Introduction
Malignant gliomas are brain tumors characterized by
high proliferation and escape from immunosurveillance via numerous
mechanisms. Clinical vaccination trials aimed to decrease immune
tolerance against high grade gliomas have been conducted (1,2). Tumor
regression appears to be associated with the absence of a large
tumor mass secreting tumor growth factor (TGF)-β2 and on the
maturation status of dendritic cells inside and around the tumor
(3,4). Therapeutic strategies targeting the
immune response in the brain are therefore of particular interest
in the search for efficient treatments of malignant gliomas. The
central nervous system (CNS) is considered to be a unique
immunological site due to the presence of the blood-brain barrier,
and low immune reactivity prevents accidental inflammation within
the CNS (5–7). However, in the case of a CNS tumor,
strong immune responses against the invading pathogens develop
indicating that potent immune responses may occur against tumor
homeostasis (8).
Interleukin (IL)-22 is a major cytokine member of
the IL-10 cytokine super family, which also includes IL-19, IL-22,
IL-22, IL-24, IL-26, IL-28 and IL-29, and is secreted by T helper
(Th)17 (9,10). However, IL-22 exhibits potent
pro-inflammatory properties, unlike IL-10 (11). A previous study reported that IL-22
induced by IL-23 had an important role in psoriasis, since IL-22
was demonstrated to be required for imiquimod-induced psoriasiform
skin inflammation in mice (11).
IL-22 triggers an inflammatory response by activating signal
transducer and activator of transcription (STAT)3 signaling, and is
able to promote hepatocellular carcinoma (HCC) tumor-infiltrated
leukocytes due to high expression in this cell type (12). However, the effect of IL-22 on brain
tumors remains to be elucidated. In 2002, a study reported that
IL-22 is able to positively regulate signaling pathways such as
p38/extracellular signal-regulated kinase/c-Jun N-terminal
kinase/mitogen-activated protein kinase and Janus kinase (JAK)/STAT
in hepatoma cells (13). However,
few papers report its role in brain tumors. IL-22 was observed to
have an anti-apoptosis effect in lung cancer, acting in an
autocrine manner (14). In addition,
IL-22 was demonstrated to trigger inflammation and drive tumor
progression via IL-22R1 signaling in large cell lymphoma (15). In HCC, long term STAT3 activation by
IL-22 may promote tumor growth by targeting damaged hepatocytes and
tumor cells, similar to HCC promotion by IL-6 (12). However, self-reactive Th cells
coexpress IL-17 and IL-22, and the latter does not appear to be
directly involved in autoimmune pathogeneses of the CNS (16,17).
Materials and methods
Cell culture and drug treatment
The GL261 murine glioma cell line was obtained from
the American Type Culture Collection (Manassas, VA, USA). Cells
were cultured in vitro at 37°C (5% CO2) in
Iscove's Modified Dulbecco's Medium (Invitrogen; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) supplemented with 10% fetal
calf serum (Sigma-Aldrich; Merck Millipore, Darmstadt, Germany), 1%
100 U/ml penicillin and 1% 100 g/ml streptomycin (Invitrogen;
Thermo Fisher Scientific, Inc.) and 20 M β-mercaptoethanol
(complete medium). IL-22 protein was purchased from PeproTech, Inc.
(Rocky Hill, NJ, USA). Anti-IL-22 neutralising polyclonal rabbit
antibodies (ab109819) were purchased from Abcam (Cambridge, MA,
USA).
Animal model
A total of 50 female C57BL/6 mice (age, 6–12 weeks;
weight, 20–25 g) were obtained from Charles River Laboratories
(Wilmington, MA, USA). A brain tumor model was set up as described
previously (11). A total of
1×104 GL261 glioma cells were washed twice in
phosphate-buffered saline (PBS) and adjusted to 5 µl PBS in a
26-gauge Hamilton syringe. The mice were anesthetized with 1.2%
isoflurane (792632; Sigma-Aldrich). Following shaving, an incision
was made in the scalp, and a burr hole was made in the skull 2 mm
lateral to the midline and 2 mm anterior to the bregma using a
dental drill. Subsequently, GL261 glioma cells were incubated with
anti-IL-22 neutralising polyclonal rabbit antibodies at 4°C for 24
h. Following neutralization, GL261 glioma cells were injected over
1 min at a depth of 2.5 mm below the dura mater into the right
cerebral hemisphere. The mice were observed daily and sacrificed by
cervical dislocation when characteristic symptoms such as hunched
posture, reduced mobility, and significant weight loss (20%)
occurred within 10 days of glioma implantation. Animals without
such symptoms were regarded as long-term survivors after 90 days. A
total of 50 IL-22-deficient [knock-out (KO)] mice were generated by
targeting exons 1–3 and backcrossed onto C57BL/6 >8 times, as
described previously (16). The
targeting vector was constructed to replace the exons 1a, 1b, 2,
and a part of exon 3 of the IL-22a gene by a neomycin-resistant
gene. A 5′ arm of 1,521 bp was amplified using a mutated sense
primer with a XhoI site (5′-CTTCGGCTCGAGATGGCCAC-3′) and a mutated
antisense primer also containing a XhoI site
(5′-GCCCTCGAGACACCAGGGTT-3′) to allow the direct insertion into the
pPNT vector. The 3′ arm consisted of a 3,559-bp KpnI fragment,
containing the end of exon 3 and exon 4, and was cloned.
Mice were divided into GL261 glioma implantation +
IL-22 and GL261 glioma implantation + vehicle groups (n=6 per
group) and the brain tissues were harvested. The mice were bred
under specific pathogen-free conditions, and all experimental
protocols were approved by the Institutional Animal Care and Use
Committee of Hubei Cancer Hospital (Wuhan, China).
Evaluation of proliferation
GL261 glioma cells were analyzed for proliferation
using a Cell Counting kit-8 (CCK8; Dojindo Molecular Technologies,
Inc., Shanghai, China). Cells were seeded into 96-well plates at
densities of 1×104 cells/well, and incubated in a
humidified atmosphere containing 5% CO2 and 95% air
overnight. Normal cell medium containing either IL-22 or 0.01 M PBS
vehicle at the desired concentration were added to the cells. After
72 h incubation, 10 µl WST-8 from CCK8 (5 g/l in PBS) was added.
The plates were incubated for 4 h and the blue dye formed was
dissolved in 100 µl dimethyl sulfoxide. Absorbance at 450 nm was
recorded using an ELISA reader.
Evaluation of cell death
The cells were stained with propidium iodide (PI; BD
Biosciences, San Jose, CA, USA) and cell death was evaluated
according to the manufacturer's instructions. Briefly, cells were
collected, washed with cold PBS and suspended in binding buffer
(0.1M Hepes (pH 7.4), 1.4M NaCl and 25 mM CaCl2 in
solution; BD Biosciences). Following staining with 10 µl PI, the
cells were analyzed using a FACScan flow cytometer (BD
Biosciences).
Cytokine content measurement in the
tissue
The levels of IL-6, IL-1β, and tumor necrosis factor
(TNF)-α in the brains of the mice were measured in brain tissue
using ELISA kits (R&D Systems, Inc., Minneapolis, MN, USA),
according to the manufacturer's instructions.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
GL261 glioma cells were treated with IL-22 in
vitro and cultured for 8 h. IL-22 and IL-22 receptor (IL-22BP)
mRNA expression levels in the brains of GL261 glioma-inoculated
mice on days 0, 7 and 14 were evaluated by RT-qPCR. Total RNA was
extracted from the cells using an RNeasy mini kit (Qiagen China
Co., Ltd., Beijing, China) according to the manufacturer's
instructions. RT to cDNA was carried out using a Superscript III
First Strand Synthesis kit (Invitrogen; Thermo Fisher Scientific,
Inc.). qPCR was performed on an amplifier using real time PCR mix
(Bio-Rad Laboratories, Inc., Hercules, CA, USA), RT products, 7.5
µl 2X iQSYBR Green mix (Bio-Rad Laboratories, Inc.), 300 nM forward
and reverse primers and nanopure water to a final volume of 15 µl.
Primer sequences were as follows: IL-22, forward AAG CAT TGC CTT
CTA GGT CTCC and reverse TCA GAG ATA CAC GAG CTG GTT; IL-22BP,
forward CAT TGC CTT CTA GGT CTC CTCA and reverse CCT GCT TGC CAG
TGC AAAAT; STAT3, forward CAA TAC CAT TGA CCT GCC GAT and reverse
GAG CGA CTC AAA CTG CCCT; STAT4, forward GCA GCC AAC ATG CCT ATCCA
and reverse TGG CAG ACA CTT TGT GTT CCA; STAT6, forward CTC TGT GGG
GCC TAA TTT CCA and reverse CAT CTG AAC CGA CCA GGAAC; Ki67,
forward CGC AGG AAG ACT CGC AGTTT and reverse CTG AAT CTG CTA ATG
TCG CCAA; and GAPDH, forward AAT GGA TTT GGA CGC ATT GGT and
reverse TTT GCA CTG GTA CGT GTT GAT. PCR cycling conditions were as
follows: 3 min at 95°C for the polymerase activation, 45 cycles of
10 sec at 95°C for denaturation, and 30 sec at 60°C for annealing
and extension, followed by a DNA dissociation curve for the
determination of the amplicon specificity. Analyses were performed
in triplicate and water was used to replace DNA in samples used as
negative controls. Data were analyzed using the Cq value normalized
to the GAPDH endogenous reference gene. Data was collected and
computed by iQ5 BioRad software with an automated analysis of the
baseline and threshold of each run, and then exported in Excel
files for further analyses.
Statistical analysis
The data are presented as the means ± standard
deviation. Statistical differences were determined using a
two-tailed paired Student's t-test. SPSS software version 17.0
(SPSS, Inc., Chicago, IL, USA) was used to carry out the
statistical analyses. P<0.05 was considered to indicate a
statistically significant result.
Results
IL-22 promotes glioma development in
vivo
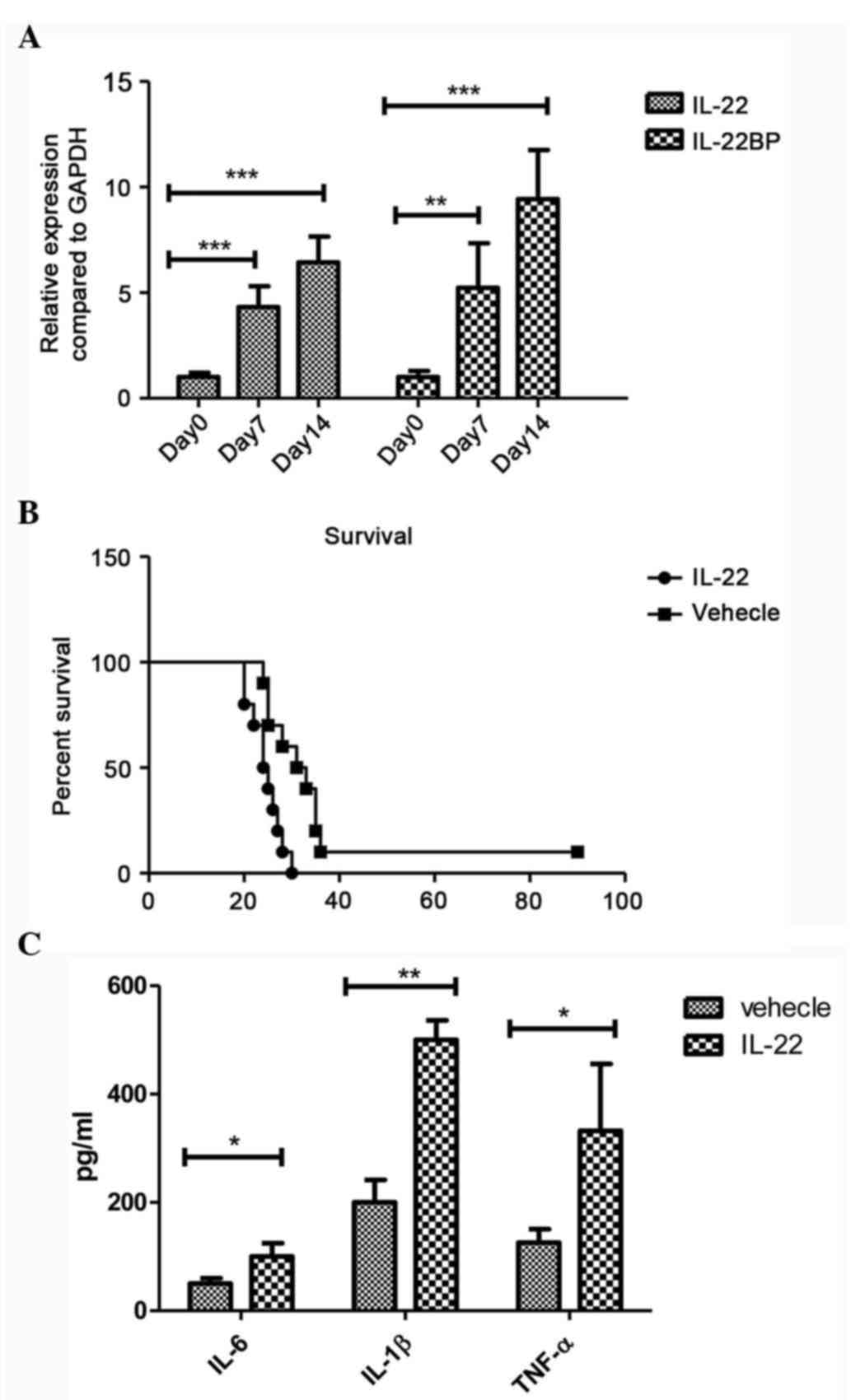
To determine the efficacy of IL-22 cytokine as a
therapeutic treatment in the murine model of glioma, the expression
levels of IL-22 were measured in the brain of GL261 glioma
cell-inoculation mice at days 7 and 14. Significantly increased
mRNA expression levels of IL-22 and IL-22BP were detected at day 7
and 14 compared with day 0 in the mouse glioma model (IL-22: day 7
vs. day 0, P=0.001; day 14 vs. day 0, P<0.001; and IL-22BP: day
7 vs. day 0, P=0.009; day 14 vs. day 0, P<0.001; Fig. 1A). To detect the biological function
of IL-22 in the brain, IL-22 or vehicle were directly injected into
the brain of normal mice to exclude the cytotoxic effect of IL-22
on the brain, the mice survived after local IL-22 treatment (data
not shown). To assess the cytotoxic of IL-22 in vivo, IL-22
was injected into the healthy mice without glioma implantation, and
the mice did not die after the single IL-22 injection. However,
when the GL261 glioma cell was implanted into the cerebral
hemisphere, the mice displayed severe disease following IL-22
injection, and significantly increased numbers of mice died
compared with the vehicle-treated group (P=0.008; Fig. 1B). In addition, treatment with IL-22
significantly increased the expression levels of IL-6 (P=0.011),
IL-1β (P<0.001) and TNF-α (P=0.018) in the brains of the mice,
suggesting that IL-22 amplifies the immune response which is
responsible for the brain tumor development in vivo
(Fig. 1C)
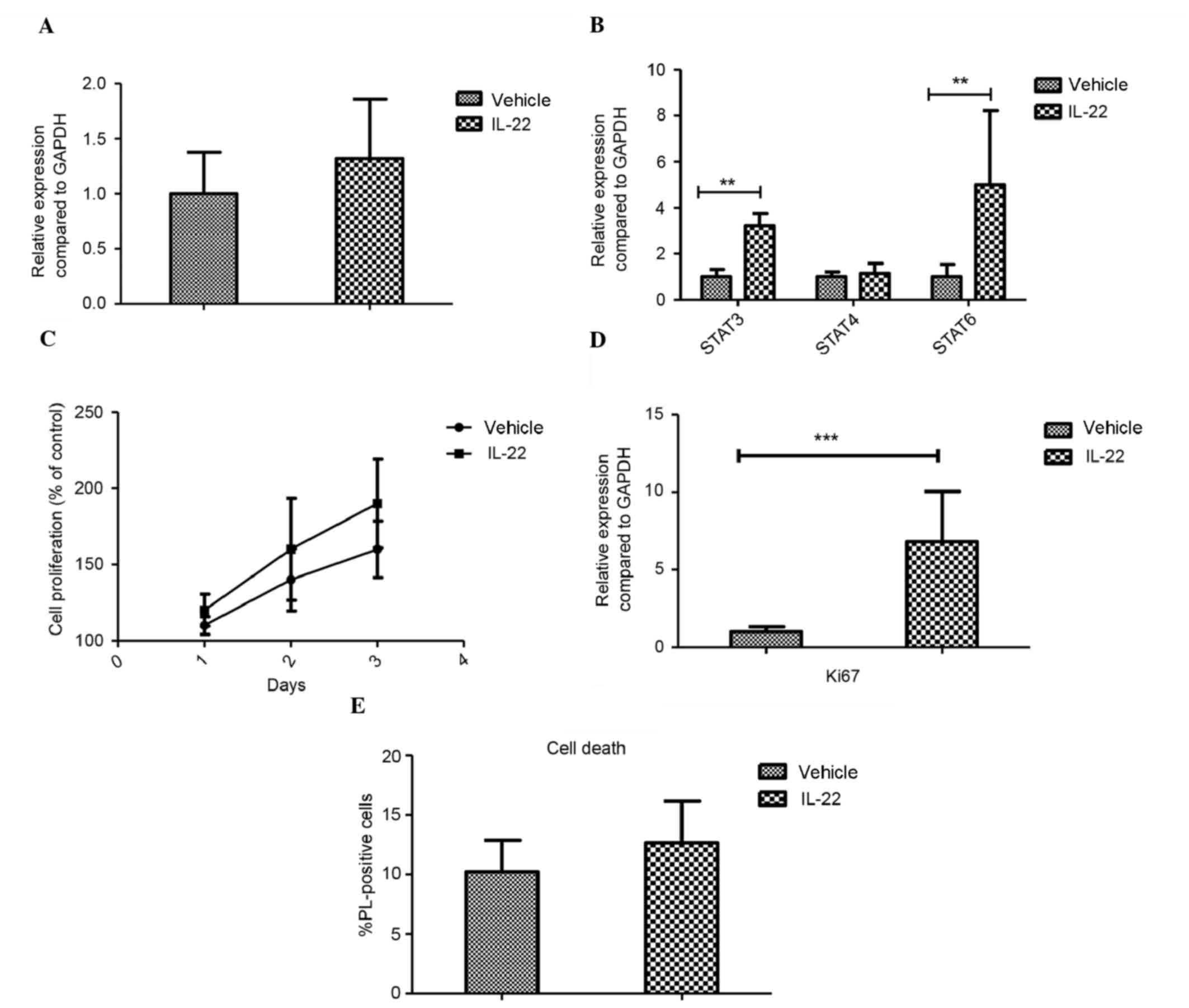
IL-22 modulates cell proliferation
through JAK-STAT-related gene expression
To elucidate the mechanism underlying the pathogenic
role of IL-22 in glioma, the effect of IL-22 was examined in
vitro. Firstly, the expression levels of the IL-22 receptor
(IL-22BP) were determined. The expression of IL-22BP in the GL261
glioma cell line was detected, but IL-22 itself did not change the
expression after IL-22 stimulation in vivo (Fig. 2A). The expression levels of the genes
of the JAK/STAT signaling pathway were also investigated, and the
cells were treated with 100 ng/ml IL-22 in vitro. IL-22
promoted the proliferation of glioma in vitro, which was
indicated by the high expression levels of Ki67. As shown in
Fig. 2B, IL-22 induced the
expression of STAT3 (P=0.001) and STAT6 (P=0.006), but had no
effect on STAT4 expression in GL261 cells, suggesting that IL-22
modulates neuronal inflammatory proteins through activation of
STAT3/STAT6 in the JAK/STAT signaling pathway. Furthermore CCK8
staining demonstrated that IL-22 promoted cell proliferation
(Fig. 2C), results which were
supported by the increase in Ki67 expression observed using qPCR
(P<0.001; Fig. 2D). In addition,
the cell death of the IL-22-treated cells was examined, and no
significant difference was observed (Fig. 2E).
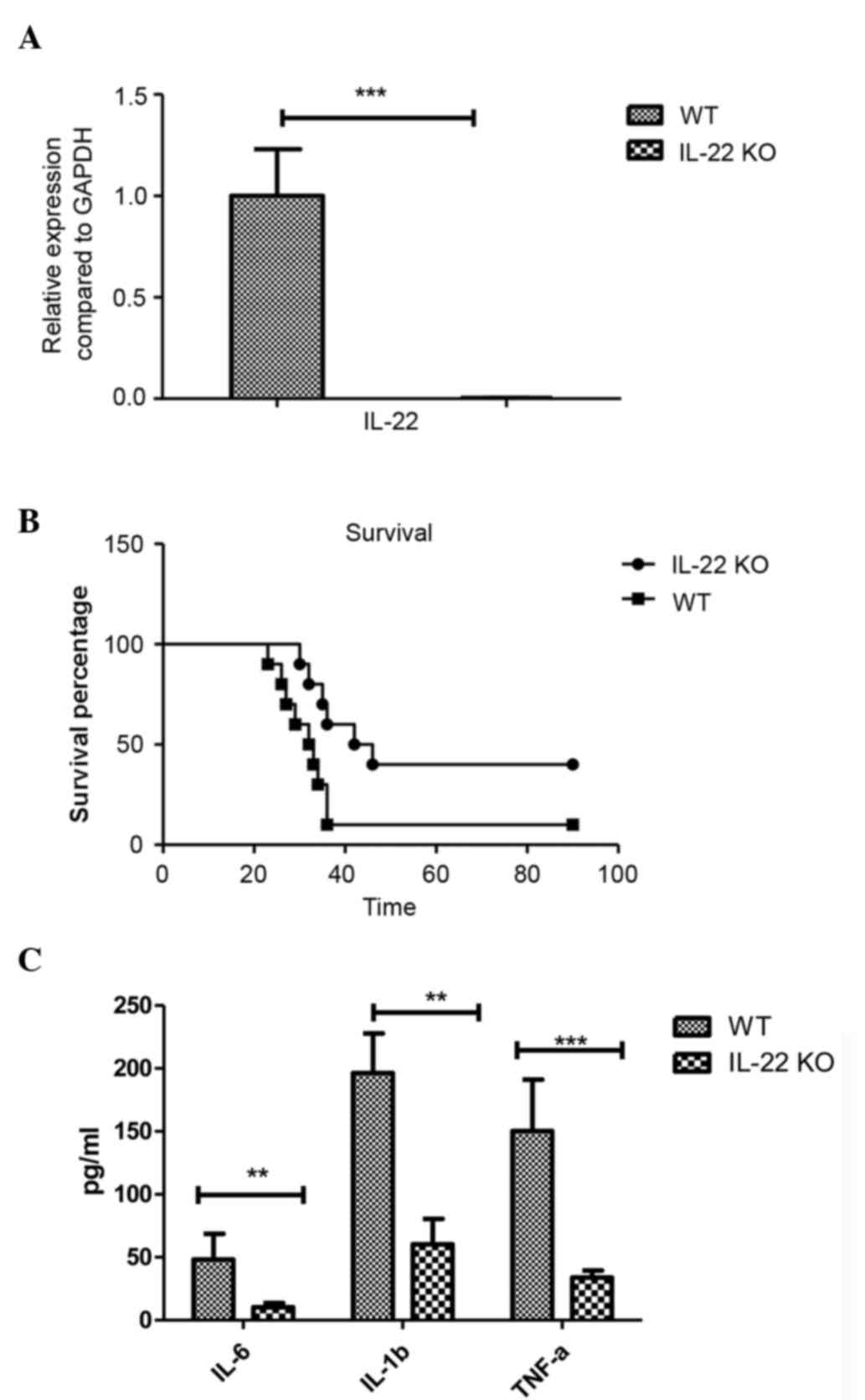
IL-22 KO attenuates glioma
progression
To further evaluate the effect of IL-22 on glioma,
the phenotype of IL-22 KO and IL-22 wild-type (WT) mice were
compared in vivo. First, the expression levels of IL-22 in
the glioma and brain of the recipient mice were investigated to
confirm the efficacy of the IL-22 KO, which indicated that IL-22 KO
mice exhibited significantly diminished IL-12 expression in the
brain (P<0.001; Fig. 3A). The KO
and WT mice were inoculated intracerebrally with GL261 glioma, and
then the mice were observed for clinical symptoms. The survival of
the IL-22 KO mice with glioma was significantly prolonged compared
with the IL-22 WT mice (Fig. 3B).
None of the surviving animals exhibited neurological disabilities.
Tumor growth was observed to be the cause of death for all of the
deceased animals. Furthermore, the inflammatory cytokines including
IL-6 (P=0.004), IL-1β (P=0.001) and TNF-α (P<0.001) were
significantly reduced in the IL-22 KO mice (Fig. 3C), suggesting that the absence of
IL-22, inflammatory cytokines which may be pathogenic for tumor
development have decreased in the brain of glioma mice.
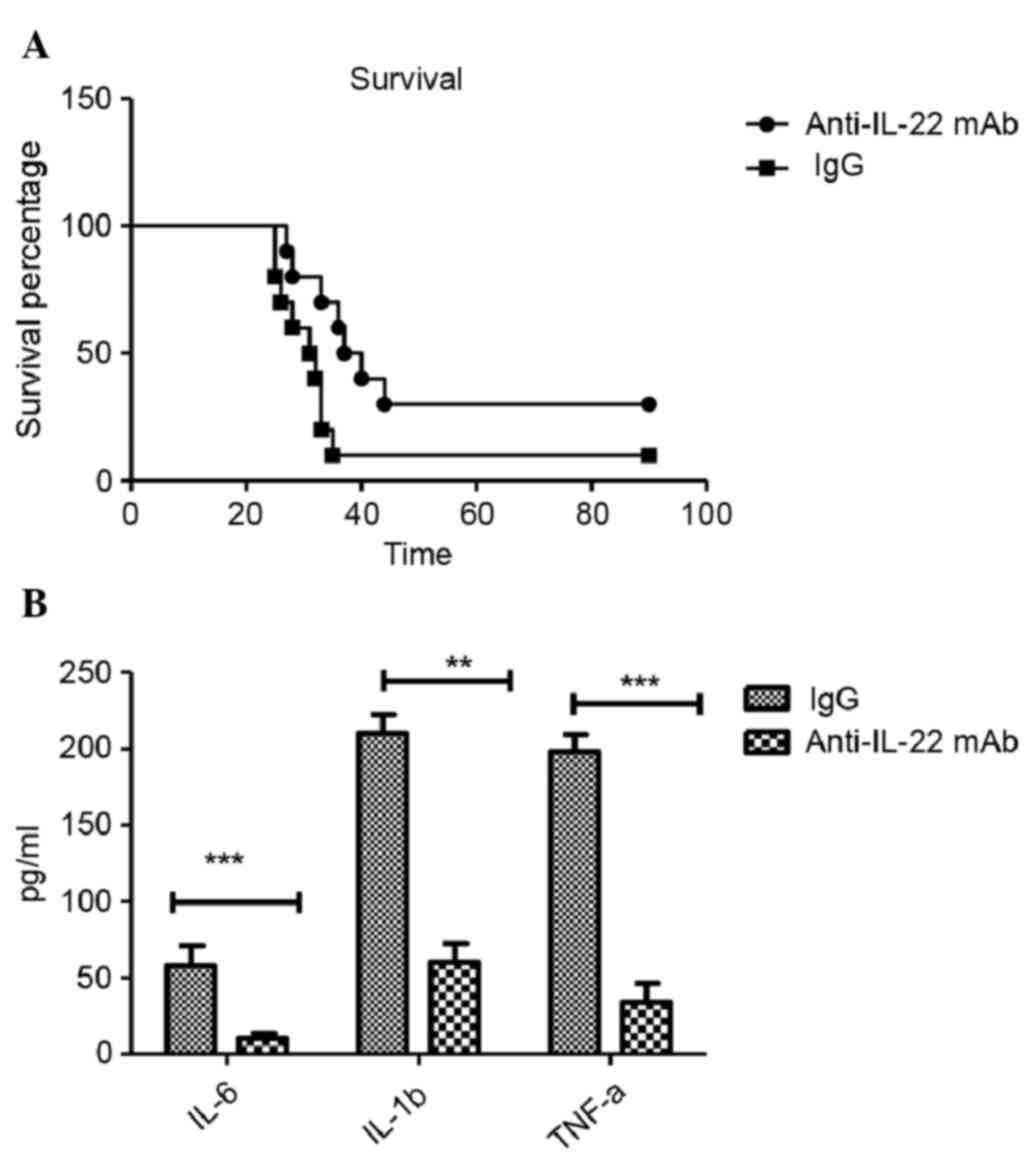
IL-22 blockade has a therapeutic
effect on glioma
In order to develop a therapeutic approach for the
treatment of IL-22, anti-IL-22 mAb was utilized. Similar to IL-22
KO mice, the anti-IL-22 antibody alleviated the symptoms of mice
glioma model, as identified by the higher survival percentage
(Fig. 4A), and anti-IL-22 mAb
reduced the levels of IL-6 (P<0.001), IL-1β (P<0.001) and
TNF-α (P<0.001) inflammatory cytokines in the brain tissue,
supporting the evidence for a role of anti-IL-22 mAb in glioma
(Fig. 4B).
Discussion
In the US, ~30,000 new patients are diagnosed with
glioma every year; glioblastoma is the most malignant form of
glioma with a median survival of 14 months (18). Previous studies have demonstrated
that gliomas retain many features of neuronal progenitor cells,
including the ability to grow as neurospheres in culture, and the
ability to self-renew and migrate in the brain (19,20).
However, inflammation-related cytokines and chemokines may have
important roles in different types of cancer. It has been reported
that miR-124 inhibits STAT3 signaling to enhance T cell-mediated
immune clearance of glioma by recruiting IL-2, IFN-γ and TNF-α
cytokines (21,22). The results of the present study
demonstrated that IL-22 promotes tumor growth in the brain by
regulating inflammatory cytokine production and cell proliferation,
suggesting that IL-22 may be a candidate for the therapeutic
targeting of glioma.
Higher expression levels of IL-22 and IL-22 receptor
(IL-22BP) were observed in the mouse glioma model. IL-22 injection
reduced the survival percentage of glioma-inoculated mice,
suggesting a role for IL-22 in tumor growth. To further confirm the
effect of IL-22 on glioma, inflammatory cytokine levels were
measured using ELISA. It was revealed that IL-6, IL-1β, and TNF-α
were induced in vivo by IL-22 injection. Concordant with
these findings, the expression of IL-22BP were detected in the
glioma cell line, and cells were treated with IL-22 in vitro
to elucidate the mechanism underlying IL-22 function in
vivo. It was demonstrated that IL-22 was able to increase the
mRNA expression levels of STAT3 and STAT6, thus activating the
JAK/STAT signaling pathway. In fact, various cytokines mediated
STAT activation, including IL-4 that was observed to specifically
activate STAT6, and IL-12 that modulated STAT4 (23–25). In
addition, IL-22 induced the phosphorylation of STAT3 (17,26).
Previous studies reported that JAK1 and STAT3 expression levels
were higher in low grade gliomas, as compared with high grade
gliomas, although the factors that induce STAT3 gene expression
remain to be determined (27,28).
Consequently, the results of the present study demonstrated that
IL-22 promoted the proliferation of glioma in vitro, which
was indicated by the high expression levels of Ki67. These results
suggest that STAT3 and STAT6 are involved in the process of
IL-22-mediated proliferation and regulation of the JAK/STAT
signaling pathway in vitro, could result in the phenotype
in vivo.
In addition, the effects of IL-22 on glioma were
further confirmed using the IL-22 KO mice. Mice with glioma
deficient in IL-22 showed alleviated disease severity.
Consistently, decreased levels of inflammatory cytokines were
observed in the IL-22 KO mice. It was previously reported that
glioma cells produce cytokines with an anti-inflammatory phenotype,
including IL-10, IL-4, IL-6, TGF-β, and prostaglandin E2 (29). TGF-β in particular suppresses the
activation and proliferation of microglia (30); the results of the present study
demonstrated that IL-6, IL-1 and TNF-α expression was downregulated
in the IL-22 KO mouse glioma model, thus IL-22 may globally
regulate different cytokines, including IL-6 and IL-1. Indeed,
IL-22 secreted by Th17 cells may elicit the production of IFN-γ,
which is also different from the cytokines in the CNS. Similarly,
using IL-22-blocking antibodies was demonstrated to protect mice
from glioma growth with a higher survival percentage, whilst
preventing the secretion of inflammatory cytokines and an immune
response in the brain. Both the IL-22 KO and anti-IL-22 antibody
protected the mice from glioma. To our knowledge, the present study
is the first to demonstrate IL-22 efficacy in a mice glioma model
in vivo and in vitro. The results demonstrated that
IL-22 has an important role in glioma via the regulation of
neuronal apoptosis, inflammatory cytokines, neural proliferation,
and JAK/STAT signaling in the tumor microenvironment of the CNS.
Anti-IL-22 antibody may prove useful in the treatment of gliomas in
clinical settings.
Acknowledgements
The present study was supported by a grant from the
Natural Science Foundation of Hubei Province, China (grant no.
050612036).
References
|
1
|
Yamanaka R, Abe T, Yajima N, Tsuchiya N,
Homma J, Kobayashi T, Narita M, Takahashi M and Tanaka R:
Vaccination of recurrent glioma patients with tumour lysate-pulsed
dendritic cells elicits immune responses: Results of a clinical
phase I/II trial. Br J Cancer. 89:1172–1179. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yu JS, Liu G, Ying H, Yong WH, Black KL
and Wheeler CJ: Vaccination with tumor lysate-pulsed dendritic
cells elicits antigen-specific, cytotoxic T-cells in patients with
malignant glioma. Cancer Res. 64:4973–4979. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Liau LM, Prins RM, Kiertscher SM, Odesa
SK, Kremen TJ, Giovannone AJ, Lin JW, Chute DJ, Mischel PS,
Cloughesy TF and Roth MD: Dendritic cell vaccination in
glioblastoma patients induces systemic and intracranial T-cell
responses modulated by the local central nervous system tumor
microenvironment. Clin Cancer Res. 11:5515–5525. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Valle RD, de Cerio AL, Inoges S, Tejada S,
Pastor F, Villanueva H, Gallego J, Espinos J, Aristu J, Idoate MA,
et al: Dendritic cell vaccination in glioblastoma after
fluorescence-guided resection. World J Clin Oncol. 3:142–149. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fabry Z, Raine CS and Hart MN: Nervous
tissue as an immune compartment: The dialect of the immune response
in the CNS. Immunol Today. 15:218–224. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Carmen J, Gowing G, Julien JP and Kerr D:
Altered immune response to CNS viral infection in mice with a
conditional knock-down of macrophage-lineage cells. Glia. 54:71–80.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Andersson U and Tracey KJ: Neural reflexes
in inflammation and immunity. J Exp Med. 209:1057–1068. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Palucka K and Banchereau J: Cancer
immunotherapy via dendritic cells. Nat Rev Cancer. 12:265–277.
2012. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Almolda B, Costa M, Montoya M, Gonzàlez B
and Castellano B: Increase in Th17 and T-reg lymphocytes and
decrease of IL22 correlate with the recovery phase of acute EAE in
rat. PloS One. 6:e274732011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cheng F, Guo Z, Xu H, Yan D and Li Q:
Decreased plasma IL22 levels, but not increased IL17 and IL23
levels, correlate with disease activity in patients with systemic
lupus erythematosus. Ann Rheum Dis. 68:604–606. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Van Belle AB, de Heusch M, Lemaire MM,
Hendrickx E, Warnier G, Dunussi-Joannopoulos K, Fouser LA, Renauld
JC and Dumoutier L: IL-22 is required for imiquimod-induced
psoriasiform skin inflammation in mice. J Immunol. 188:462–469.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jiang R, Tan Z, Deng L, Chen Y, Xia Y, Gao
Y, Wang X and Sun B: Interleukin-22 promotes human hepatocellular
carcinoma by activation of STAT3. Hepatology. 54:900–909. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lejeune D, Dumoutier L, Constantinescu S,
Kruijer W, Schuringa JJ and Renauld JC: Interleukin-22 (IL-22)
activates the JAK/STAT, ERK, JNK and p38 MAP kinase pathways in a
rat hepatoma cell line. Pathways that are shared with and distinct
from IL-10. J Biol Chem. 277:33676–33682. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang W, Chen Y, Wei H, Zheng C, Sun R,
Zhang J and Tian Z: Antiapoptotic activity of autocrine
interleukin-22 and therapeutic effects of interleukin-22-small
interfering RNA on human lung cancer xenografts. Clin Cancer Res.
14:6432–6439. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sabat R, Ouyang W and Wolk K: Therapeutic
opportunities of the IL-22-IL-22R1 system. Nat Rev Drug Discov.
13:21–38. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kreymborg K, Etzensperger R, Dumoutier L,
Haak S, Rebollo A, Buch T, Heppner FL, Renauld JC and Becher B:
IL-22 is expressed by Th17 cells in an IL-23-dependent fashion, but
not required for the development of autoimmune encephalomyelitis. J
Immunol. 179:8098–8104. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Backert I, Koralov SB, Wirtz S, Kitowski
V, Billmeier U, Martini E, Hofmann K, Hildner K, Wittkopf N, Brecht
K, et al: STAT3 activation in Th17 and Th22 cells controls
IL-22-mediated epithelial host defense during infectious colitis. J
Immunol. 193:3779–3791. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Davis FG and McCarthy BJ: Current
epidemiological trends and surveillance issues in brain tumors.
Expert Rev Anticancer Ther. 1:395–401. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Colleoni F and Torrente Y: The new
challenge of stem cell: Brain tumour therapy. Cancer Lett.
272:1–11. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Singh SK, Hawkins C, Clarke ID, Squire JA,
Bayani J, Hide T, Henkelman RM, Cusimano MD and Dirks PB:
Identification of human brain tumour initiating cells. Nature.
432:396–401. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Silber J, Lim DA, Petritsch C, Persson AI,
Maunakea AK, Yu M, Vandenberg SR, Ginzinger DG, James CD, Costello
JF, et al: miR-124 and miR-137 inhibit proliferation of
glioblastoma multiforme cells and induce differentiation of brain
tumor stem cells. BMC Med. 6:142008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wei J, Wang F, Kong LY, Xu S, Doucette T,
Ferguson SD, Yang Y, McEnery K, Jethwa K, Gjyshi O, et al: MiR-124
inhibits STAT3 signaling to enhance T cell-mediated immune
clearance of glioma. Cancer Res. 73:3913–3926. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kaplan MH, Schindler U, Smiley ST and
Grusby MJ: Stat6 is required for mediating responses to IL-4 and
for development of Th2 cells. Immunity. 4:313–319. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Monteleone G, Holloway J, Salvati VM,
Pender SL, Fairclough PD, Croft N and MacDonald TT: Activated STAT4
and a functional role for IL-12 in human Peyer's patches. J
Immunol. 170:300–307. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Morinobu A, Gadina M, Strober W, Visconti
R, Fornace A, Montagna C, Feldman GM, Nishikomori R and O'Shea JJ:
STAT4 serine phosphorylation is critical for IL-12-induced
IFN-gamma production but not for cell proliferation. Proc Natl Acad
Sci USA. 99:12281–12286. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sovran B, Loonen LM, Lu P, Hugenholtz F,
Belzer C, Stolte EH, Boekschoten MV, van Baarlen P, Kleerebezem M,
de Vos P, et al: IL-22-STAT3 pathway plays a key role in the
maintenance of ileal homeostasis in mice lacking secreted mucus
barrier. Inflamm Bowel Dis. 21:531–542. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang L, Liu W, Alizadeh D, Zhao D,
Farrukh O, Lin J, Badie SA and Badie B: S100B attenuates microglia
activation in gliomas: Possible role of STAT3 pathway. Glia.
59:486–498. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lo HW, Cao X, Zhu H and Ali-Osman F:
Constitutively activated STAT3 frequently coexpresses with
epidermal growth factor receptor in high-grade gliomas and
targeting STAT3 sensitizes them to Iressa and alkylators. Clin
Cancer Res. 14:6042–6054. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zisakis A, Piperi C, Themistocleous MS,
Korkolopoulou P, Boviatsis EI, Sakas DE, Patsouris E, Lea RW and
Kalofoutis A: Comparative analysis of peripheral and localised
cytokine secretion in glioblastoma patients. Cytokine. 39:99–105.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Suzumura A, Sawada M, Yamamoto H and
Marunouchi T: Transforming growth factor-beta suppresses activation
and proliferation of microglia in vitro. J Immunol. 151:2150–2158.
1993.PubMed/NCBI
|