Introduction
Hand, foot, and mouth disease (HFMD) is an
infectious disease, which is mainly induced by coxsackievirus A16
(CVA16) and enterovirus 71 (EV71), and commonly occurs in children
<5 years old (1,2). Wang et al (3) indicated that cytokines are the
mediators of the severe systemic inflammatory response associated
with EV71-induced pulmonary edema (PE). Several cases with HFMD are
mild and reversible, while other cases exhibit rapid progression to
neurogenic PE (4). PE is associated
with a high early mortality rate of 30–40% (5). In the past three decades, Asian
countries have experienced several widespread and severe outbreaks
of HFMD with deaths predominantly occurring among children
(6). At present, there are no
satisfactory treatments for EV71-induced PE. Thus, more effective
pharmacological strategies are urgently required to treat the
neurological complications of this potentially fatal viral
infection.
The underlying mechanisms of PE have been not
completely elucidated due to its complex etiology. However,
activation of the sympathetic nervous system, particularly the
massive stimulation of α-adrenoceptors following the discharge of
catecholamines in response to endothelin receptor activation in the
central nervous system, is considered to be the major factor
resulting in cardiopulmonary dysfunction (7–9). Thus,
blocking the activation of α-adrenergic receptors is crucial for
alleviating the symptoms of PE. Phentolamine is a reversible and
nonselective α-adrenergic antagonist (10). Krishnamoorthy and Weinberg (11) have indicated that phentolamine can
attenuate or completely prevent epinephrine infusion-induced
catecholamine release. Moreover, phentolamine has been indicated to
competitively block α-adrenergic receptors and decrease
hypertension by antagonism of the catecholamines epinephrine and
norepinephrine to further improve the cardiopulmonary function in
patients with PE (12). These
results are encouraging but not definitive since additional
mechanisms may be involved. A former study has demonstrated that
phentolamine only partly protects lung function, indicating that
other mediators discharged during sympathetic activation might play
important roles in increasing the permeability of the lung
(13). Due to the lack of clarity of
these results, the present study aimed to provide
clarification.
Therefore, the present study tested the assumption
that phentolamine alleviates the symptoms of HFMD combined with PE
by attenuating the associated changes in cardiopulmonary function.
In this study, preliminary evidence that phentolamine reduces
mortality and relieves the symptoms of EV71-induced PE is provided.
The favorable therapeutic outcome supports the potential of
phentolamine as a candidate therapeutic agent for HFMD with PE.
However, this requires evaluation in prospective clinical
studies.
Materials and methods
Study design and ethics
statements
This was a single center, open-label, randomized
trial. The study was approved by and implemented under the
guidelines of the ethics committees of Cangzhou Central Hospital
(Cangzhou, China) as well as institutional review boards of
Cangzhou Central Hospital. Randomization was conducted using a
random digital table by assigning odd numbers to the phentolamine
treatment group and even numbers to the control group. The
investigators did not obtain prior notice of which participants
were assigned to take either treatment. The next of kin of the
children involved in this trial signed and provided written
informed consent on the children's behalf prior to participating in
the study.
Patients
Children in Cangzhou Central Hospital were recruited
from May 2008 to December 2012. Eligible patients were required to
have a clinical diagnosis with severe HFMD combined with PE based
on the diagnosis and treatment guidelines for HFMD (2010) issued by
the Ministry of Health of China (14). Children were excluded when they
exhibited complications including chronic hepatitis, epilepsy,
congenital heart disease, nephritis and serious metabolic or
hematological diseases. Further exclusion criteria included chronic
diarrhea, a history of allergy to the study drugs and participation
in other clinical trials.
Intervention
All patients were treated with ventilation support
using high levels of positive end expiratory pressure (5–15 cm
H2O), as well as intravenous γ-globulin at a dose of 1
g/kg/day for two days (Shandong Taibang Biological Products Co.,
Ltd., Taibang, China). In-patient children received methyl
prednisolone at a dose of 3–5 mg/kg/time once daily for five
consecutive days (Pfizer, Inc., New York, NY, USA). Furthermore,
all patients were treated with mannitol to reduce intracranial
pressure. Mannitol was administered at a dose of 0.5–1 g/kg/time
six times daily for seven consecutive days (Shandong Qidu
Pharmaceutical Co., Ltd., Zibo, China). Concurrently, in the
phentolamine treatment group, a phentolamine infusion (10 mg/ml;
Shanghai Xudong Haipu Pharmaceutical Co., Ltd., Shanghai, China)
was initiated at a dosage of 3 µg/kg/min and titrated for the
control of stress ulcer-related gastrointestinal bleeding during
the physiological stress of HFMD. Additionally, phentolamine was
administered intravenously to control the indices of blood
pressure, heart rate and blood gas at a loading dose of 5
µg/kg/min. When the indicators of blood pressure, heart rate and
blood gas stabilized, phentolamine administration was gradually
decreased and then stopped within 24 h. The dosage was adjusted
from high to low as necessary to stabilize the vital signs. Therapy
was continued for 3–5 days. Patients in the phentolamine treatment
and control groups were followed up for 6 months.
Pathogen detection
Samples obtained from the stool, throat or
cerebrospinal fluid were immediately sent to the laboratory of the
Chinese Center for Disease Control and Prevention (Beijing, China)
to confirm the association of EV71 infection with the illness by
means of reverse transcription-polymerase chain reaction
(RT-PCR).
Total viral RNA was isolated from the samples using
TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA). Prior to RT-PCR, RNA samples were treated with
DNase (Thermo Fisher Scientific Inc.). RT-PCR amplification was
conducted using the following protocol: 10 µl 10X PCR buffer
(Thermo Fisher Scientific, Inc.), 3 µl upstream primer, 3 µl
downstream primer and 8 µl dNTPs (Thermo Fisher Scientific, Inc.),
1 µl MgCl2 50 mM (Thermo Fisher Scientific, Inc.), 1 µl Taq DNA
polymerase (Sigma-Aldrich; Merck Millipore, Darmstadt,
Germany).
VP1-specific cDNA was synthesized and was used as a
template for EV71 viral mRNA amplification using primers
(EV2449-EV2780) with the sequences: Forward,
5′-GGAGATAGGGTRGCAGATGTAAT-3′ and reverse,
5′-ATTTCCCAAGAGTAGTGATCGC-3′. β-actin was used as reference gene.
For β-actin, forward: 5′-CTCCATCCTGGCCTCGCTGT-3′ and reverse,
5′-GCTGTCACCTTCACCGTTCC-5′. The amplification reactions were as
follows: Initial denaturation at 95°C for 5 mins, followed by 15
cycles comprising denaturation at 95°C for 35 sec, annealing at
50°C for 16 sec and extension at 72°C for 35 sec, followed by 40
cycles consisting of denaturation at 95°C for 35 sec, annealing at
48°C for 16 sec and extension at 72°C for 35 sec, and final
extension at 72°C for 10 min. Subsequently, RT-PCR-positive samples
were confirmed by sequence analysis within the VP-1 region of EV71
using BigDye v3.0 Sequencing kits (Thermo Fisher Scientific, Inc.)
as well as an ABI 3730 automated sequencer (Applied Biosystems;
Thermo Fisher Scientific, Inc.).
Outcomes
Venous blood samples were collected from all
patients who were treated by ventilation support for 6 and 72 h.
These blood samples were used to measure the levels of creatine
kinase (CK), CK-MB and cardiac troponin I (cTnI). The investigator
recorded results for CK, CK-MB and cTnI, in the two groups. In
addition, heart rate and systolic blood pressure (SBP) in the two
groups were recorded every 6 h, until these indexes dropped to the
normal level. Moreover, the ventilation time and the duration of
hospitalization in these two groups were collected. In addition,
the investigator used a standard adverse-event case report form to
gather information about adverse events if they occurred. The
investigator also assessed whether or not the side events were
associated with the drug.
Statistical analysis
SPSS 13.0 software (SPSS, Inc., Chicago, IL, USA)
was utilized for all statistical analyses. All results are
expressed as the means ± standard deviations. All categorical
variables were compared using a paired Student's t-test. Continuous
variables were compared by means of an unpaired Student's t-test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Subject characteristics
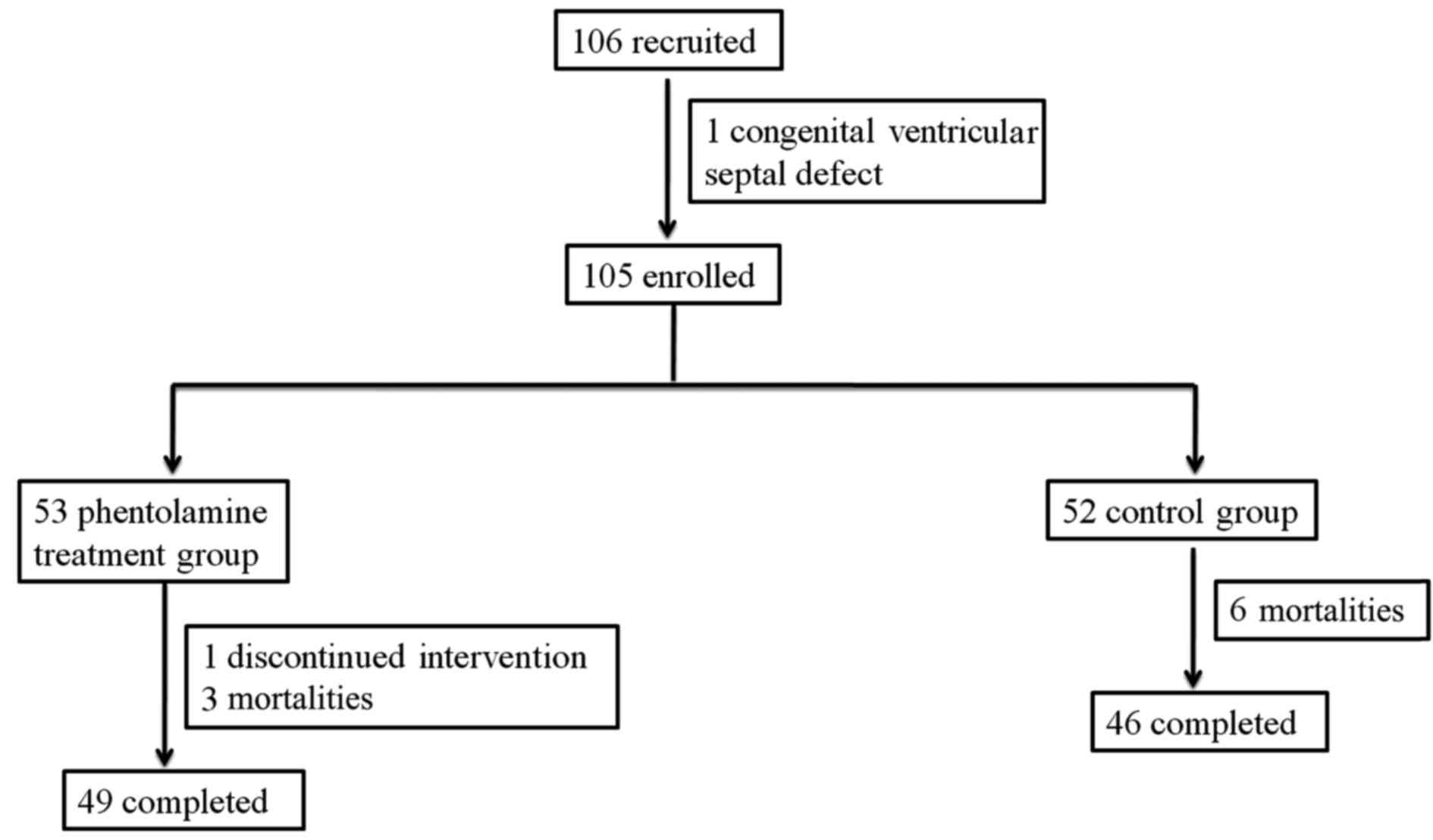
Between May 2008 and December 2012, a total of 106
subjects were enrolled in this trial. However, one of these
participants was excluded due to a congenital ventricular septal
defect. Thus, a total of 105 patients were eligible and
participated in the treatment, as shown in Fig. 1.
The baseline characteristics of the phentolamine and
control groups were similar. Of the 105 patients, 53 were assigned
to the phentolamine treatment group while 52 served as controls.
Among the 53 patients of the phentolamine treatment group, 32
(60.4%) were male and 21 (39.6%) were female. The median age of the
patients at disease onset was 1.6±0.8 years, and 52 (98%) patients
were <3 years of age at the time of the study. Moreover, in the
control group, 30 (57.7%) were male and 22 (42.3%) were female. The
median age was 1.5±0.7 years, and 50 (96%) patients were <3
years of age. No significant differences were observed in gender,
age and clinical disease degree between the two groups.
In addition, a total of 48 (91%) and 51 (98%)
subjects tested positive for EV71 by RT-PCR and were confirmed to
be acutely infected with EV71 in the phentolamine treatment and
control groups, respectively.
Clinical outcomes
The phentolamine-treated patients exhibited
significantly lower CK, CK-MB and cTnI levels, heart rate and SBP
than did the control patients (P=0.004, 0.008, 0, 0.002 and 0.005
for each parameter, respectively), as depicted in Table I.
 | Table I.Outcomes for children with severe
hand, foot and mouse disease plus pulmonary edema a randomized
trial evaluating the efficacy of phentolamine. |
Table I.
Outcomes for children with severe
hand, foot and mouse disease plus pulmonary edema a randomized
trial evaluating the efficacy of phentolamine.
| Groups | No. | CK-MB (U/l) | CK (U/l) | cTnl (µg/l) | HR (beats/min) | SBP (mmHg) |
|---|
| Phentolamine
treatment | 53 | 17.61±1.31 | 120.53±6.32 | 0.45±0.08 | 140.00±30.21 | 126±16 |
| Control | 52 | 28.12±3.98 | 450.75±8.56 | 0.95±0.05 | 160.22±35.34 | 140±20 |
| t |
| −17.736 | −20.082 | −20.71 | −3.079 | −3.865 |
| P-value |
|
0.008 |
0.004 | 0 |
0.002 |
0.005 |
The key events during the period of hospitalization
are summarized in Table II. The
average durations of ventilator dependence and hospital time were
shorter (3.41±1.72 vs. 4.95±2.16 days; 15.52±3.51 vs. 18.51±8.56
days, respectively) in the phentolamine treatment group than in the
control group. Significant differences were observed between the
groups in requirement for mechanical ventilation and the duration
of hospitalization (P=0.004 and 0.002 for ventilation application
time and the duration of hospitalization, respectively).
 | Table II.Clinical course of children with
severe hand, foot and mouse disease plus pulmonary edema treated
with phentolamine or standard therapy. |
Table II.
Clinical course of children with
severe hand, foot and mouse disease plus pulmonary edema treated
with phentolamine or standard therapy.
| Groups | No. | Ventilator dependence
(days) | Hospitalization
(days) |
|---|
| Phentolamine
treatment | 53 | 3.41±1.72 | 15.52±3.51 |
| Control | 52 | 4.95±2.16 | 18.51±8.56 |
| t |
| −3.596 | −5.462 |
| P-value |
|
0.004 |
0.002 |
Safety
It was found that the overall mortality rate was
lower in the phentolamine group (3 mortalities, 5.8%) than in the
control group (6 mortalities, 11.5%). All other patients survived
the 6-month follow-up. Other than these mortalities, no adverse
events were observed in either group.
Discussion
The attributes of EV71-induced HFMD with central
nervous system involvement are brain-stem encephalitis complicated
by PE as well as a high mortality rate (15). There is no available antiviral
treatment for EV71-induced disorders. We suggest that a more
favorable outcome might result from blocking the stimulation of
α-adrenergic receptors to alleviate the symptoms of PE.
Phentolamine, a reversible and nonselective α-adrenergic
antagonist, is recommended as an appropriate treatment to attenuate
the vasoconstricting effects of excess catecholamine secretion
(16). The present trial was
designed to investigate the potential effects of phentolamine on
severe HFMD combined with PE. The results indicate that
phentolamine markedly decreased the mortality rate, and improved
the cardiopulmonary functions in patients with this disorder.
Phentolamine has been utilized for the treatment of
patients with PE (11). Phentolamine
has been reported to induce relative hemodynamic stability
(17,18). Usually, the administration of
α-adrenergic blockers reduces and stabilizes blood pressure and
heart rate by reducing circulating catecholamine levels and
increasing intravascular volume (19,20).
Furthermore, α-adrenergic blockers have inotropic and vasodilatory
properties (21). In the present
trial, it was demonstrated that phentolamine treatment
significantly reduced the mortality rate, the average duration of
ventilator dependence and duration of hospitalization, CK, CK-MB
and cTnI levels, heart rate and SBP. Furthermore, a low incidence
of side events in the phentolamine treatment group suggested that
this drug may be a well-tolerated and safe therapy for children
with HFMD plus PE.
The potential action mechanisms of phentolamine
appear to be multifactorial. Firstly, EV71 may cause multiple organ
damage (22). A previous study has
indicated that phentolamine competitively blocks postsynaptic (α-1)
and presynaptic (α-2) adrenergic receptors, thereby causing
vasodilation and a reduction in peripheral resistance (23). Pharmacological effects of α-blockers
also include relaxation of vascular smooth muscle, and
antihypertensive effects, via antagonism of the catecholamines
epinephrine and norepinephrine, to further improve the
cardiopulmonary function of patients with PE (12). Sympathetic blockade using
phentolamine also prevents myocardial necrosis (24). Sympathetic hyperactivity is a primary
characteristic of EV71-induced PE. Norepinephrine activation is
thought to be the cause of sympathetic control of the heart,
leading to increased contractility force and heart rate (25). The present observation of reductions
in heart rate, CK levels and SBP following the infusion of
phentolamine in patients with HFMD and PE supports this
hypothesis.
Phentolamine, as a vasodilator, plays important
roles in the reduction of blood pressure (26). It is worthy of note that the change
in vascular tone associated with α-blocker treatment necessitates
fluid infusion to prevent the occurrence of considerable
hypotension (27). In the current
study, the infusion rate of phentolamine was maintained at a level
of 5 µg/kg/min, so that its antihypertensive effect was relatively
weak and its main role was in improvement of the microcirculation.
Furthermore, no severe phentolamine-related hypotension or other
side effects were evident in this study. Moreover, phentolamine
treatment significantly lowered the overall mortality rate relative
to that of the control group. This low incidence of side events of
phentolamine further indicates that this drug is a well-tolerated
and safe therapy for children with HFMD plus PE.
Several shortcomings in the current trial should be
noted. The relatively small sample size limited the analysis.
Additionally, eligible children were recruited in a single clinical
center. Finally, the effects of phentolamine on cytokine levels
were not performed. PE induced by EV71 is caused by aberrant
cytokine activation that generates a systemic inflammatory
reaction, which results in increased pulmonary vascular
permeability (5). Due to these
limitations, a randomized trial in multiple centers with a larger
sample size, and more measurement indicators is required to
identify the safety and effectiveness of phentolamine in the
treatment of EV71-induced PE.
In conclusion, the present randomized study provides
evidence that phentolamine markedly decreases mortality, and
improves the cardiopulmonary functions in children with HFMD and PE
induced by EV71. These favorable findings indicate that
phentolamine has potential as a therapeutic drug for treating this
disease. However, dose-response as well as time-course studies for
phentolamine in animal models of EV71-induced HFMD with PE are
further warranted.
References
|
1
|
World Health Organization (WHO), . A guide
to clinical management and public health response for hand, foot
and mouth disease (HFMD). WHO; Geneva: 2011
|
|
2
|
Cardosa MJ, Perera D, Brown BA, Cheon D,
Chan HM, Chan KP, Cho H and McMinn P: Molecular epidemiology of
human enterovirus 71 strains and recent outbreaks in the
Asia-Pacific region: Comparative analysis of the VP1 and VP4 genes.
Emerg Infect Dis. 9:461–468. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wang SM, Lei HY, Huang MC, Wu JM, Chen CT,
Wang JN, Wang JR and Liu CC: Therapeutic efficacy of milrinone in
the management of enterovirus 71-induced pulmonary edema. Pediatr
Pulmonol. 39:219–223. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Choi CS, Choi YJ, Choi UY, Han JW, Jeong
DC, Kim HH, Kim JH and Kang JH: Clinical manifestations of CNS
infections caused by enterovirus type 71. Korean J Pediatr.
54:11–16. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wang SM, Lei HY, Huang MC, Wu JM, Chen CT,
Wang JN, Wang JR and Liu CC: Therapeutic efficacy of milrinone in
the management of enterovirus 71-induced pulmonary edema. Pediatr
Pulmonol. 39:219–223. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ma E, Lam T, Chan K, Wong C and Chuang S:
Changing epidemiology of hand, foot, and mouth disease in Hong
Kong, 2001–2009. Jpn J Infect Dis. 63:422–426. 2010.PubMed/NCBI
|
|
7
|
Fontes RB, Aguiar PH, Zanetti MV, Andrade
F, Mandel M and Teixeira MJ: Acute neurogenic pulmonary edema: Case
reports and literature review. J Neurosurg Anesthesiol. 15:144–150.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Theodore J and Robin ED: Pathogenesis of
neurogenic pulmonary oedema. Lancet. 2:749–751. 1975. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Poulat P and Couture R: Increased
pulmonary vascular permeability and oedema induced by intrathecally
injected endothelins in rat. Eur J Pharmacol. 344:251–259. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Raja SN, Treede RD, Davis KD and Campbell
JN: Systemic alpha-adrenergic blockade with phentolamine: A
diagnostic test for sympathetically maintained pain.
Anesthesiology. 74:691–698. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Krishnamoorthy V and Weinberg G:
Phentolamine for neurogenic pulmonary edema: Bench to bedside
progress. Chest. 142:809–810. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Davison DL, Chawla LS, Selassie L, Tevar
R, Junker C and Seneff MG: Neurogenic pulmonary edema: Successful
treatment with IV phentolamine. Chest. 141:793–795. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hirabayashi A, Nishiwaki K, Shimada Y and
Ishikawa N: Role of neuropeptide Y and its receptor subtypes in
neurogenic pulmonary edema. Eur J Pharmacol. 296:297–305. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ministry of Health of the People's
Republic of China, . Guidelines for the diagnosis and treatment of
hand, foot and mouth disease (version 2010). Beijing: 2010
|
|
15
|
Liu CC, Tseng HW, Wang SM, Wang JR and Su
IJ: An outbreak of enterovirus 71 infection in Taiwan, 1998:
Epidemiologic and clinical manifestations. J Clin Virol. 17:23–30.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kobal SL, Paran E, Jamali A, Mizrahi S,
Siegel RJ and Leor J: Pheochromocytoma: Cyclic attacks of
hypertension alternating with hypotension. Nat Clin Pract
Cardiovasc Med. 5:53–57. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Pacak K, Eisenhofer G, Ahlman H, Bornstein
SR, Gimenez-Roqueplo AP, Grossman AB, Kimura N, Mannelli M, McNicol
AM and Tischler AS: International Symposium on Pheochromocytoma:
Pheochromocytoma: Recommendations for clinical practice from the
first international symposium. October 2005. Nat Clin Pract
Endocrinol Metab. 3:92–102. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kinney MA, Narr BJ and Warner MA:
Perioperative management of pheochromocytoma. J Cardiothorac Vasc
Anesth. 16:359–369. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Duka I, Gavras I, Johns C, Handy DE and
Gavras H: Role of the postsynaptic alpha(2)-adrenergic receptor
subtypes in catecholamine-induced vasoconstriction. Gen Pharmacol.
34:101–106. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sparks JW, Seefelder C, Shamberger RC and
McGowan FX: The perioperative management of a patient with complex
single ventricle physiology and pheochromocytoma. Anesth Analg.
100:972–975. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Osnes JB: Positive inotropic effect
without cyclic AMP elevation after alpha-adrenergic stimulation of
perfused hearts from hypothyroid rats. Acta Pharmacol Toxicol
(Copenh). 39:232–240. 1976. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sun LM, Zheng HY, Zheng HZ, Guo X, He JF,
Guan DW, Kang M, Liu Z, Ke CW, Li JS, et al: An enterovirus 71
epidemic in Guangdong Province of China, 2008: Epidemiological,
clinical, and virogenic manifestations. Jpn J Infect Dis. 64:13–18.
2011.PubMed/NCBI
|
|
23
|
van Zwieten PA: The influence of
antihypertensive drug treatment on the prevention and regression of
left ventricular hypertrophy. Cardiovasc Res. 45:82–91. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Neil-Dwyer G, Walter P, Cruickshank J,
Doshi B and O'Gorman P: Effect of propranolol and phentolamine on
myocardial necrosis after subarachnoid haemorrhage. Br Med J.
2:990–992. 1978. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Drummond G and Severson D: Cyclic
nucleotides and cardiac function. Circ Res. 44:145–153. 1979.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bek MJ, Wang X, Asico LD, Jones JE, Zheng
S, Li X, Eisner GM, Grandy DK, Carey RM, Soares-da-Silva P and Jose
PA: Angiotensin-II type 1 receptor-mediated hypertension in D4
dopamine receptor-deficient mice. Hypertension. 47:288–295. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
McMillian WD, Trombley BJ, Charash WE and
Christian RC: Phentolamine continuous infusion in a patient with
pheochromocytoma. Am J Health Syst Pharm. 68:130–134. 2011.
View Article : Google Scholar : PubMed/NCBI
|