
the gene expression of MMP9 was triggered by RC but not by RAB,
indicating this as the minor difference in angiogenic mechanisms
between these two extracts. Overall, the extracts of RAB and RC
possess angiogenic effects through similar mechanisms, which should
be one of the reasons why they can be used interchangeably in
different TCM formulae for treating numerous illnesses (7).
As indicated in the Chinese Pharmacopoeia 2010, both
RAB and RC are believed to treat amenorrhea and bone-related
disease within the TCM system, including bone fracture healing. In
the treatments of these diseases, the role of angiogenesis has been
demonstrated in numerous scientific reports (3–5). For
example, physiological angiogenesis always occurs in the
endometrium during the menstrual cycle, and appears in the basalis
layer during menstruation and in the functionalis and subepithelial
capillary plexus during the proliferative and early secretory
stages (3). In addition, it is
well-known that blood vessel formation is associated in bone
regeneration (23). During bone
fracture healing, ingrowth of blood vessels are responsible for
supplying nutrients and the influx of osteoblasts (24). In summary, the pro-angiogenic
capacity of RAB and RC is hypothesized to be responsible for one of
the therapeutic mechanisms of their traditional uses.
It is concluded that whole extracts of RAB and RC
promoted angiogenesis in HUVECs in vitro and in zebrafish
in vivo mainly through promoting cell migration. As
mentioned above, angiogenesis is important in stimulating
menstruation and bone healing. Therefore, from a modern
pharmacology point of view, the present study revealed for the
first time that pro-angiogenesis may be among the pharmacological
mechanisms underlying the purported traditional therapeutic effects
of RAB and RC.
Acknowledgements
The present study was financially supported by the
Innovation and Technology Commission, the government of Hong Kong
SAR (grant no. GHX/002/11) and Alberta Technology Limited.
References
|
1
|
Polverini PJ: Angiogenesis in health and
disease: Insights into basic mechanisms and therapeutic
opportunities. J Dent Educ. 66:962–975. 2002.PubMed/NCBI
|
|
2
|
Lin CM, Chiu JH, Wu IH, Wang BW, Pan CM
and Chen YH: Ferulic acid augments angiogenesis via VEGF, PDGF and
HIF-1 alpha. J Nutr Biochem. 21:627–633. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gargett CE and Rogers PA: Human
endometrial angiogenesis. Reproduction. 121:181–186. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Beamer B, Hettrich C and Lane J: Vascular
endothelial growth factor: An essential component of angiogenesis
and fracture healing. HSS J. 6:85–94. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Folkman J: Angiogenesis in cancer,
vascular, rheumatoid and other disease. Nat Med. 1:27–31. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Shohet RV and Garcia JA: Keeping the
engine primed: HIF factors as key regulators of cardiac metabolism
and angiogenesis during ischemia. J Mol Med (Berl). 85:1309–1315.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
C.P. Commission, . 2010, The Pharmacopoeia
of the People's Republic of China version 2010. China Medical
Science Press; Beijing, China:
|
|
8
|
Gao L, Cai G and Shi X: Beta-ecdysterone
induces osteogenic differentiation in mouse mesenchymal stem cells
and relieves osteoporosis. Biol Pharm Bull. 31:2245–2249. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang X, Xu X, Xu T and Qin S:
β-Ecdysterone suppresses interleukin-1β-induced apoptosis and
inflammation in rat chondrocytes via inhibition of NF-kB signaling
pathway. Drug Dev Res. 75:195–201. 2014.PubMed/NCBI
|
|
10
|
Li J, Li P, Li HJ, Song Y, Bi ZM and Li
YJ: Simultaneous qualification and quantification of eight
triterpenoids in radix achyranthis bidentatae by high-performance
liquid chromatography with evaporative light scattering detection
and mass spectrometric detection. J Sep Sci. 30:843–850. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li J, Qi H, Qi LW, Yi L and Li P:
Simultaneous determination of main phytoecdysones and triterpenoids
in radix achyranthis bidentatae by high-performance liquid
chromatography with diode array-evaporative light scattering
detectors and mass spectrometry. Anal Chim Acta. 596:264–272. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liu CL, Kwok HF, Cheng L, Ko CH, Wong CW,
Ho TW, Leung PC, Fung KP and Lau CB: Molecular mechanisms of
angiogenesis effect of active sub-fraction from root of Rehmannia
glutinosa by zebrafish sprout angiogenesis-guided fractionation. J
Ethnopharmacol. 151:565–575. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gebäck T, Schulz MM, Koumoutsakos P and
Detmar M: TScratch: A novel and simple software tool for automated
analysis of monolayer wound healing assays. Biotechniques.
46:265–274. 2009.PubMed/NCBI
|
|
14
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hong SJ, Wan JB, Zhang Y, Hu G, Lin HC,
Seto SW, Kwan YW, Lin ZX, Wang YT and Lee SM: Angiogenic effect of
saponin extract from Panax notoginseng on HUVECs in vitro and
zebrafish in vivo. Phytother Res. 23:677–686. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lam HW, Lin HC, Lao SC, Gao JL, Hong SJ,
Leong CW, Yue PY, Kwan YW, Leung AY, Wang YT and Lee SM: The
angiogenic effects of Angelica sinensis extract on HUVEC in vitro
and zebrafish in vivo. J Cell Biochem. 103:195–211. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li S, Lou S, Lei BU, Chan TF, Kwan YW,
Chan SW, Leung GP, Tsui SK and Lee SM: Transcriptional profiling of
angiogenesis activities of calycosin in zebrafish. Mol Biosyst.
7:3112–3121. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhou X, Siu WS, Fung CH, Cheng L, Wong CW,
Zhang C, Liu CL, Kwok HF, Lau CP, Wat E, et al: Pro-angiogenic
effects of Carthami Flos whole extract in human microvascular
endothelial cells in vitro and in zebrafish in vivo. Phytomedicine.
21:1256–1263. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ridley AJ, Schwartz MA, Burridge K, Firtel
RA, Ginsberg MH, Borisy G, Parsons JT and Horwitz AR: Cell
migration: Integrating signals from front to back. Science.
302:1704–1709. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gontero P, Banisadr S, Frea B and Brausi
M: Metastasis markers in bladder cancer: A review of the literature
and clinical considerations. Eur Urol. 46:296–311. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liebner S, Cavallaro U and Dejana E: The
multiple languages of endothelial cell-to-cell communication.
Arterioscler Thromb Vasc Biol. 26:1431–1438. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Reis M and Liebner S: Wnt signaling in the
vasculature. Exp Cell Res. 319:1317–1323. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sun H, Jung Y, Shiozawa Y, Taichman RS and
Krebsbach PH: Erythropoietin modulates the structure of bone
morphogenetic protein 2-engineered cranial bone. Tissue Eng Part A.
18:2095–2105. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Khosla S, Westendorf JJ and Mödder UI:
Concise review: Insights from normal bone remodeling and stem
cell-based therapies for bone repair. Stem Cells. 28:2124–2128.
2010. View
Article : Google Scholar : PubMed/NCBI
|