Introduction
Human adipose-derived stem cells (ASCs) were
initially isolated from the aspirate of human fat by Zuk et
al (1). ASCs are convenient to
obtain, have broad sources, a long culturing time, strong breeding
ability and are not associated with ethical issues. Furthermore,
ASCs possess identical immunosuppressive effects to bone
marrow-derived mesenchymal stem cells, along with the potential to
differentiate into multiple blastophyllums, including cardiac
cells. ASCs are adult stem cells that have the characteristics of
mesenchymal stem cells and an extremely strong ability for external
amplification, as well as the potential for multi-directional
differentiation. In recent years, researchers have also shown that
ASCs may have a paracrine mechanism. ASCs secrete large volumes of
vascular endothelial growth factor, transforming growth factor-β,
hepatocyte growth factor and other active factors to promote
angiogenesis and improve ischemia when transplanted into areas of
myocardial necrosis. Due to their advantages in various fields,
ASCs have a strong potential for application in future stem cell
treatments and are beneficial for allogenic transplantation
treatments. ASCs may emerge as the ideal seed stem cells in cell
transplantation and tissue engineering clinical treatments
(2–5).
In accordance with the summary of worldwide
experience in obtaining, isolating, cultivating and identifying
ASCs, the present study has improved existing methods for these
processes in order to reduce damage to and simplify the
identification of ASCs.
Materials and methods
Reagents
Fetal bovine serum (FBS), type I collagenase and
trypsin were obtained from Sigma-Aldrich (Merck Millipore,
Darmstadt, Germany). Osteogenic and adipogenic differentiation
cultivating media were purchased from Cyagen Biosciences (Santa
Clara, CA, USA). Oil Red O dye and Dulbecco's Modified Eagle's
Medium (DMEM)-F12 cultivating medium were obtained from Bio Teke
Corporation (Beijing, China). Mouse anti-cluster of differentiation
(CD)44, anti-CD105 and anti-CD45 monoclonal antibodies were
purchased from Kangchen Bio-tech Corporation (Shanghai, China).
Separation and cultivation of
ASCs
Conventional liposuction using negative pressure
suction with abdominal distention was performed under aseptic
conditions on five female volunteers aged between 25 and 29 years
at the Department of Gynaecology and Obstetrics, The Second
Hospital of Tianjin Medical University (Tianjin, China), according
to a previous study (6). The present
study was approved by The Second Hospital of Tianjin Medical
University. The body mass indexes of the patients ranged from
25.7–29.9 kg/m2. Fat aspirate (50 ml/patient) was
obtained via the injector negative-pressure method (6), in the absence of excessive distention
solution and the assistance of ultrasonic emulsification or
resonance technology. Prior to the cessation of the anesthetic (100
mg lidocaine), the same volume of PBS (5 ml) was administered and
hemocytes in the liposuction were centrifuged at 1,200 × g
for 10 min at 37°C. The stock solution of collagenase I was diluted
to 0.075% using PBS, after which the adipose tissue was transferred
into a 50 ml centrifuge tube containing collagenase I. The tissue
was shredded using a small pair of scissors, after which the tube
was sealed with sealing film and centrifuged at 37°C and 1,200 ×
g for 30 min. The resulting pellet was resuspended in DMEM
containing 10% FBS (complete medium) with the same volume of
digestive enzyme (BioTeke Corporation, Beijing, China) to terminate
the collagenase-mediated digestion, after which centrifugation at
1,200 × g for 10 min was performed to subside the cells.
After removal of the supernatant, the cells were sedimented by
centrifugation at 1,200 × g for 10 min. Complete medium that
was 10 times the cell sedimentation volume was placed in a
centrifuge tube. Subsequently, the cells were inoculated into a
10-cm culture vessel at a density of 30–50%, followed by the
addition of complete medium to a final volume of 10 ml. After 24 h,
the medium was replaced to remove non-adherent cells; half of the
medium was replaced every 2 days until the cells reached a
confluency of 80–90%. The offspring produced in this step were
called the first passage (P1) cells and were frozen.
Differentiation was induced upon reaching 80–90% confluency.
ASCs oriented differentiation into
osteogenic cells
Second passage (P2) ASCs were seeded into 6-well
plates at a density of 20–30%, followed by the addition of
osteogenic-inducing medium (HUXMD-90021; Cyagen Biosciences). The
medium was replaced every 2 days during the 2 weeks of constant
culture when the cells were not proliferating exponentially. After
2 weeks, the cells were removed for alkaline phosphatase staining,
and the cells were visualized under a confocal microscope.
ASCs oriented differentiation into
adipogenic cells
P2 ASCs were seeded into 6-well plates at a
confluency of 20–30%, followed by the addition of
adipogenic-inducing medium (HUXMD-90031; Cyagen Biosciences). The
medium was replaced every other day for 1 week, after which the
cell climbing was removed for Oil Red staining, and the cells were
visualized under a microscope.
Proliferation activity of ASCs and
their directional differentiated cells
P2 ASCs (3,000 cells) were placed into wells
containing 100 µl DMEM culture medium, after which 100 µl
adipogenic- or osteogenic-inducing differentiation media were added
to some of the wells. In addition, 10 µl cell counting kit (CCK)-8
solution and 10 µl cell culturing solution were added to each well.
The wells without allocated cells served as the blank control. The
cells were incubated for 2 h at 37°C, after which the absorbance
was measured at 450 nm using a spectrophotometer.
Identifying the surface antigens of
ASCs by flow cytometry
P2 ASCs were digested with trypsin and then rinsed
twice with the Stain Buffer (Merck Millipore), which had been
pre-cooled at 4°C, prior to re-suspension to adjust the cell
density to 2×107 cells/ml. The cell suspension (50 µl;
1×106 cells) was added to 1.5 ml EP tubes, after which
the cells were incubated with 1 µg anti-CD44 (cat no.
GD-x0297M-AF567), anti-CD45 (cat no. GD-x0297M-AF468) and
anti-CD105 (cat no. GD-x0297M-AF555) antibodies (1:100 dilution) in
the dark for 20 min at 37°C. Subsequently, the cell suspension was
centrifuged at 300 × g for 5 min, followed by removal of the
supernatant and resuspension of the sediment in 200 µl Stain
Buffer. All steps were repeated twice prior to analysis in the flow
cytometer.
Results
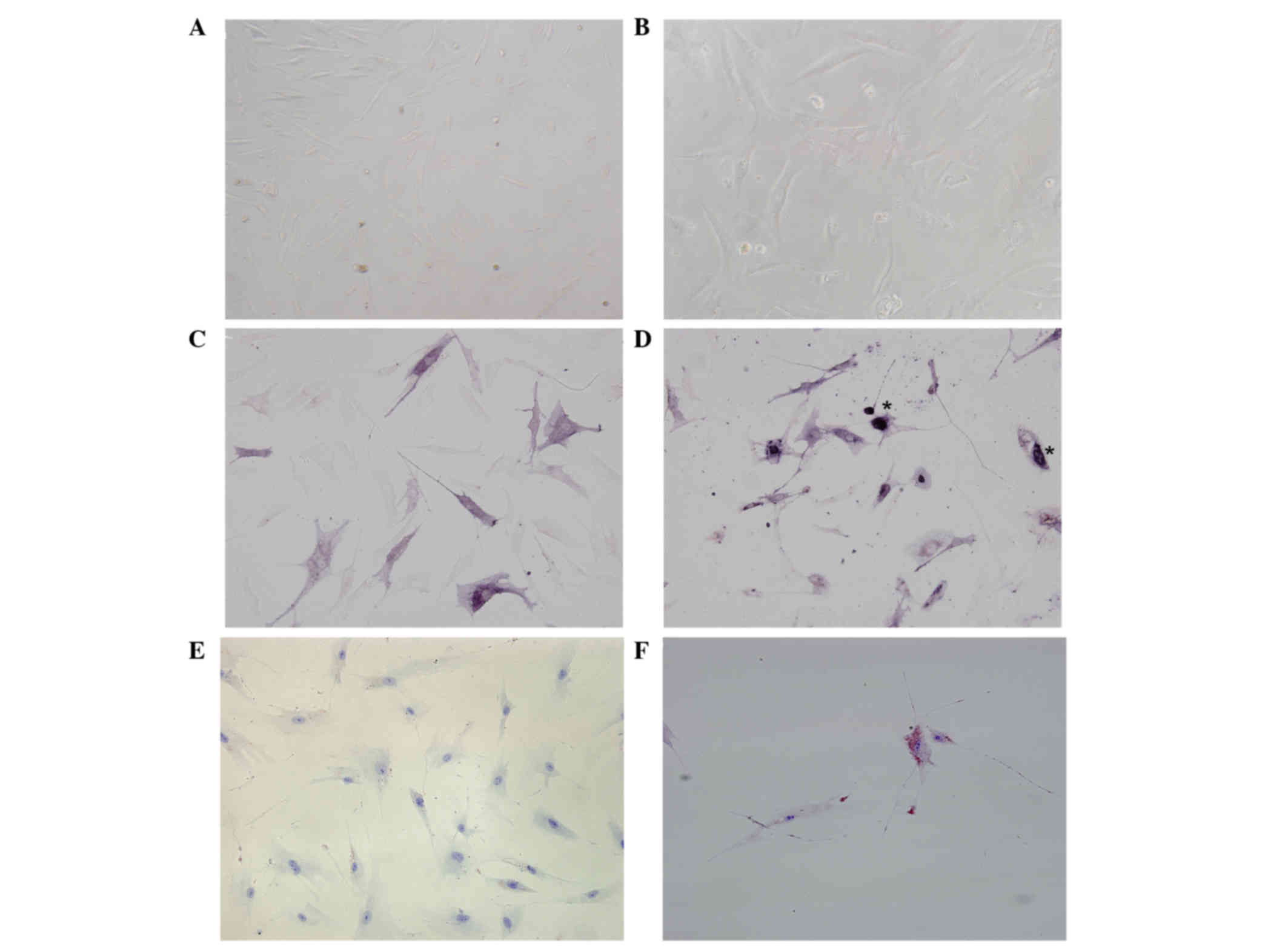
Morphology and growth of ASCs
When observed under a microscope, a small number of
the original cells had adhered to the culture vessel walls after 72
h. In addition, on day 14, the cells were short fusiform and
polygon in appearance. Over time, the morphology of the ASCs
altered into a long shuttle shape that resembled fiber cells and
bone marrow derived mesenchymal stem cells. Lastly, the cells
merged into sheets and exhibited spiral growth upon reaching
confluence. Notably, the P2 cells grew at a faster rate compared
with the P1 cells (Fig. 1A and
B).
Identification of
multi-differentiation potential
During the 2-week incubation in osteogenic-inducing
medium, the number of irregularly shaped cells increased in the
experimental group and alkaline phosphatase staining was positive,
indicating successful osteogenic induction of ASCs. Conversely, the
control group retained in their fiber cell appearance and alkaline
phosphatase staining was as negative (Fig. 1C and D).
After the 1-week incubation in adipogenic-inducing
medium, lipid droplets of various sizes could be seen in the
experimental group cells, and Oil Red O dye staining was positive.
In contrast, the control group cells were negative for Oil Red O
dye staining (Fig. 1E and F).
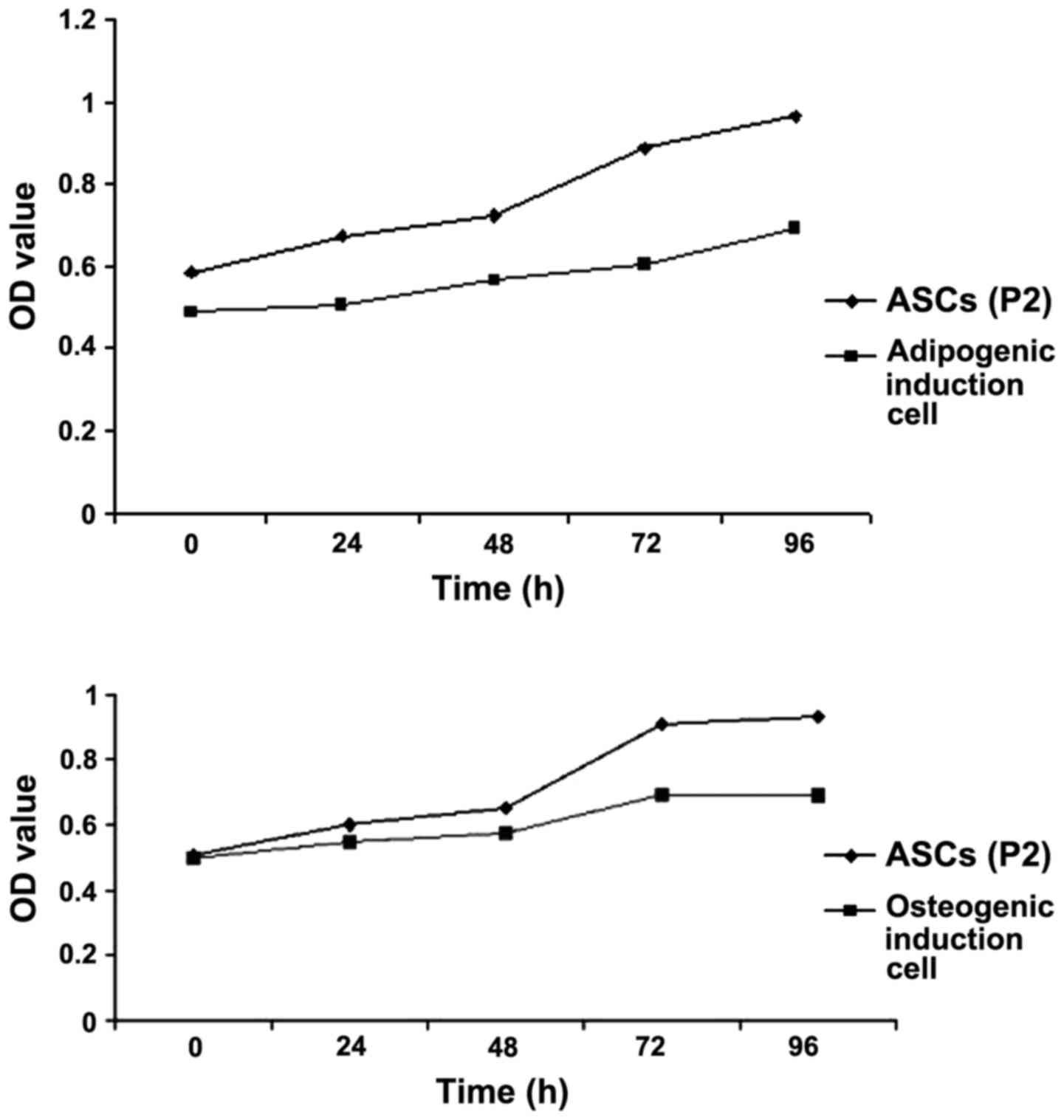
Proliferation activity of ASCs and
their directional differentiation cells
Osteogenic and adipogenic inductions were performed
on the P2 ASCs. ASCs cultured in DMEM were used as a control. The
proliferation of the cells was assessed using the CCK-8 assay. P2
cells cultured in DMEM proliferated at a faster rate, as compared
with the P2 cells cultured in osteogenic and adipogenic induction
medium (Fig. 2).
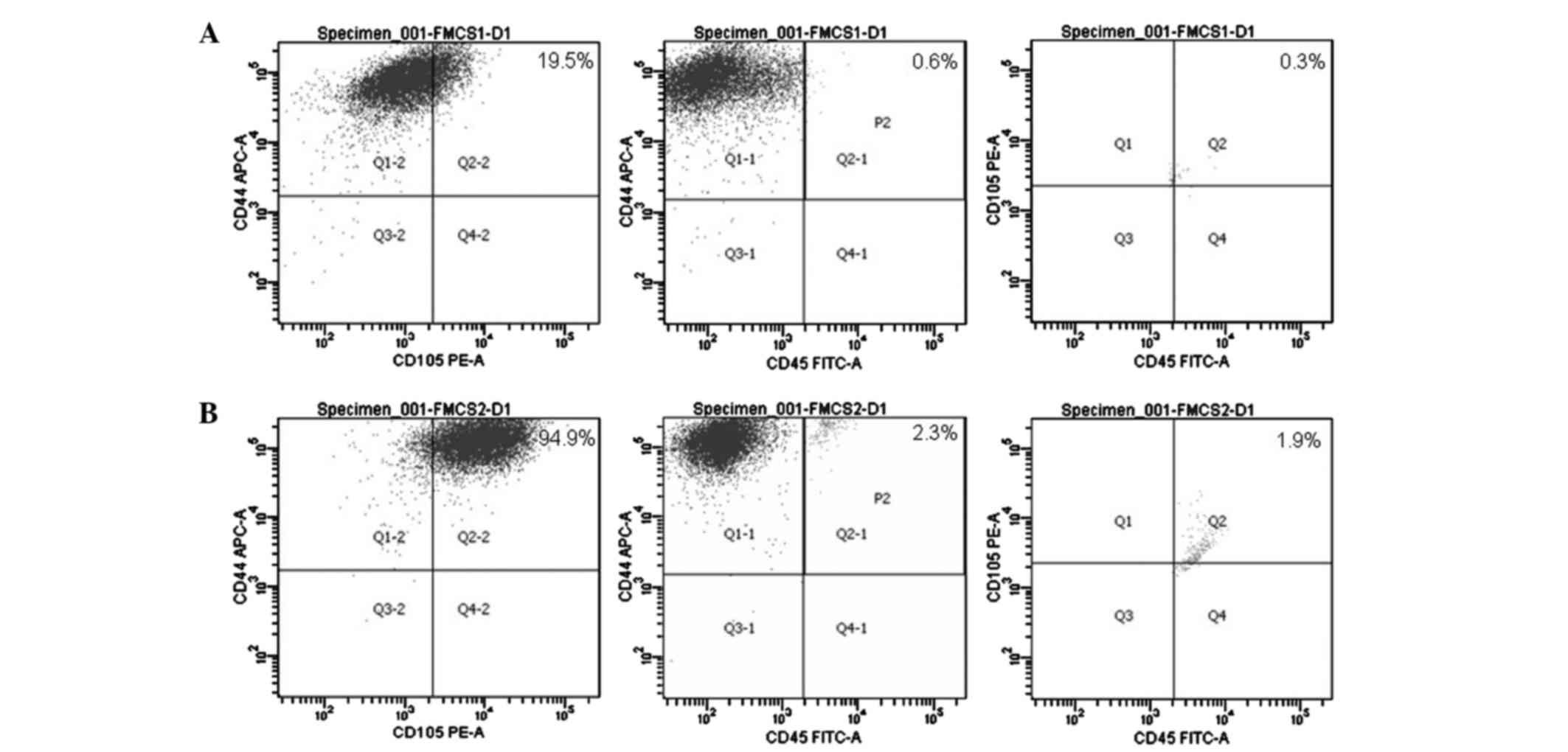
Identifying the surface antigens of
ASCs with flow cytometry
Flow cytometric analysis of the surface markers on
both P1 and P2 ASCs demonstrated that the percentage of
CD44+CD45−CD105+ cell subsets was
18.6% in P1 and 90.7% in P2 ASCs (Fig.
3).
Discussion
Adult ASCs are convenient to obtain, have broad
sources, a long culturing time, strong breeding ability and are not
associated with ethical issues. Furthermore, they have the
potential of differentiating into multiple blastophyllums,
including cardiac cells (7,8), and they possess identical
immunosuppressive effects to bone marrow-derived mesenchymal stem
cells (9–12), making them beneficial for allogenic
transplantation treatments. Therefore, ASCs may emerge as the ideal
seed stem cells in future cell transplantation and tissue
engineering clinical practices.
A set of highly practical methods for extracting,
culturing and identifying ASCs have been improved in this study.
First, when selecting the donor tissues, the search was limited to
young female volunteers aged between 25 and 30 years who had BMIs
of 25–30 kg/m2, in order to minimize the confounding
effects of underlying conditions. It has previously been reported
that BMI and ASC yield are negatively correlated (r=−0.44,
P<0.05), and that age does not influence ASC yield
(r=−0.17, P=0.27) (13).
However, Yu, et al (14)
hypothesized that there are positive correlations among BMI, age
and ASC yield. Secondly, liposuction was selected as the method to
obtain adipose tissues, as the amount of subcutaneous fat obtained
by conventional surgeries is usually limited and a lot of time is
spent on repeated shearing during the separation process (15). The present study adopted the method
used by Panfilov et al (6),
in which a fixed amount of fat is initially extracted using a 50-ml
injector under negative pressure and in the absence of external
ultrasonic emulsification technology (16), resonance technology or the excessive
expansion solution method in order to prevent damage to the adipose
cells. Therefore, in comparison with existing surgical and
liposuction methods involving additional techniques, the method
used in the present study allowed time to be saved when obtaining
fat tissues, as well as the number of living cells to be maximized,
thereby improving the acquisition rate of ASCs.
During the separation phase of the ASCs,
low-concentration (0.075%) collagenase was used to separate the
cells. Although this method increased the digesting time, as
compared with the combined enzyme digestion method and
high-concentration trypsin solution method chosen by previous
studies (17,18), it avoided excessive damage to the
cells. In addition, the fluid replacement method was selected in
order to remove red blood cells from the suspension and thereby
avoid the use of the conventional NH4CI method (19), which typically harms the cells; this
also increased the number and activity of the ASCs. Based on our
calculations, 1×105 ASCs was acquired from every 50 ml
of fat aspirate. Furthermore, it was discovered that the breeding
ability of the ASCs was increased; by observing two 25-cm culturing
vessels initially containing ~5×104 cells, a logarithmic
growth period was observed as commencing after 72 h, and the time
needed for fusion was ~7 days, which was similar to a previous
study (20).
Taking into consideration the current lack of
identification methods specific for ASCs, the present study
referred to the newest joint declaration of the International
Federation for Adipose Therapeutics and Science and the
International Society for Cellular Therapy (21), which describes the conformation and
simplification of the identification of ASCs as its fundamental
purpose. This declaration suggests that ASC identification should
involve comprehensive identification involving assessment of tissue
origin, cellular morphology, surface markers and their
multi-differentiation potential (21). Particularly important is selecting
and identifying the surface markers of ASCs, as the surface markers
of stem cells transform over successive generations of cells
(22). Therefore, the present study
selected three comparably stable markers according to the joint
declaration (21), along with a
number of global experimental reports (23,24).
Among them, CD45 has commonly been used as a surface marker for
hemopoietic stem cells; thus, the persistent negative result in the
present study helped to rule out this type of stem cells (24). CD105 is a marker of mesenchymal stem
cells, while CD44 is a stable marker for ASCs; both are highly
expressed in ASCs and, therefore, the identification of ASCs based
on surface markers was simplified, while the cost of and time spent
on screening for markers was reduced (25). Furthermore, desmocytes, which are
similar to ASCs, could be effectively distinguished through the
multi-differentiation ability of ASCs.
By using P1 and P2 ASCs in the experiments, the
results suggested that a low positive rate of P1 ASCs should be
related to adherent cells and contamination with other cells. In
accordance to relevant documents and experimental reports, ASCs
show signs of aging after being cultured for 10 generations
(3,4). On the other hand, the present study has
demonstrated that P2 stem cells show satisfying purity and breeding
activity, and, hence, P2 cells have been selected to be stored for
future use.
In conclusion, the present study has improved
methods for the isolation, cultivation and identification of ASCs,
thereby reducing damage to ASCs, simplifying their identification
and increasing the ASC yield from adipose aspirate. This has
allowed the establishment of mature and stable stem cell sources
for future research.
References
|
1
|
Zuk PA, Zhu M, Mizuno H, Huang J, Futrell
JW, Katz AJ, Benhaim P, Lorenz HP and Hedrick MH: Multilineage
cells from human adipose tissue: Implications for cell-based
therapies. Tissue Eng. 7:211–228. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
LapPar DJ, Kron IL and Yang Z: Stem cell
therapy for ischemic heart disease: Where are we? Curr Opin Organ
Transplant. 14:79–84. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yang Z, Hao J and Hu ZM: MicroRNA
expression profiles in human adipose-derived stem cells during
chondrogenic differentiation. Int J Mol Med. 35:579–586.
2015.PubMed/NCBI
|
|
4
|
Laflamme MA and Murry CE: Heart
regeneration. Nature. 473:326–335. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Singh D, Nayak V and Kumar A:
Proliferation of myoblast skeletal cells on three-dimensional
supermacroporous cryogels. Int J Biol Sci. 6:371–381. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Panfilov IA, de Jong R, Takashima S and
Duckers HJ: Clinical study using adipose-derived mesenchymal-like
stem cells in acute myocardial infarction and heart failure.
Methods Mol Biol. 1036:207–212. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Miyahara Y, Nagaya N, Kataoka M, Yanagawa
B, Tanaka K, Hao H, Ishino K, Ishida H, Shimizu T, Kangawa K, et
al: Monolayered mesenchymal stem cells repair scarred myocardium
after myocardial infarction. Nat Med. 12:459–465. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Planat-Bénard V, Menard C, André M, Puceat
M, Perez A, Garcia-Verdugo JM, Pénicaud L and Casteilla L:
Spontaneous cardiomyocyte differentiation from adipose tissue
stromal cells. Circ Res. 94:223–229. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
McIntosh K, Zvonic S, Garrett S, Mitchell
JB, Floyd ZE, Hammill L, Kloster A, Di Halvorsen Y, Ting JP, Storms
RW, et al: The immunogenicity of human adipose-derived cells:
Temporal changes in vitro. Stem Cells. 24:1246–1253. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Puissant B, Barreau C, Bourin P, Clavel C,
Corre J, Bousquet C, Taureau C, Cousin B, Abbal M, Laharrague P, et
al: Immunomodulatory effect of human adipose tissue-derived adult
stem cells: Comparison with bone marrow mesenchymal stem cells. Br
J Haematol. 129:118–129. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Han ZM, Huang HM and Wang FF:
Brain-derived neurotrophic factor gene-modified bone marrow
mesenchymal stem cells. Exp Ther Med. 9:519–522. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Di C and Zhao Y: Multiple drug resistance
due to resistance to stem cells and stem cell treatment progress in
cancer (Review). Exp Ther Med. 9:289–293. 2015.PubMed/NCBI
|
|
13
|
Aust L, Devlin B, Foster SJ, Halvorsen YD,
Hicok K, du Laney T, Sen A, Willingmyre GD and Gimble JM: Yield of
human adipose-derived adult stem cells from liposuction aspirates.
Cytotherapy. 6:7–14. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yu G, Wu X, Dietrich MA, Polk P, Scott LK,
Ptitsyn AA and Gimble JM: Yield and characterization of
subcutaneous human adipose-derived stem cells by flow cytometric
and adipogenic mRNA analyzes. Cytotherapy. 12:538–546. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jin J, Jeong SI, Shin YM, Lim KS, Shin Hs,
Lee YM, Koh HC and Kim KS: Transplantation of mesenchymal stem
cells within a poly(lactide-co-epsilon-caprolactone) scaffold
improves cardiac function in a rat myocardial infarction model. Eur
J Heart Fail. 11:147–153. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Oedayrajsingh-Varma MJ, van Ham SM,
Knippenberg M, Helder MN, Klein-Nulend J, Schouten TE, Ritt MJ and
van Milligen FJ: Adipose tissue-derived mesenchymal stem cell yield
and growth characteristics are affected by the tissue-harvesting
procedure. Cytotherapy. 8:166–177. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kang S, Han J, Song SY, Kim WS, Shin S,
Kim JH, Ahn H, Jeong JH, Hwang SJ and Sung JH: Lysophosphatidic
acid increases the proliferation and migration of adipose-derived
stem cells via the generation of reactive oxygen species. Mol Med
Rep. 12:145–149. 2015.
|
|
18
|
Pan F, Liao N, Zheng Y, Wang Y, Gao Y,
Wang S, Jiang Y and Liu X: Intrahepatic transplantation of
adipose-derived stem cells attenuates the progression of
non-alcoholic fatty liver disease in rats. Mol Med Rep.
26:3725–3733. 2015.
|
|
19
|
Lee RH, Kim B, Choi I, Kim H, Choi HS, Suh
K, Bae YC and Jung JS: Characterization and expression analysis of
mesenchymal stem cells from human bone marrow and adipose tissue.
Cell Physiol Biochem. 14:311–324. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Boquest AC, Shahdadfar A, Brinehmann JE
and Collas P: Isolation of stromal stem cells from human adipose
tissue. Methods Mol Biol. 325:35–46. 2006.PubMed/NCBI
|
|
21
|
Bourin P, Bunnell BA, Casteilla L,
Dominici M, Katz AJ, March KL, Redl H, Rubin JP, Yoshimura K and
Gimble JM: Stromal cells from the adipose tissue-derived stromal
vascular fraction and culture expanded adipose tissue-derived
stromal/stem cells: A joint statement of the international
federation for adipose therapeutics and science (IFATS) and the
international society for cellular therapy (ISCT). Cytotherapy.
15:641–648. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Park HS, Kim JH, Sun BK, Song SU, Suh W
and Sung JH: Hypoxia induces glucose uptake and metabolism of
adipose-derived stem cells. Mol Med Rep. 14:4706–4714.
2016.PubMed/NCBI
|
|
23
|
Gimble JM, Katz AJ and Bunnell BA:
Adipose-derived stem cells for regenerative medicine. Circ Res.
100:1249–1260. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zuk PA, Zhu M, Ashjian P, De Ugarte DA,
Huang JI, Mizuno H, Alfonso ZC, Fraser JK, Benhaim P and Hedrick
MH: Human adipose tissue is a source of multipotent stem cells. Mol
Biol Cell. 13:4279–4295. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Fu X, Tong Z, Li Q, Niu Q, Zhang Z, Tong
X, Tong L and Zhang X: Induction of adipose-derived stem cells into
Schwann-like cells and observation of Schwann-like cell
proliferation. Mol Med Rep. 14:1187–1193. 2016.PubMed/NCBI
|