Introduction
Ischemic-reperfusion injury often occurs during
surgical procedures for heart disease, including bypass heart
surgery and coronary revascularization, and affects treatment
efficacy (1). The symptoms of
ischemic-reperfusion injury include myocardial cell edema, changes
in the ultrastructure of cells, microvascular damage, a persistent
low ventricular systolic function, myocardial enzyme release and
reperfusion arrhythmias (2). Murry
et al (3) demonstrated that
the myocardium develops increased resistance against permanent
injury following repeated, transient episodes of ischemia and
reperfusion; this is known as ischemic preconditioning (IPC). A
previous study demonstrated that IPC was able to alleviate
reperfusion injury, reduce the extent of myocardial necrosis, and
prevent the occurrence of reperfusion arrhythmias (4). However, since it is difficult to
accurately determine when ischemia may arise, the potential
therapeutic application of IPC is greatly limited. Zhao et
al (5) initially proposed the
concept of ischemic postconditioning (IPO), which involves brief
periods of ischemia during reperfusion (6,7), in
2003. It is thought that IPO may reduce the extent of myocardial
infarction and myocardial enzyme release, and the occurrence of
reperfusion arrhythmias.
Opioids, including morphine, ohmefentanyl and
enkephalin, have been used prior to and following coronary artery
bypass surgery to treat acute myocardial ischemia, in order to
treat patients with pain and post-operative analgesia (8). It has previously been demonstrated that
the application of opioids during cardiac surgery imitates the
process of IPO, thereby providing broad therapeutic potential
(9).
In the present study, IPO was stimulated using the
Langendorff isolated heart perfusion working model (10), in conjunction with use of the opioid
receptor agonist remifentanil. In this model, the cardiac output
and cardiac enzyme levels were measured, and the changes to
myocardial cells and the mitochondria were observed. The present
study aimed to investigate the effects of remifentanil during IPO,
as well as its therapeutic potential in the treatment of
ischemic-reperfusion injury.
Materials and methods
Animals and grouping
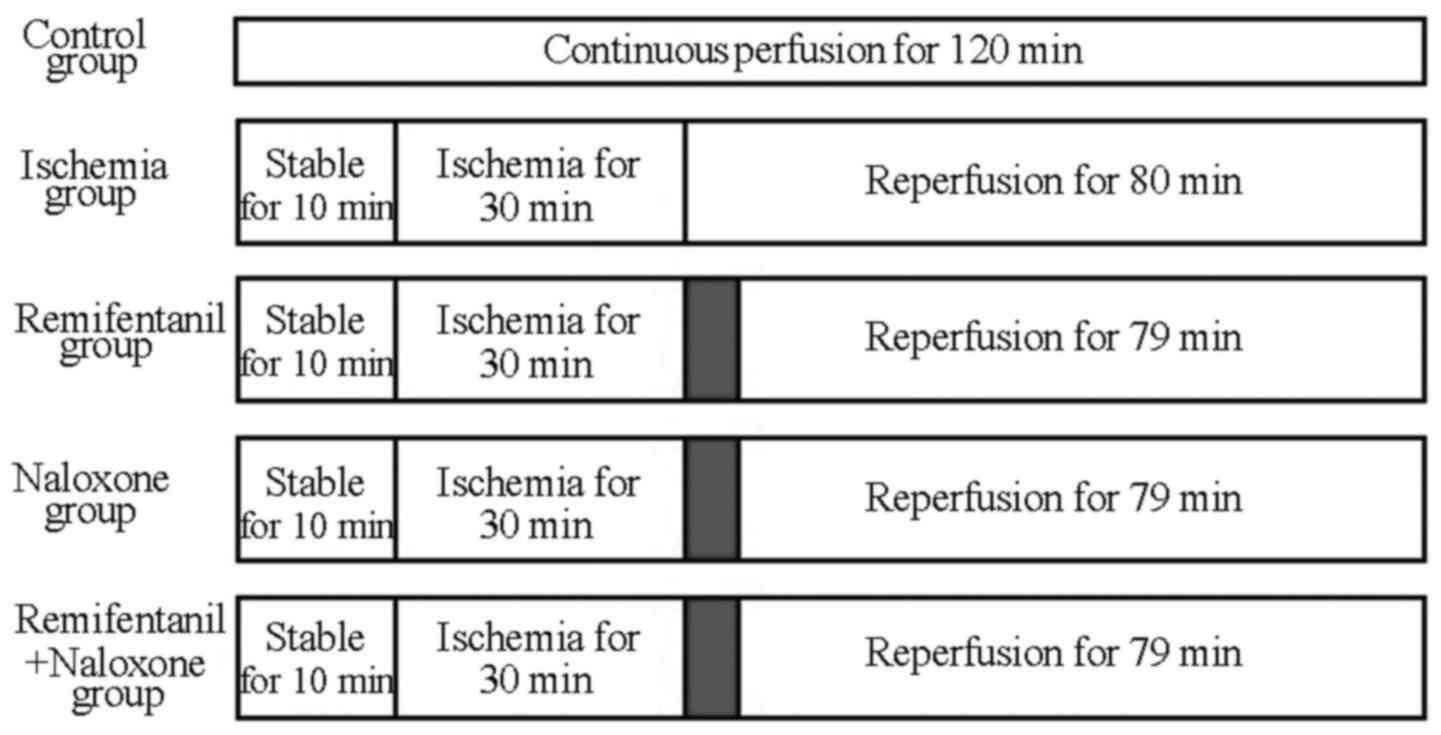
A total of 75 healthy, 6-week-old male rats with
body weights of 200–300 g (China Medical University Experimental
Animal Center, Beijing, China) were randomly divided into 5 groups
(Fig. 1). In the control group (n=15
rats), the rat hearts were perfused continuously with blood using a
Langendorff heart perfusion system (Beijing Zhishuduobao Biological
Technology Co., Ltd., Beijing, China), with no additional treatment
and without ischemia, for the same duration as the treatment of the
other groups. In the ischemia-reperfusion group (n=15 rats), stable
perfusion was implemented for 10 min and stopped for 30 min to
cause global ischemia, then reperfusion was applied for 80 min with
no additional treatment. In the treatment groups (n=15 rats each),
stable perfusion was again implemented for 10 min and stopped for
30 min to cause global ischemia. The rats were then continuously
administered a fixed concentration of drug for 1 min at a pressure
of 50 mmHg; following this, reperfusion with blood was implemented,
as aforementioned, for the remaining 79 min. The agonist group
received 100 µg/l remifentanil (Yichang Humanwell Pharmaceutical
Co., Ltd., Yichang, China), the naxolone (opioid antagonist) group
were administered 300 µg/l naloxone (Hebei Aoxing Pharmaceutical
Group Co., Ltd., Shijiazhuang, China) and the agonist plus
antagonist group (n=15 rats) received 100 µg/l remifentanil and 300
µg/l naloxone simultaneously. The present study was performed in
strict accordance with the recommendations in the Guide for the
Care and Use of Laboratory Animals of the National Institutes of
Health (Bethesda, MA, USA), and the animal use procedure was
reviewed and approved by the Institutional Animal Care and Use
Committee of the General Hospital of Shenyang Military Region
(Shenyang, China).
Establishment of an isolated heart
model
To anesthetize the rats, 4 ml/kg 10% chloral hydrate
(Sigma-Aldrich, St. Louis, MO, USA) was intraperitoneally injected
5 min prior to subsequent treatment. Heparinization was then
performed by intraperitoneally injecting 500 U/kg heparin
(Sigma-Aldrich) to prevent blood clotting. The rats were fixed in a
supine position and operated upon under sterile conditions. An
incision was made transversely along the anterior abdominal wall
and along the costal margin. The abdomen was incised between the
head end and the xiphoid process of the sternum, and across the
diaphragm. Cuts were then made longitudinally across the chest,
along the center line of the clavicle, on each side of the body.
The anterior mediastinal tissues were separated, the anterior chest
wall was opened over the head and the heart was exposed. The heart
was rapidly and carefully removed by incising the aorta and other
vasculature, starting from 4–5 mm into the initial portion of the
aorta. The heart was immediately placed in the 4°C, pre-treated
Krebs-Henseleit (K-H) solution (Sigma-Aldrich), saturated with 95%
O2 and 5% CO2 at a rate of 1.5 l/min and
rinsed, and then the blood was gently extruded.
The aorta was opened using ophthalmic forceps for
cannulation and the Langendorff heart perfusion system was placed
at the perfusing entrance. This system consisted of a double hollow
glass tube; an outer section was supplied with heated water, and
upper and lower entrances were connected to a thermostatic water
pump pipe. The inner layer was filled with K-H solution, and the
inner tube had two entry ports. The upper entrance was used for the
addition of K-H solution, in order to maintain a constant perfusion
pressure, and the lower port was connected to the aorta. The aortic
cannula was connected to the heart via 3 tubes. The aortic root was
below the surface of the water during the whole experiment and the
insertion position of the perfusion fluid output port catheter into
the aortic root was higher than the aortic valve level. The
apparatus was fixed in place with a fourth silk ligature, following
which constant cardiac perfusion was performed. The perfusion
pressure was maintained at 80 cm H2O, and the beating
heart was observed at random intervals. Bilateral pulmonary vein
ligation was performed by inserting the horn cone catheter (with a
diameter of 3 mm) into the left atrial appendage; this was
connected to a constant pressure reservoir bottle, and its height
was adjusted to cause a left atrial load of 13 cm H2O.
The isolated heart was subjected to Langendorff perfusion with an
aortic load of 80 cm H2O. Perfusion was performed as
aforementioned.
Hemodynamic measurement
The volumes of aortic fluid and coronary flow were
measured by collecting the aortic and coronary effluent,
respectively, and the cardiac output was calculated as a control.
Following reperfusion, these metrics were recorded following 10 and
30 min of activity by the left ventricle. These were compared with
corresponding values prior to cardiac arrest to attain the
percentage change resulting from post-ischemic reperfusion [change
(%) = (value of reperfusion / value prior to cardioplegia) ×
100].
Enzyme determination
The coronary effluent at 5 min prior to cardiac
arrest and the left ventricular output at 10 and 30 min after
reperfusion were collected. An RT-200C Plus Automated Chemistry
Analyzer (Shenzhen Leidu Life Sciences Co., Ltd., Shenzhen, China)
was used to measure lactate dehydrogenase (LDH) and levels of 2
phosphocreatine kinase (CK-MB) variants.
Electron microscopic observation
A total of 3 specimens from each group were randomly
selected for examination by electron microscopy. Preparation
involved multiple steps, as follows: Apical slices of ~1 mm of
myocardial tissue from each group were sectioned, placed in 2.5%
glutaraldehyde (Sigma-Aldrich) and rinsed with phosphate-buffered
saline (PBS) three times (with a duration of 30 min for each
rinse). Samples were fixed with 1% osmium tetroxide (Sigma-Aldrich)
for 2 h and rinsed with PBS three times. Sections were then
subjected to alcohol and acetone gradient dehydration, involving
treatment with 50% ethanol for 30 min, 70% ethanol for 30 min, 80%
acetone for 30 min (Sigma-Aldrich) and 90% acetone for 30 min,
followed by three washes (of 30 min each) with 100% acetone. These
sections were embedded and permeated in epoxy resin (Wuxi Guangming
Chemical Co., Ltd., Wuxi, China) and ultra-thin 70-nm slices were
prepared using an LKB microtome (LKB Corp., Stockholm, Sweden). The
slices were stained with uranyl acetate (Sigma-Aldrich) for 15 min
and with lead citrate (Yingkou Tianyuan Chemical Research Institute
Co., Ltd., Yingkou, China) for 20 min. Flameng scoring of the
mitochondria was performed under a 1200 EX Transmission Electron
Microscope (JEOL, Ltd., Tokyo, Japan), according to a previous
study (11). A total of 5 fields of
view were randomly selected from each slice, and each field
included 20 mitochondria. The mean ± standard deviation of the
mitochondrial scores for each field were calculated and scored as
follows: Normal mitochondrial structure and intact particle, 0
points; normal mitochondrial structure but missing particles, 1
point; mitochondrial swelling and transparent matrix, 2 points;
steep rupture and transparent and concentrated matrix, 3 points;
and rupture, and incomplete inner and outer mitochondrial
membranes, 4 points.
Statistical analysis
Statistical analysis was performed using the SPSS
13.0 software package (SPSS Inc., Chicago, IL, USA). Data are
expressed as the mean ± standard deviation and comparisons among
groups were performed using an analysis of variance. P<0.05 was
considered to indicate a statistically significant difference.
Results
General data
In total, 75 rats were used in the present study.
The body weight of each rat was between 229 and 246 g, with no
significant difference between the groups. The measured heart rate
prior to and following perfusion fluctuated within a range of 300
beats/min, but no significant difference between the groups was
observed.
Enzymatic changes
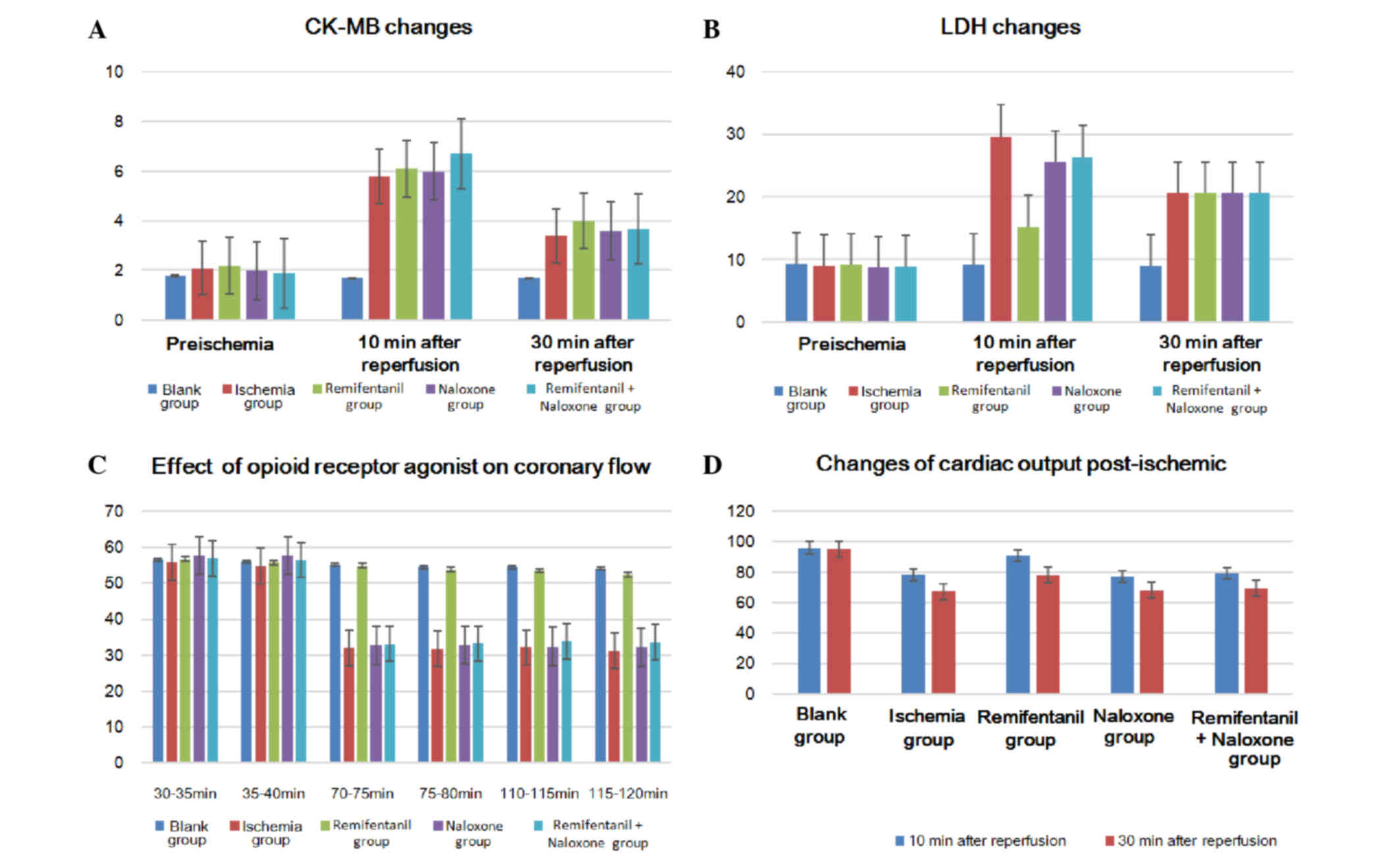
LDH and CK-MB levels prior to ischemia were not
significantly different between the rats from each group. However,
LDH levels in the coronary effluent at 5 min and 10 min of
reperfusion following ischemia were significantly lower in the
remifentanil treatment group than the other ischemia-reperfusion
groups (P<0.05); the differences between the other
ischemia-reperfusion groups were not significant (P>0.05)
(Table I; Fig. 2A and B). CK-MB values measured prior
to ischemia and after reperfusion for 5 min and 10 min were also
not identified as significantly different.
 | Table I.Myocardial enzyme changes prior to
ischemia (n=15 rats per group; mean ± standard deviation). |
Table I.
Myocardial enzyme changes prior to
ischemia (n=15 rats per group; mean ± standard deviation).
| Index/ group | Prior to
ischemia | Reperfusion for 10
min | Reperfusion for 30
min |
|---|
| LDH, U/l |
|
|
|
| Control | 9.3±1.2 | 9.2±1.1 | 9.0±1.5 |
| Ischemia | 9.1±2.1 | 29.7±8.3 | 20.6±8.0 |
| Remifentanil | 9.2±1.8 | 15.3±7.1a | 13.2±6.8a |
| Naloxone | 8.8±1.5 | 25.6±8.1 | 19.8±7.5 |
| Remifentanil +
naloxone | 8.9±1.7 | 26.4±6.9 | 21.4±7.1 |
| CK-MB, U/l |
|
|
|
| Control | 1.8±0.5 | 1.7±1.0 | 1.7±0.7 |
| Ischemia | 2.1±0.7 | 5.8±1.5 | 3.4±1.2 |
| Remifentanil | 2.2±0.8 | 6.1±2.0 | 4.0±2.0 |
| Naloxone | 2.0±0.4 | 6.0±1.4 | 3.6±2.2 |
| Remifentanil +
naloxone | 1.9±0.3 | 6.7±2.1 | 3.7±1.3 |
Heart function
The cardiac output of the non-ischemic control group
during the entire reperfusion did not significantly alter. When
examining the cardiac output between 10 and 30 min of reperfusion
following ischemia, the remifentanil-treated group demonstrated a
significantly higher cardiac output recovery rate than the other
ischemia-reperfusion groups (P<0.05), but no significant
difference was identified between the other groups (P>0.05;
Table II; Fig. 2C). Coronary blood flow of the
remifentanil-treated group following ischemia-reperfusion decreased
by ~4 ml/min when compared with the control group not exposed to
ischemia, but the reduction in coronary blood flow reached up to
20–24 ml/min less than the corresponding non-ischemic values in the
other groups (P<0.05; Tables II
and III; Fig. 2D).
 | Table II.Cardiac output volume changes
following ischemia (n=15 rats per group; mean ± standard
deviation). |
Table II.
Cardiac output volume changes
following ischemia (n=15 rats per group; mean ± standard
deviation).
|
|
| Cardiac output volume
recovery rate, % |
|---|
|
|
|
|
|---|
| Groups | Output prior to
ischemia, ml/min | 10-min
reperfusion | 30-min
reperfusion |
|---|
| Control | 30.3±2.5 | 96.1±7.5 | 95.2±7.1 |
| Ischemia | 29.4±4.5 | 78.4±7.9 | 67.2±8.2 |
| Remifentanil | 28.9±5.3 | 91.1±8.1a | 78.1±7.5a |
| Naloxone | 31.7±2.3 | 77.2±7.1 | 68.1±7.3 |
| Remifentanil +
naloxone | 28.1±3.3 | 79.1±8.3 | 69.6±9.1 |
 | Table III.Effects of opioid receptor agonist on
cardiac coronary flow, ml (n=15 rats per group; mean ± standard
deviation). |
Table III.
Effects of opioid receptor agonist on
cardiac coronary flow, ml (n=15 rats per group; mean ± standard
deviation).
| Groups | 30–35 min | 35–40 min | 70–75 min | 75–80 min | 110–115 min | 115–120 min |
|---|
| Control | 56.60±3.42 | 56.07±3.33 | 55.13±3.00 | 54.47±2.42 | 54.53±3.27 | 54.13±3.54 |
| Ischemia | 55.93±4.43 | 54.73±3.15 | 32.00±4.61 | 31.87±3.87 | 32.20±3.61 | 31.27±4.91 |
| Remifentanil | 56.73±4.86 | 55.87±4.26 |
54.93±4.64a |
53.87±5.17a |
53.53±5.14a |
52.47±5.67a |
| Naloxone | 57.73±4.27 | 57.67±3.98 | 32.73±2.99 | 32.87±3.85 | 32.40±4.00 | 32.20±3.90 |
| Remifentanil +
naloxone | 56.93±3.77 | 56.53±3.46 | 33.20±2.54 | 33.27±1.71 | 33.93±2.58 | 33.67±2.85 |
Myocardial ultrastructure
The myocardial fibers and mitochondria were observed
using electron microscopy (Table
IV). In the control group, muscle fibers were appropriately
arranged, the organelles were clear, and the mitochondrial outer
membrane and crests were intact (Fig.
3A). In the ischemic control group, muscle fibers were
disordered, mitochondrial crests were reduced and the mitochondrial
outer membranes were damaged (Fig.
3B). The remifentanil group tissues exhibited
appropriately-arranged, mitochondria-rich muscle fibers, and the
mitochondrial crest and outer membrane had maintained their
integrity (Fig. 3C). Treatment with
naloxone led to disorganized muscle fibers, reduced mitochondrial
crests and vacuolar degeneration (Fig.
3D). In the remifentanil and naloxone co-treatment group,
myocardial lines were blurred, and the mitochondrial crest and
outer membrane were damaged (Fig.
3E).
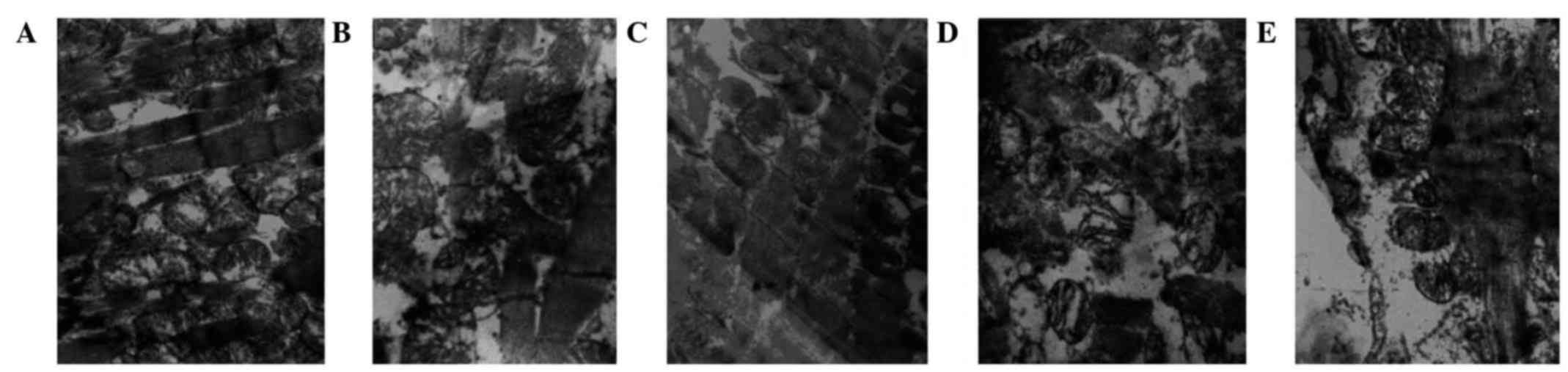 | Figure 3.Changes to myocardial ultrastructure,
observed using electron microscopy (magnification, 1,300,000x). (A)
Non-ischemic control group; muscle fibers were correctly arranged,
the sensitive sectors were clear, and the outer mitochondrial
membrane and crests were complete. (B) Ischemic control group;
muscle fibers were disordered, the mitochondrial crests were
reduced and the outer mitochondrial membranes were damaged. (C)
Remifentanil-treated group; muscle fibers were correctly arranged
and rich in mitochondria, and the mitochondrial crests and outer
membranes had retained their integrity. (D) Naloxone-treated group;
muscle fibers were disorganized, the mitochondrial crests were
reduced and vacuoles were degenerated. (E) Remifentanil and
naloxone co-treated group; cardiac lines were blurred, and the
mitochondrial crests and outer membranes were defective. The white
arrows indicate the mitochondria and mitochondrial cristae of the
myocardial cells. |
 | Table IV.Effect of opioid receptor agonist on
mitochondria in cardiac muscle cells (mean ± standard
deviation). |
Table IV.
Effect of opioid receptor agonist on
mitochondria in cardiac muscle cells (mean ± standard
deviation).
| Group | Flameng score for
mitochondria |
|---|
| Control | 0.41±0.29 |
| Ischemia | 3.14±0.17 |
| Remifentanil |
1.25±0.31a |
| Naloxone | 2.98±0.16 |
| Remifentanil +
naloxone | 3.05±0.11 |
Discussion
Zhao et al (5)
first proposed IPO as a potent endogenous protective mechanism in
2003; the molecular details of this mechanism remain unclear, but
are an area of active study in the field of cardiology (12). Sun et al (13) hypothesized that the reduction of
oxygen free radicals during early reperfusion may be the main cause
of post-reperfusion injury; this argument was supported and refuted
by subsequent studies. Tsang et al (14) used the phosphoinositide 3-kinase
(PI3K) inhibitor wortmannin to successfully attenuate the
cardioprotective effect of ischemic post-adaptation, hypothesizing
that PI3K-Akt-endothelial nitric oxide synthase, not PI3K, is the
primary mediator of post-ischemic adaptation. Ischemic adaptation
may, therefore, be mediated by multiple signal transduction
pathways (15). Previous studies of
adaptation mechanisms indicated that opioid receptor agonists have
cardioprotective effects, and receptor activation and intracellular
information transmission are the predominant focus of these
(16–18).
In the present study, the aortic effluent volume was
measured using isolated heart working models. The cardiac output in
the opioid receptor agonist-treated group recovered to ~91% of the
cardiac output measurement of the group not subjected to ischemia,
which was far higher than that of the ischemic control group (78%).
Remifentanil was able to significantly increase the volume of
aortic outflow and cardiac output when compared with the ischemic
control group. This myocardial protective effect disappeared upon
treatment with opioid receptor antagonists, which resulted in a
cardiac output recovery at 79% of that of the group not subjected
to ischemia. These results indicate that the cardioprotective
effects of post-conditioning may be accomplished, at least in part,
by opioid receptors. The coronary blood flow following reperfusion
in the remifentanil-treated group was less than that of the group
not subjected to ischemia (~4 ml/min), but the equivalent data from
the ischemic control group demonstrated a greater, 20–24 ml/min
reduction. There were significant differences in coronary blood
flow between the groups following ischemia-reperfusion, which may
explain the cardioprotective effect of the opioid receptor
agonist.
A previous study has indicated that opioid receptor
agonists can protect diastolic and systolic function following
ischemia-reperfusion (19). Kin
et al (20) revealed that an
opioid receptor agonist had no effect on myocardial diastolic and
systolic function prior to ischemia, but that the left ventricular
end-diastolic pressure (LVEDP) of the agonist group prior to
ischemia increased more slowly and that cardiac spasmodic
contraction occurred later than in the control group. In this
study, the left ventricular pressure in the opioid receptor agonist
group after 30 min reperfusion recovered to 85% of its value prior
to ischemia, but in the control group, the left ventricular
pressure only reached 45% of its pre-ischemia pressure. The LVEDP
of the agonist group was significantly lower than that of the
control group.
The present study simulated adaptation following
myocardial ischemia, using an opioid receptor agonist remifentanil
to demonstrate that this has a cardioprotective effect. Heart
coronary blood flow and cardiac output were significantly different
to the ischemic control group; due to the significant improvement
in cardiac function in the agonist group and the attenuation of
this effect upon treatment with an opioid receptor antagonist, it
can be concluded that the cardioprotective effect was accomplished
by opioid receptors. The remifentanil used in this experiment was a
specific receptor agonist, whereas naloxone was a non-selective
opioid receptor antagonist, suggesting that certain opioid receptor
agonists can significantly reduce the impact of
ischemia-reperfusion injury on cardiac function, in addition to
improving heart function and increasing coronary blood flow and
cardiac output. These roles were performed via opioid receptors,
but the exact mechanism behind this effect remains unknown.
Mitochondria have an important function in the
energy provision for cells, and can also regulate the osmotic
pressure, calcium balance, pH value and intracellular signal
transduction pathways of the cell (21). The present study noted the appearance
of the mitochondrial ion channels and scored the protective effect
of the opioid receptor agonist remifentanil upon the mitochondria.
The mitochondrial score of the agonist-treated group was 1.25±0.31,
which was significantly lower than the score of 3.14±0.17 observed
in the ischemic control group. The LDH and CK-MB levels were
determined in order to deduce the protective effect of the opioid
receptor agonist upon the myocardial cells. The measured LDH level
of the remifentanil-treated group following 10 and 30 min of
reperfusion was 15.3±7.1 and 10.2±6.8 U/l, respectively, while the
ischemic control group demonstrated significantly higher values of
29.7±8.3 and 20.6±6.8 U/l, respectively. It could thus be concluded
that remifentanil had roles in protecting the myocardial
mitochondria when administered during myocardial adaptation
following ischemia, associated with enzyme release during this
process. The corresponding values in the naxolone and naxolone +
remifentanil groups were 25.6±8.1 and 26.4±6.9 U/l, supporting the
hypothesis that this process is mediated by opioid receptors.
Mitochondrial function is essential to a positive
cardiac outcome following ischemia-reperfusion (22); if mitochondria are damaged during
reperfusion, this can result in an insufficient adenosine
triphosphate (ATP) supply, meaning that the ischemic myocardial
damage cannot be repaired. If mitochondria are protected, however,
myocardial cell function can gradually recover to avoid causing
irreversible damage (23). It has
previously been suggested that mitochondrial calcium overload is an
important contributory factor to myocardial injury during
ischemia-reperfusion (24). Free and
stable Ca2+ regulation is necessary for ATP production
in the mitochondria (25). A
previous study has indicated that myocardial ischemia-reperfusion
is accompanied by Ca2+ influx into the mitochondria and
concomitant calcium orthophosphate deposition, following which
mitochondrial oxidative phosphorylation and thus energy metabolism
are disturbed, leading to a reduction in ATP production (26).
Calcium influx causes the mitochondrial permeability
transition pore (MPTP) in the inner mitochondrial membrane to open,
causing the destruction of the mitochondria and the hydrolysis of
ATP (27,28). MPTP opening, resulting from
mitochondrial matrix calcium overload and often associated with
oxidative stress, is also hypothesized to be causative of
myocardial ischemia-reperfusion injury (29). A previous study indicated that, under
low mitochondrial matrix ATP and high Pi conditions, opioid
receptor agonists protect myocardial mitochondrial function in
response to ischemia-reperfusion injury (30). This may be directly implemented by
the mitochondria to prevent calcium uptake, or may be due to
inhibition of myocardial MPTP opening by oxygen free radical
scavengers (29,31).
In the present study, electron microscopy and
Flameng scoring demonstrated that the degree of mitochondrial
swelling and the number of fragmented mitochondria in the
experimental group was significantly decreased compared with that
in the ischemic control group, and myocardial enzyme level
measurements supported these conclusions. Remifentanil may thus
have a cardioprotective role in myocardial cells, functioning by
reducing the release of cardiac enzymes and protecting mitochondria
in myocardial cells, as supported by the evidence for these roles
in the current study; however, its specific mechanism of action
remains to be studied.
The current study revealed that opioid receptor
agonists had a significant role in improving heart function and
protecting myocardial cells, increasing coronary blood flow and
cardiac output, reducing myocardial enzyme release and protecting
mitochondria. These effects were hypothesized to occur by the
activation of these receptors in myocardial cells; this may present
therapeutic potential, if administered during early cardiac
reperfusion, in improving cardiac function and protecting the
myocardium. For instance, remifentanil may be used for myocardial
protection in early reperfusion during cardiac bypass surgery or in
early revascularization in the treatment of coronary heart disease.
However, the present study was performed in an animal model,
meaning that these effects may not be replicated in humans;
therefore, the recovery of cardiac function in patients following
surgery and the improvement of post-operative outcome require
further investigation.
References
|
1
|
Carden DL and Granger DN: Pathophysiology
of ischaemia-reperfusion injury. J Pathol. 190:255–266. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Naito H, Furukawa Y, Chino D, Yamada C and
Hashimoto K: Effects of zatebradine and propranolol on canine
ischemia and reperfusion-induced arrhythmias. Eur J Pharmacol.
388:171–176. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Murry CE, Jennings RB and Reimer KA:
Preconditioning with ischemia: A delay of lethal cell injury in
ischemic myocardium. Circulation. 74:1124–1136. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Miura T, Miura T, Kawamura S, Goto M,
Sakamoto J, Tsuchida A, Matsuzaki M and Shimamoto K: Effect of
protein kinase C inhibitors on cardioprotection by ischemic
preconditioning depends on the number of preconditioning episodes.
Cardiovasc Res. 37:700–709. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhao ZQ, Corvera JS, Halkos ME, Kerendi F,
Wang NP, Guyton RA and Vinten-Johansen J: Inhibition of myocardial
injury by ischemic postconditioning during reperfusion: Comparison
with ischemic preconditioning. Am J Physiol Heart Circ Physiol.
285:H579–H588. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jennings RB: Historical perspective on the
pathology of myocardial ischemia/reperfusion injury. Circ Res.
113:428–438. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hausenloy DJ: Cardioprotection techniques:
Preconditioning, postconditioning and remote conditioning (basic
science). Curr Pharm Des. 19:4544–4563. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Umegaki H and Tagami N: Anesthetic
management of an ALS patient with remifentanil. Masui.
57:1139–1142. 2008.(In Japanese). PubMed/NCBI
|
|
9
|
Zhang G, Sun Y, Wang Y, Bai J, Li T, Li X,
Su S and Liu X: An improved postconditioning algorithm: Gradually
increased reperfusion provides improved cardioprotection in rats.
Mol Med Rep. 8:696–702. 2013.PubMed/NCBI
|
|
10
|
Finegan BA, Gandhi M and Clanachan AS:
Phentolamine prevents the adverse effects of adenosine on
glycolysis and mechanical function in isolated working rat hearts
subjected to antecedent ischemia. J Mol Cell Cardiol. 32:1075–1086.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Flameng W, Borgers M, Daenen W and
Stalpaert G: Ultrastrutural and cytochemical correlates of
myocardial protection by cardiac hypothermia in man. J Thorac
Cardiovasc Surg. 79:413–424. 1980.PubMed/NCBI
|
|
12
|
Tong G, Aponte AM, Kohr MJ, Steenbergen C,
Murphy E and Sun J: Postconditioning leads to an increase in
protein S-nitrosylation. Am J Physiol Heart Circ Physiol.
306:H825–H832. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sun K, Liu ZS and Sun Q: Role of
mitochondria in cell apoptosis during hepatic ischemia-reperfusion
injury and protective effect of ischemic postconditioning. World J
Gastroenterol. 10:1934–1938. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tsang A, Hausenloy DJ, Mocanu MM and
Yellon DM: Postconditioning: A form of ‘modified reperfusion’
protects the myocardium by activating the phosphatidylinositol
3-kinase-Akt pathway. Circ Res. 95:230–232. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhao Q, Shao L, Hu X, Wu G, Du J, Xia J
and Qiu H: Lipoxin a4 preconditioning and postconditioning protect
myocardial ischemia/reperfusion injury in rats. Mediators Inflamm.
2013:2313512013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Vinten-Johansen J, Zhao ZQ, Zatta AJ, Kin
H, Halkos ME and Kerendi F: Postconditioning - A new link in
nature's armor against myocardial ischemia-reperfusion injury.
Basic Res Cardiol. 100:295–310. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhao TC, Du J, Zhuang S, Liu P and Zhang
LX: HDAC inhibition elicits myocardial protective effect through
modulation of MKK3/Akt-1. PLoS One. 8:e654742013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Takata K, Tomiyama Y, Tanaka K and Oshita
S: Cardioprotective effects of hyperkalemia during simulated
ischemia/reperfusion in neonatal rat cardiomyocytes - Preservation
of Na+/K+-ATPase activity. J Med Invest. 60:66–76. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Babiker FA, Joseph S and Juggi J: The
protective effects of 17beta-estradiol against ischemia-reperfusion
injury and its effect on pacing postconditioning protection to the
heart. J Physiol Biochem. 70:151–162. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kin H, Zatta AJ and Jiang R: Activation of
opioid receptors mediates the infarct postconditioning. J Mol Cell
Cardiol. 38:8272005.
|
|
21
|
Yellon DM and Hausenloy DJ: Myocardial
reperfusion injury. N Engl J Med. 357:1121–1135. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Widgerow AD: Ischemia-reperfusion injury:
Influencing the microcirculatory and cellular environment. Ann
Plast Surg. 72:253–260. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Perrelli MG, Tullio F, Angotti C, Cerra
MC, Angelone T, Tota B, Alloatti G, Penna C and Pagliaro P:
Catestatin reduces myocardial ischaemia/reperfusion injury:
Involvement of PI3K/Akt, PKCs, mitochondrial KATP channels and ROS
signalling. Pflugers Arch. 465:1031–1040. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cui J, Li Z, Qian LB, Gao Q, Wang J, Xue
M, Lou XE, Bruce IC, Xia Q and Wang HP: Reducing the oxidative
stress mediates the cardioprotection of bicyclol against
ischemia-reperfusion injury in rats. J Zhejiang Univ Sci B.
14:487–495. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Argaud L, Gateau-Roesch O, Raisky O,
Loufouat J, Robert D and Ovize M: Postconditioning inhibits
mitochondrial permeability transition. Circulation. 111:194–197.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Di Benedetto G, Scalzotto E, Mongillo M
and Pozzan T: Mitochondrial Ca2+ uptake induces cyclic AMP
generation in the matrix and modulates organelle ATP levels. Cell
Metab. 17:965–975. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Staat P, Rioufol G, Piot C, Cottin Y, Cung
TT, L'Huillier I, Aupetit JF, Bonnefoy E, Finet G, André-Fouët X
and Ovize M: Postconditioning the human heart. Circulation.
112:2143–2148. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Maslov LN, Naryzhnaia NV, Hanuš L, Pei JM,
Baĭkov AN, Zhang I, Wang H and Khaliulin IG: Problem of
end-effector of ischemic postconditioning of the heart. Ross Fiziol
Zh Im I M Sechenova. 99:555–574. 2013.(In Russian). PubMed/NCBI
|
|
29
|
Yang XM, Proctor JB, Cui L, Krieg T,
Downey JM and Cohen MV: Multiple, brief coronary occlusions during
early reperfusion protect rabbit hearts by targeting cell signaling
pathways. J Am Coll Cardiol. 44:1103–1110. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Bround MJ, Wambolt R, Luciani DS, Kulpa
JE, Rodrigues B, Brownsey RW, Allard MF and Johnson JD:
Cardiomyocyte ATP production, metabolic flexibility, and survival
require calcium flux through cardiac ryanodine receptors in vivo. J
Biol Chem. 288:18975–18986. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Darling CE, Jiang R, Maynard M, Whittaker
P, Vinten-Johansen J and Przyklenk K: Postconditioning via
stuttering reperfusion limits myocardial infarct size in rabbit
hearts: Role of ERK1/2. Am J Physiol Heart Circ Physiol.
289:H1618–H1626. 2005. View Article : Google Scholar : PubMed/NCBI
|