Introduction
Thyroid cancer is the most common type of malignant
tumor in the endocrine system. I-131 therapy has been used to treat
differentiated thyroid cancer (DTC) (1). With demonstrable curative effects, this
therapy has been recognized by clinical practice (2). However, the iodine uptake capacities of
tumor nidi in ~10% of patients are decreased or depleted entirely,
which causes a reduction of treatment efficacy, resulting in a
higher severity of anaplastic thyroid carcinoma (ATC) (3). Furthermore, with short median survival
time and high mortality rates, these patients are less sensitive to
various traditional therapies (for example, surgery and
endocrinotherapy) (4).
DTC and ATC describe two possible endpoints of
thyroid cancer (5). In fact, changes
from DTC to ATC do not occur at once. As far as differentiated
degrees, cell morphology and biological tumor behavior are
concerned, there is a progression from DTC to ATC, i.e., from
differentiated to poorly differentiated and then to
dedifferentiated (6). The World
Health Organization considers poorly differential thyroid carcinoma
(PDTC) to be an intermediate between DTC and ATC (7).
With a length of <10 amino acids,
cell-penetrating peptide is a micromolecular polypeptide that is
able to penetrate through the cytomembrane into cytoplasm or the
cell nucleus without damaging structures of the cell membrane
(8). Studies in recent years have
shown that cell-penetrating peptides may carry a series of
substances with biological activities into living cells, including
proteins, polypeptides, nucleic acids and oligonucleotides
(9,10). Hairpin-shaped cell-penetrating
peptide is a recently identified transmembrane small peptide
(11). Its hairpin structure can
construct numerous protease sites, which may provide novel
approaches for molecular targeted diagnosis and therapy.
As a glycoprotein, the Na+/I-symporter
(NIS), also known as an iodine pump, is located in the membrane of
epithelial cells of the thyroid follicle (12). NIS participates in the iodine uptake
process and plays a rate-limiting role in iodine transportation
(13). For normal human bodies,
expression levels of NIS in thyroid tissues are higher than those
in other tissues (14). However, it
has been hypothesized that due to obstructions to NIS positioning
and reductions in NIS protein expression levels, iodine uptake
capacities for certain patients with thyroid cancer are reduced or
depleted entirely, which may influence the efficacy of I-131
treatment and worsen prognosis (15). By improving functional position
expression levels of NIS, iodine uptake capacities of thyroid
cancer tissues may be increased (16). Thus, in the present study we
investigated whether the cell penetrating peptide of NIS affects
the efficacy of I-131 radiotherapy in human thyroid cancer
cells.
Materials and methods
Cell lines and cell culture
Human thyroid carcinoma TPC-1 cells were purchased
from the Cell Bank of Type Culture Collection of Chinese Academy of
Sciences (Shanghai, China). TPC-1 cells were maintained in
RPMI-1640 medium (Invitrogen; Thermo Fisher Scientific, Inc.,
Carlsbad, CA, USA) and 10% fetal bovine serum (Gibco; Thermo Fisher
Scientific, Inc., Grand Island, NY, USA), 100 U/ml penicillin and
50 µg/ml streptomycin (Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany), and incubated at 37°C in a humidified atmosphere of 5%
CO2.
dTAT nanoparticle (NP)-NIS
transfection
dTAT NP-NIS and the negative control (NC) plasmids
were purchased from Shanghai GenePharma, Co., Ltd. (Shanghai,
China). TPC-1 cells were incubated in six-well plates at a density
of 1–2×106 cells/well. Subsequently, 100 nmol/l plasmids
were transfected Lipofectamine 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's instructions and
transfected into TPC-1 cells.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis of dTAT NP-NIS
Following transfection, TPC-1 cells were seeded
six-well plates at a density of 1–2×106 cells/well for
24 h. Total RNA from the TPC-1 cells was extracted using TRIzol
reagent (Invitrogen; Thermo Fisher Scientific, Inc.). Following
treatment with DNase (Takara Biotechnology Co., Ltd., Dalian,
China) a TaqMan miRNA Reverse Transcription kit (Applied
Biosystems; Thermo Fisher Scientific, Inc., Waltham, MA, USA) was
used to synthesize cDNA using 1 µg RNA. Subsequently, a 7500
Real-Time PCR system (Applied Biosystems; Thermo Fisher Scientific,
Inc.) was used to conduct RT-qPCR using SYBR Premix Ex Taq (Takara
Biotechnology Co., Ltd.). The sequences were as follows: NIS
forward, 5′-ACSCACTGGAAGCACGGCGG-3′ and reverse,
5′-GTGGMRCCGTGCAKRTTGG-3′; β-actin forward, 5′-AGGCACCAGGGCGTGAT-3′
and reverse, 5′-TGCTCCCAGTTGGTGACGAT-3′. The cycling conditions
used were 95°C for 4 min, 95°C for 15 sec and 60°C for 30 sec for
40 cycles. The RT-qPCR experiment was conducted 6 times. Relative
expression of mRNA was quantified using the 2−∆∆CT
method (17).
Western blot analysis
TPC-1 cell total protein was extracted using a
radioimmunoprecipitation assay lysis buffer (Sigma-Aldrich; Merck
KGaA). Protein concentrations were determined using a Bradford
assay (Bio-Rad Laboratories, Inc., Hercules, CA, USA). Equal
quantities of total protein (50 µg) were resolved using 10–12%
SDS-PAGE (Bio-Rad Laboratories, Inc.) and transferred to
polyvinylidene difluoride membranes (Bio-Rad Laboratories, Inc.).
The membranes were blocked in 5% non-fat milk for 2 h at room
temperature and then incubated with rabbit anti-human NIS (1:500;
sc-134515), caspase-3 (1:500; sc-98785), Akt (1:200; sc-8312) and
phosphorylated-Akt (1:500; sc-33437) and PTEN primary antibodies
(1:500; sc-9145; all Santa Cruz Biotechnology, Inc., Santa Cruz,
CA, USA) overnight at 4°C. The membranes were washed with
Tris-buffered saline containing Tween 20 (Sigma-Aldrich; Merck
KGaA) and then incubated with horseradish peroxidase-conjugated
secondary goat anti-rabbit immunoglobulin G antibodies (1:1,000;
sc-2922; Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA) for 1
h at room temperature. The membranes were visualized using an
Immobilon Western chemiluminescent HRP substrate (EMD Millipore,
Billerica, CA, USA), and the blots were densitometrically analyzed
using Image Lab 3.0 software (Bio-Rad Laboratories, Inc.).
MTT assay
TPC-1 cells were seeded onto 96-well plates at a
density of 1–2×103 cells/well for 12, 24 and 48 h, and
subsequently incubated with 20 µl MTT (5 mg/ml; Sigma-Aldrich;
Merck KGaA) for 4 h. Then, 150 µl DMSO (Invitrogen; Thermo Fisher
Scientific, Inc.) was added to each well and shaken for 15 min
after the medium was removed. The optical density (OD) of each well
was detected at 490 nm using a microplate reader (BioTek
Instruments, Inc., Winooski, VT, USA).
Flow cytometry for evaluation of
apoptosis
TPC-1 cells were seeded onto six-well plates at a
density of 1–2×106 cells/well for 24 h and washed twice
using phosphate-buffered saline (PBS). TPC-1 cells were resuspended
using buffer solution (from a FITC Annexin V Apoptosis Detection
kit; BD Biosciences, Franklin Lakes, NJ, USA), and incubated with 5
µl Annexin V-fluorescein isothiocyanate and 10 µl propidium iodide
for 30 min at 4°C in the dark. Flow cytometry (FACSCalibur; BD
Biosciences) was used to measure cell apoptosis rates of the TPC-1
cells.
DAPI staining assay
TPC-1 cells were seeded onto six-well plates at a
density of 1–2×106 cells/well for 24 h and washed twice
using PBS. TPC-1 cell was fixed using 4% paraformaldehyde for 30
min at 4°C. Then, fixed TPC-1 cells were washed twice using PBS and
incubated with sodium citrate (0.1%) containing 0.1% Triton X-100
(Beyotime Institute of Biotechnology, Haimen, China) for 5 min at
4°C. TPC-1 cells were dyed by DAPI staining and incubated for 10
min at 4°C in the dark, then activated using ultraviolet. DAPI
staining was observed and images captured using a fluorescence
microscope (CKX41; Olympus Corporation, Tokyo, Japan) at 340
nm.
Statistical analysis
All values were expressed as the mean ± standard
deviation. Statistical analyses were conducted using SPSS 17.0
software (SPSS, Inc., Chicago, IL, USA). Data were analyzed using
the Student's t-test. P<0.05 was considered to indicate a
statistically significant difference between values.
Results
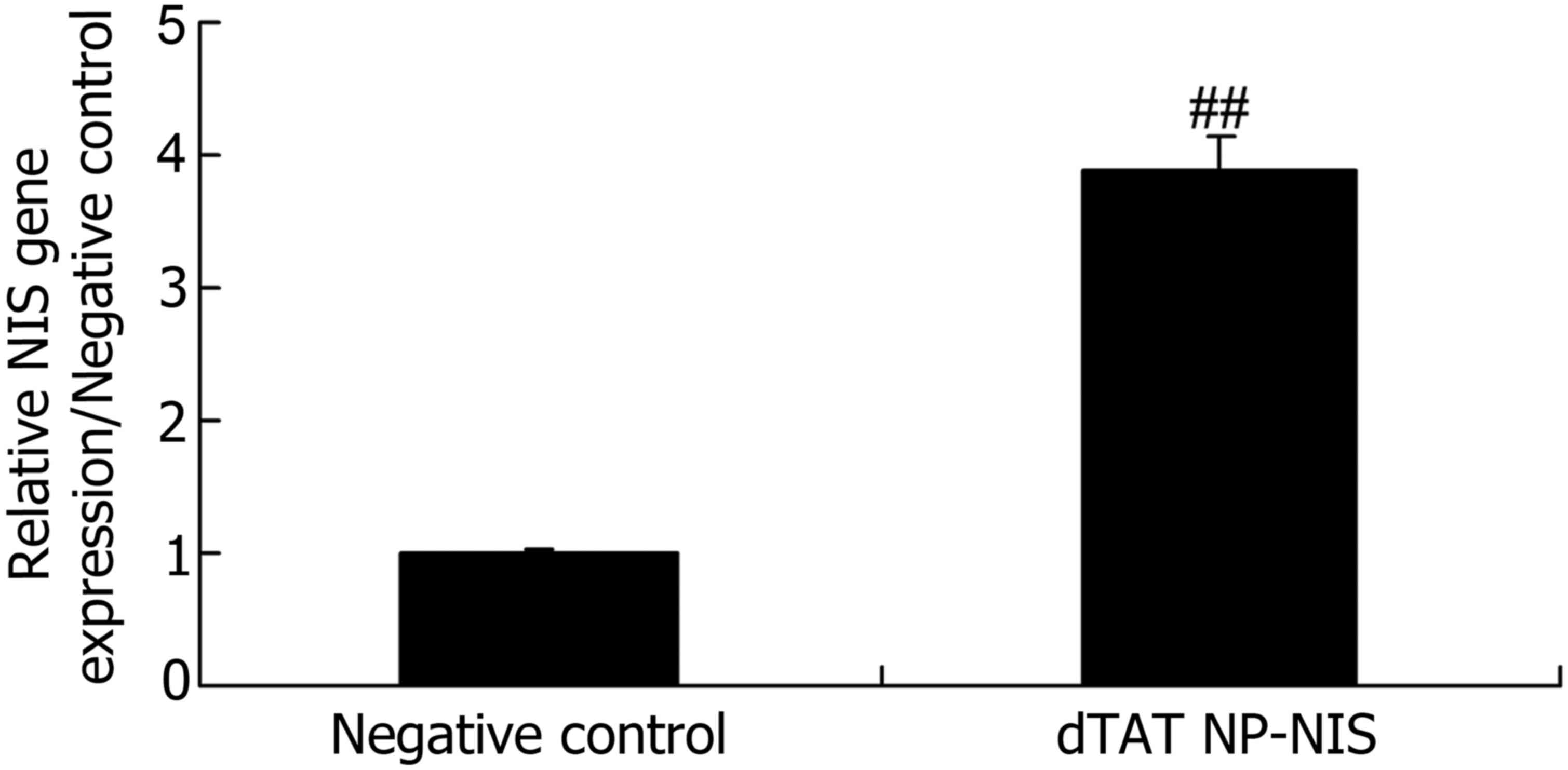
Gene expression level of NIS in TPC-1
cells
To evaluate the gene expression of NIS in TPC-1
cells transfected with dTAT NP and negative control, RT-qPCR was
performed. As shown in Fig. 1, there
was a significant increase in the relative gene expression of NIS
of dTAT NP group, compared with the NC group (P<0.05).
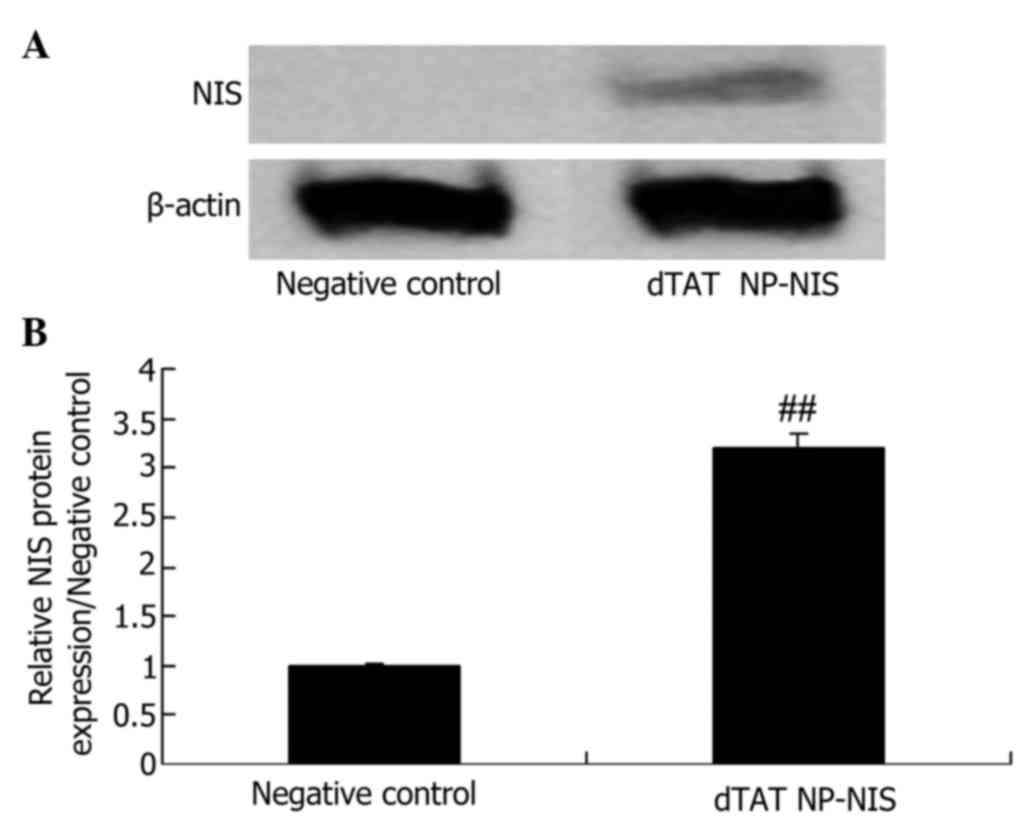
Protein expression level of NIS in
TPC-1 cells
In order to characterize the dTAT NP-NIS and NC
plasmid influence on the protein expression of NIS, western blot
analysis was performed. The expression of NIS protein was
significantly higher than that of the NC group (P<0.05; Fig. 2).
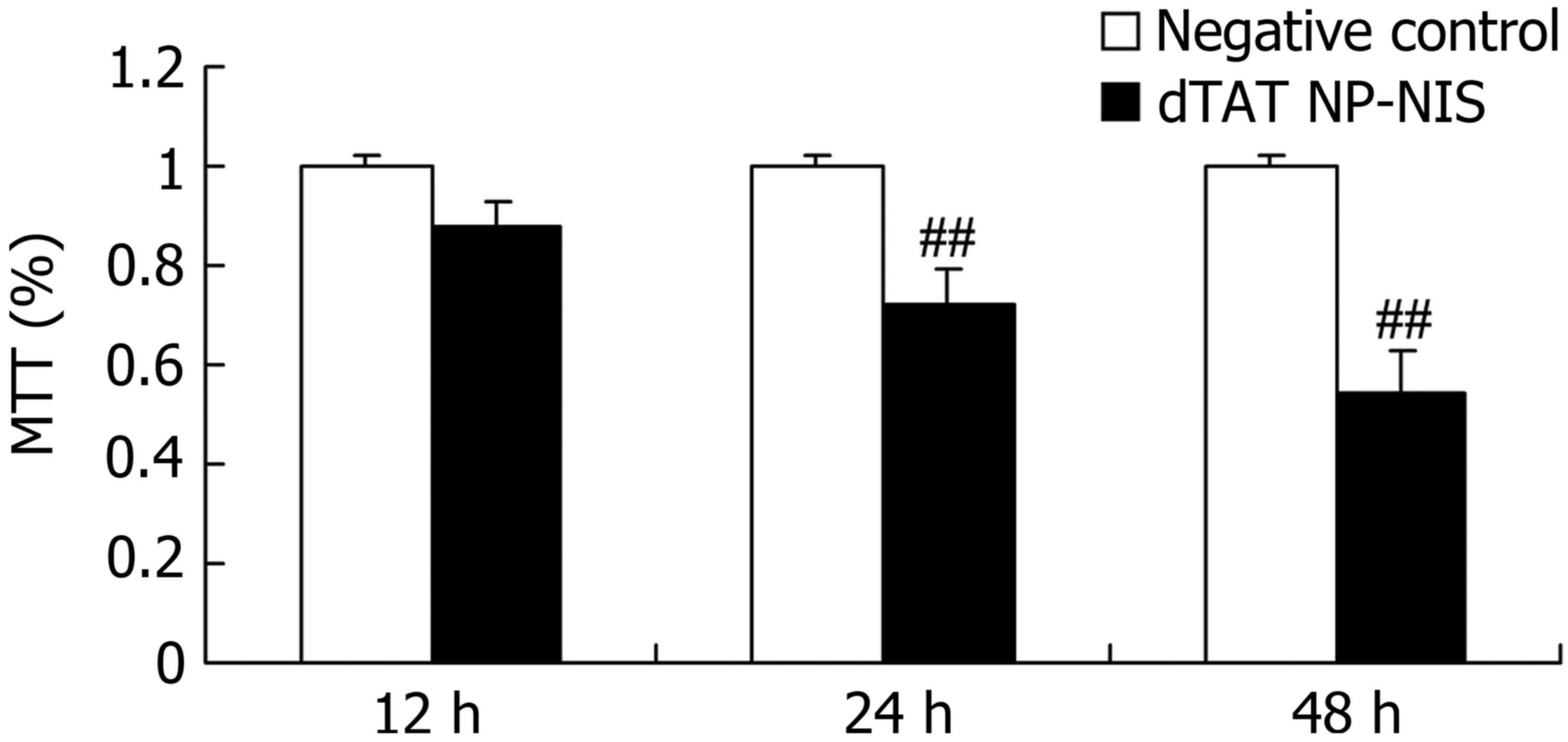
Effect of NIS expression on cell
growth in TPC-1 cells
We clarified the overexpression of NIS influence on
cell growth of TPC-1 cell. The results from MTT assay showed the
cell growth of TPC-1 cell was significantly suppressed after dTAT
NP-NIS transfection at 24 and 48 h, compared with the NC group
(P<0.05; Fig. 3).
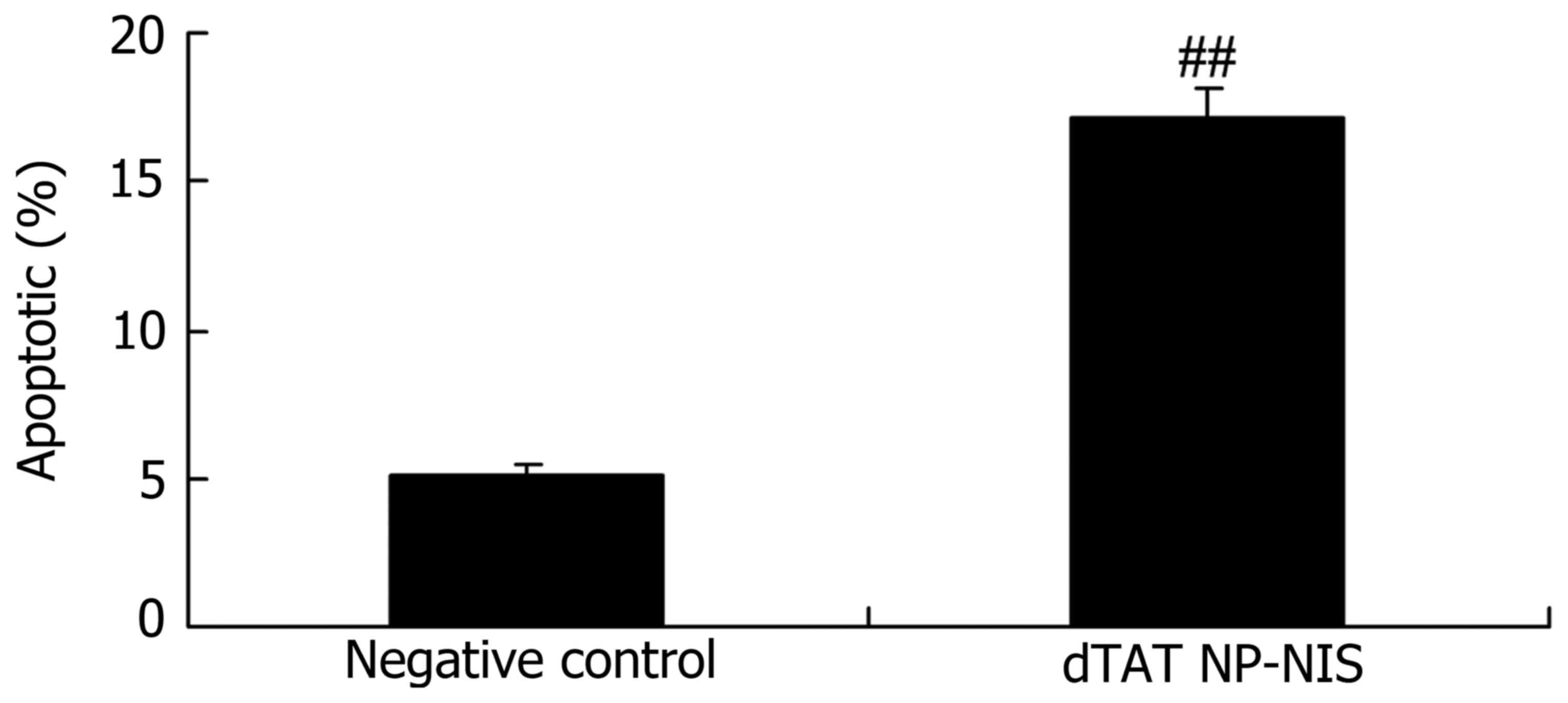
Effect of NIS expression on apoptosis
rates in TPC-1 cells
To determine the effect of the overexpression of NIS
apoptosis rates in TPC-1 cells, cellular apoptosis was observed in
TPC-1 cells using flow cytometry. As shown in Fig. 4, cellular apoptosis of dTAT NP-NIS
transfection was significantly higher than that of the NC group,
after dTAT NP-NIS transfection for 24 h (P<0.05).
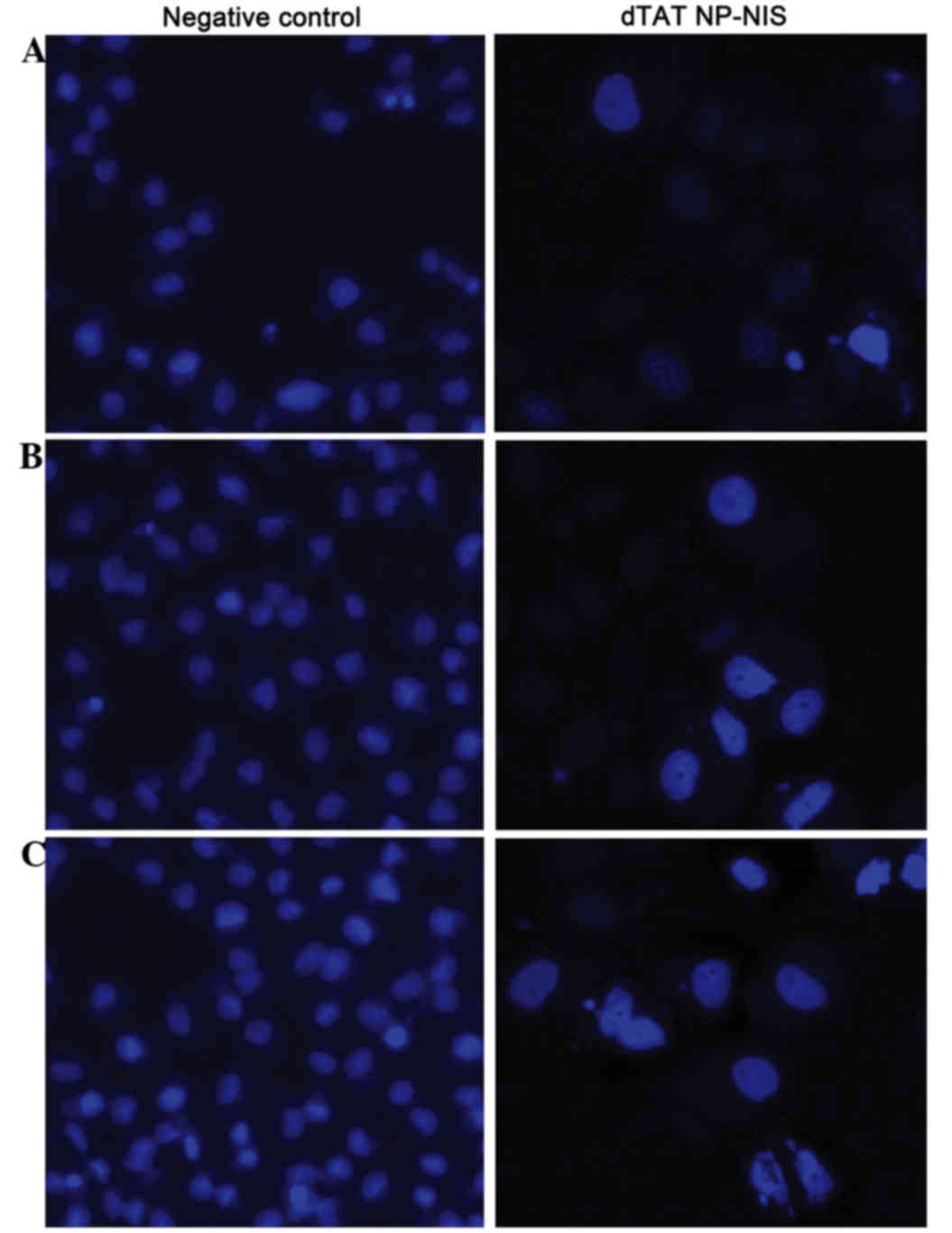
Effect of NIS expression on cell
nucleus apoptosis in TPC-1 cells
To validate the overexpression of NIS influence on
cell nucleus apoptosis of TPC-1 cell, TPC-1 cells were stained with
DAPI. After dTAT NP-NIS transfection at 24 or 48 h, there was a
visible increase in the nuclear apoptosis rate of TPC-1
cell-transfected with dTAT NP-NIS compared with the NC group
(Fig. 5).
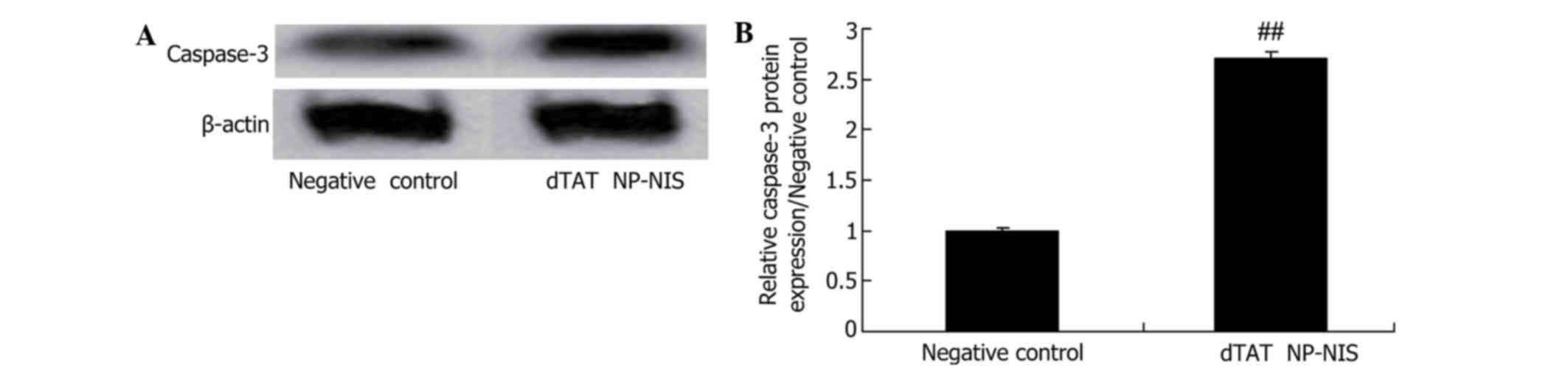
Effect of NIS expression on caspase-3
expression in TPC-1 cells
To further clarify whether the effect of NIS
expression on caspase-3 protein expression of TPC-1 cell, caspase-3
protein expression was analyzed using western blot analysis in
TPC-1 cell. Western blot analysis displayed that caspase-3 protein
expression was significantly activated in the dTAT NP-NIS
transfected cells at 24 h, compared with the NC group (Fig. 6).
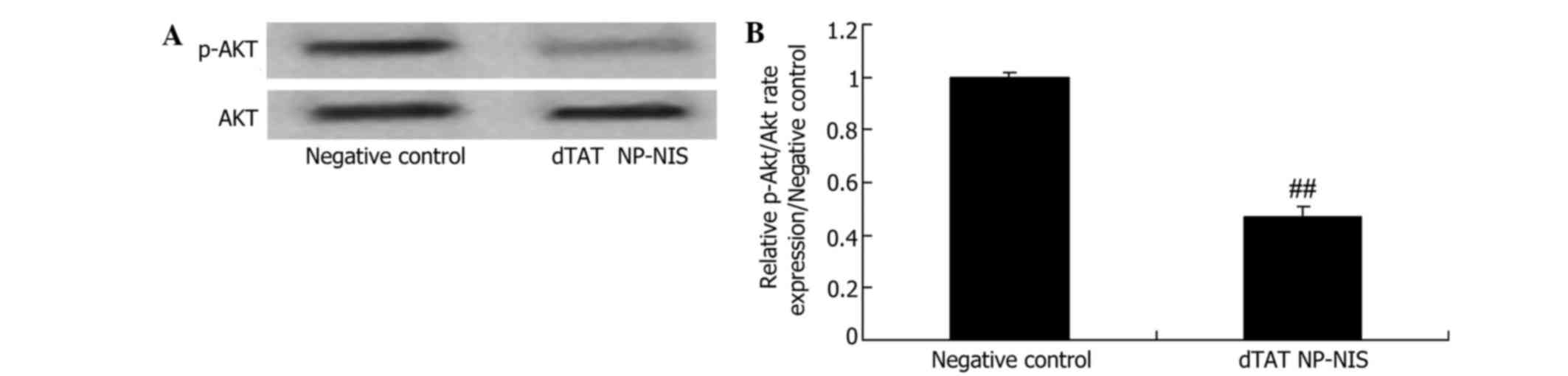
Effect of NIS expression on Akt
expression in TPC-1 cells
To further validate the effect of NIS expression on
Akt signaling in TPC-1 cells, Akt and p-Akt protein expression
levels were detected. Compared with the NC group, the relative
Akt/p-Akt rate was significantly reduced by overexpression of NIS
(Fig. 7).
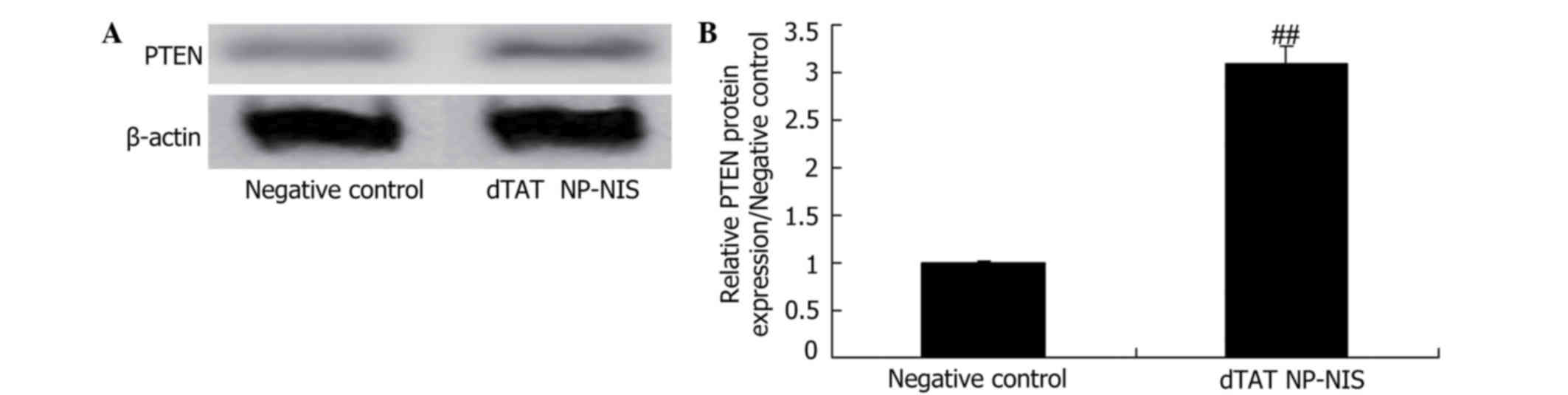
Effect of NIS expression on PTEN
expression in TPC-1 cells
To further evaluate the effects of NIS expression on
PTEN signaling in TPC-1 cells, PTEN protein expression was
evaluated. As shown in Fig. 8,
relative PTEN protein expression was significantly elevated
compared with the NC group (Fig.
8).
Discussion
Thyroid cancer is among the most common diseases
affecting the thyroid. Iodine therapy following surgery may
evidently lower recurrence rates (18). However, during the treatment process,
sensitivity of tumor nidi or metastatic tumor tissue to iodine is
decreased or lost entirely, which may be caused by
de-differentiation (19). In cases
of the greatest severity with the worst prognosis, methods as
surgery, radiotherapy and chemotherapy may not be sufficient to
successfully treat PDHC (20).
Previous studies have investigated the pathogenesis, therapeutic
strategies and drug development associated with PDHC (16,21). The
present results showed that dTAT NP-NIS inhibits cell growth and
induces cellular apoptosis in TPC-1 cells.
At present, a comprehensive therapeutic method for
PTC involves surgery assisted by endocrinotherapy and radioactive
I-131 treatment (22). Located on
the epithelial cell membrane, NIS is a type of glycoprotein, whose
genes are on the short arm of chromosome 19 (23). NIS carries iodine into cytoplasm
using a sodium potassium pump, and is important for the maintenance
of iodine uptake capacities of thyroids, while also participating
in the synthesis of thyroid hormones (24). Furthermore, NIS may be expressed in
other locations, such as mammary and prostate glands (25). The present results suggest that the
anti-cancer effect of dTAT NP-NIS may be associated with the
activation of caspase-3 pathway in TPC-1 cells.
Radioactive I-131 therapy is essential in diagnosis
and treatment of ATC and metastasis. The presupposition of the
lethal effects of radioactive I-131 to tumor cells is that tumor
cells possess increased iodine uptake capacities (26). PTEN/PI3K/AKT signal transduction
pathways play an important part in regulating NIS protein synthesis
(27). Overactivity of the
PTEN/PI3K/AKT signal transduction pathways may affect locations of
NIS proteins in cells. The present results suggests that there was
significant suppression of PI3K/AKT signaling, which may be
associated with the anti-cancer effects of dTAT NP-NIS on TPC-1
cells.
The gene for PTEN protein lie in chromosome 10q23.3
area. PTEN protein is expressed in numerous early embryonic
tissues, such as kidney, stomach, central and peripheral nervous
system tissue (28). The expression
levels of PTEN in the early stage of thyroid tissue growth and in
adults are relatively high. In normal human cells, PTEN
participates in the regulation of numerous signal transduction
pathways, including PTEN/PI3K/AKT, FAK/PI30C and MAPK (28). Through these pathways, PTEN is
involved in the maintenance of normal and regulatory substance
metabolism and stabilization of the internal environment (29). Low expression levels of PTEN protein
in cancer tissues are closely associated with the genesis and
progression of tumors (30). The
present results suggest that the anti-cancer effects of dTAT NP-NIS
increased the protein expression levels of PTEN in TPC-1 cells.
In conclusion, to our knowledge the present study is
the first to demonstrate that dTAT NP-NIS inhibits growth and
induces the apoptosis of TPC-1 cells, possibly via the caspase-3
and PTEN/PI3K/AKT pathways. Our data provide a preclinical
proof-of-concept for a novel gene delivery system that efficiently
delivers NIS to the targeted cancer cells and presents a
satisfactory efficacy. This may offer an effective strategy for
improving thyroid cancer gene therapy, and warrants further
investigation.
Acknowledgements
This study was partially supported by Guangdong
Provincial Natural Science Foundation (grant no.
S2013010015733).
References
|
1
|
Iakovou I, Chrisoulidou A, Balaris V,
Balaris C, Doumas A and Karatzas N: Acute effects of recombinant
human TSH on bone markers in differentiated thyroid cancer. Hell J
Nucl Med. 13:208–212. 2010.PubMed/NCBI
|
|
2
|
Azizmohammadi Z, Tabei F, Shafiei B,
Babaei AA, Jukandan SM, Naghshine R, Javadi H, Nabipour I, Assadi M
and Asli IN: A study of the time of hospital discharge of
differentiated thyroid cancer patients after receiving iodine-131
for thyroid remnant ablation treatment. Hell J Nucl Med.
16:103–106. 2013.PubMed/NCBI
|
|
3
|
Chen G, Nicula D, Renko K and Derwahl M:
Synergistic anti-proliferative effect of metformin and sorafenib on
growth of anaplastic thyroid cancer cells and their stem cells.
Oncol Rep. 33:1994–2000. 2015.PubMed/NCBI
|
|
4
|
Scharpf J, Tuttle M, Wong R, Ridge D,
Smith R, Hartl D, Levine R and Randolph G: Comprehensive management
of recurrent thyroid cancer: An American Head and Neck Society
consensus statement: AHNS consensus statement. Head Neck. Sep
22–2016.(Epub ahead of print). doi: 10.1002/hed.24513. View Article : Google Scholar
|
|
5
|
Waldherr C, Schumacher T, Pless M,
Crazzolara A, Maecke HR, Nitzsche EU, Haldemann A and Mueller-Brand
J: Radiopeptide transmitted internal irradiation of non-iodophil
thyroid cancer and conventionally untreatable medullary thyroid
cancer using. Nucl Med Commun. 22:673–678. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Meng S, Wu H, Wang J and Qiu Q: Systematic
Analysis of Tyrosine Kinase Inhibitor Response to RET Gatekeeper
Mutations in Thyroid Cancer. Mol Inform. 35:495–505. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Rossi ED, Straccia P, Palumbo M, Stigliano
E, Revelli L, Lombardi CP, Santeusanio G, Pontecorvi A and Fadda G:
Diagnostic and prognostic role of HBME-1, galectin-3 and β-catenin
in poorly differentiated and anaplastic thyroid carcinomas. Appl
Immunohistochem Mol Morphol. 21:237–241. 2013.PubMed/NCBI
|
|
8
|
Barkalina N, Jones C, Townley H and Coward
K: Functionalization of mesoporous silica nanoparticles with a
cell-penetrating peptide to target mammalian sperm in vitro.
Nanomedicine (Lond). 10:1539–1553. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Helmfors H, Eriksson J and Langel Ü:
Optimised luciferase assay for cell-penetrating peptide-mediated
delivery of short oligonucleotides. Anal Biochem. 484:136–142.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
D'Alessio D, Giliberti C, Benassi M and
Strigari L: Potential third-party radiation exposure from patients
undergoing therapy with 131I for thyroid cancer or
metastases. Health Phys. 108:319–325. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gautam A, Chaudhary K, Kumar R, Sharma A,
Kapoor P and Tyagi A: Open source drug discovery consortium Raghava
GP In silico approaches for designing highly effective cell
penetrating peptides. J Transl Med. 11:742013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cazarin JM, Andrade BM and Carvalho DP:
AMP-activated protein kinase activation leads to lysome-mediated
NA(+)/I(−)-symporter protein degradation in rat thyroid cells. Horm
Metab Res. 46:313–317. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Merron A, Peerlinck I, Martin-Duque P,
Burnet J, Quintanilla M, Mather S, Hingorani M, Harrington K, Iggo
R and Vassaux G: SPECT/CT imaging of oncolytic adenovirus
propagation in tumours in vivo using the Na/I symporter as a
reporter gene. Gene Ther. 14:1731–1738. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kurebayashi J, Tanaka K, Otsuki T, Moriya
T, Kunisue H, Uno M and Sonoo H: All-trans-retinoic acid modulates
expression levels of thyroglobulin and cytokines in a new human
poorly differentiated papillary thyroid carcinoma cell line, KTC-1.
J Clin Endocrinol Metab. 85:2889–2896. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Modoni S, Landriscina M, Fabiano A,
Fersini A, Urbano N, Ambrosi A and Cignarelli M: Reinduction of
cell differentiation and 131I uptake in a poorly differentiated
thyroid tumor in response to the reverse transcriptase (RT)
inhibitor nevirapine. Cancer Biother Radiopharm. 22:289–295. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Said M, Fujimoto M, Franken C, Woo S,
Vuong B and Haigh PI: Preferential use of total thyroidectomy
without prophylactic central lymph node dissection for early-stage
papillary thyroid cancer: Oncologic outcomes in an integrated
health plan. Perm J. 20:15–251. 2016.
|
|
17
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kist JW, de Keizer B, Stokkel MP, Hoekstra
OS and Vogel WV: THYROPET study group: Recurrent differentiated
thyroid cancer: Towards personalized treatment based on evaluation
of tumor characteristics with PET (THYROPET Study): Study protocol
of a multicenter observational cohort study. BMC Cancer.
14:4052014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kundu P, Lata S, Sharma P, Singh H,
Malhotra A and Bal C: Prospective evaluation of (68)Ga-DOTANOC
PET-CT in differentiated thyroid cancer patients with raised
thyroglobulin and negative (131)I-whole body scan: Comparison with
(18)F-FDG PET-CT. Eur J Nucl Med Mol Imaging. 41:1354–1362. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ghofrani M, Sosa JA, Ocal IT and Angeletti
C: Fine needle aspiration of poorly differentiated oxyphilic
(Hurthle cell) thyroid carcinoma: A case report. Acta Cytol.
50:560–562. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sherman SI, Clary DO, Elisei R,
Schlumberger MJ, Cohen EE, Schöffski P, Wirth LJ, Mangeshkar M,
Aftab DT and Brose MS: Correlative analyses of RET and RAS
mutations in a phase 3 trial of cabozantinib in patients with
progressive, metastatic medullary thyroid cancer. Cancer. Aug
15–2016.(Epub ahead of press). doi: 10.1002/cncr.30252. View Article : Google Scholar
|
|
22
|
Liepe K: Sensitivity of preparation with
rhTSH or thyroid hormone withdrawal using 131I-whole
body scans to identify metastases of differentiated thyroid cancer.
Int J Surg. 16:107–112. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Vilasdechanon N, Ua-Apisitwong S,
Chatnampet K, Ekmahachai M and Vilasdechanon J: Design of patient
rooms and automatic radioiodine-131 waste water management system
for a thyroid cancer treatment ward: ‘Suandok Model’. J Radiol
Prot. 34:699–708. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Turba UC, Sildiroglu O and Rehm PK:
Radioiodine (131I) accumulation in bronchogenic cyst in the setting
of thyroid carcinoma remission. Clin Imaging. 36:224–227. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bastos AU, Oler G, Nozima BH, Moyses RA
and Cerutti JM: BRAF V600E and decreased NIS and TPO expression are
associated with aggressiveness of a subgroup of papillary thyroid
microcarcinoma. Eur J Endocrinol. 173:525–540. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
D'Alessio D, Giliberti C, Benassi M and
Strigari L: Potential third-party radiation exposure from patients
undergoing therapy with 131I for thyroid cancer or metastases.
Health Phys. 108:319–325. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
de la Chapelle A and Jazdzewski K:
MicroRNAs in thyroid cancer. J Clin Endocrinol Metab. 96:3326–3336.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Duman BB, Kara OI, Uğuz A and Ates BT:
Evaluation of PTEN, PI3K, MTOR and KRAS expression and their
clinical and prognostic relevance to differentiated thyroid
carcinoma. Contemp Oncol (Pozn). 18:234–240. 2014.PubMed/NCBI
|
|
29
|
Yun F, Jia Y, Li X, Yuan L, Sun Q, Yu H,
Shi L and Yuan H: Clinicopathological significance of PTEN and
PI3K/AKT signal transduction pathway in non-small cell lung cancer.
Int J Clin Exp Pathol. 6:2112–2120. 2013.PubMed/NCBI
|
|
30
|
Biswas R, Mondal A and Ahn JC:
Deregulation of EGFR/PI3K and activation of PTEN by photodynamic
therapy combined with carboplatin in human anaplastic thyroid
cancer cells and xenograft tumors in nude mice. J Photochem
Photobiol B. 148:118–127. 2015. View Article : Google Scholar : PubMed/NCBI
|