Introduction
Hepatocellular carcinoma (HCC), one of the most
common types of cancer in the world, is a hypervascular carcinoma.
Angiogenesis serves an important role in HCC progression,
malignancy, metastasis and high rates of recurrence (1–3). Solid
tumors may not grow bigger than 2–3 mm3 if tumor
angiogenesis is blocked (4).
Therefore, the development of genetic engineering technologies to
target angiogenesis may be a novel and effective method of treating
HCC.
Angiogenesis enables tumor growth and metastasis to
occur. Moreover, it is the process by which tumor cells are
provided with a supply of blood and nutrients (5–7). Due to
its extensive role in inducing cancer cell growth, tumor
angiogenesis has become a novel and promising target for anticancer
therapy. Inhibiting tumor angiogenesis may be an efficient way of
preventing tumor occurrence and progression.
Tumstatin is a novel factor that inhibits vascular
endothelial cell growth. It belongs to the NC1 domain of α3 chain
of type-IV collagen and exerts anti-angiogenic activity (8,9).
Tumstatin binds to endothelial cell surface integrins and exerts
its effects through multiple mechanisms, including inhibition of
endothelial cell protein synthesis, which results in endothelial
cell apoptosis, inhibition of tumor blood vessel formation, and
inhibition of tumor cell growth, invasion and metastasis (9). The anti-angiogenic activity of
tumstatin is localized to its 54–132 amino acid region (Tum-5),
which exhibits similar biological activity to the parent protein
(10–12).
The aim of the present study was to evaluate whether
gene therapy with Tum-5 is an effective strategy to treat patients
with HCC. The Tum-5 gene fragment was cloned and inserted into a
pLXSN retroviral vector following the protocol of a previous study
by our group (13). To identify the
role Tum-5 serves in the process of HCC growth, the anti-angiogenic
and antitumor effects of pLXSN-Tum-5 virus transfection were
assessed in vitro and in vivo.
Materials and methods
Reagents
The rabbit anti-mouse CD31 monoclonal antibody was
purchased from eBioscience, Inc. (cat. no. 13-0311-81; San Diego,
CA, USA). The Ready-to-Use Immunohistochemistry Hypersensitivity
UltraSensitive™ S-P kit was purchased from Maixin Biotech. Co.,
Ltd. (Fuzhou, China). MTT and Polybrene® were purchased
from Sigma-Aldrich (Merck Millipore, Darmstadt, Germany).
Cells and culture
The retroviral packaging mouse fibroblast cell line
PA317, NIH3T3 fibroblasts, human umbilical vein endothelial cells
(HUVECs), the HepG2 human hepatocarcinoma cell line and the H22
mouse hepatocarcinoma cell line were all obtained from American
Type Culture Collection (Manassas, VA, USA). Cells were cultured at
37°C with 5% CO2 in complete Dulbecco's Modified Eagle's
medium (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
containing 10% FBS (Gibco; Thermo Fisher Scientific, Inc.).
Production of viral particles and
determination of viral titer
The human pLXSN-Tum-5 plasmid was constructed as
previously described (13). A total
of 5×105 PA317 packaging cells were seeded in 6-well
plates. After 24 h, pLXSN-Tum-5 and pLXSN were transfected into the
PA317 cells using Lipofectamine® 2000 (Invitrogen;
Thermo Fisher Scientific, Inc.) for 6 h. Infected cells were split
and grown with 400 µg/ml G418-containing medium. After 2 weeks,
G418-resistant colonies were picked, the virus-containing
supernatant was collected and passed through a 0.45 µm filter,
subsequently frozen and stored at −80°C. The titer of viral stocks
was determined by infection of the NIH3T3 cells using a previously
described technique (14). The
titers of pLXSN-Tum-5 and pLXSN virus were 8.2×106
colony-forming units/ml and 7.6×106 colony-forming
units/ml, respectively.
MTT assay for cell proliferation in
vitro
Cell growth was evaluated using an MTT assay. HUVECs
or HepG2 cells were seeded in 96-well plates at 8×103
cells/well (0.2 ml/well) and cultured at 37°C in 5% CO2
overnight to allow for cell attachment. Cells were subsequently
infected with pLXSN-Tum-5 virus at 0, 1, 5, 10, 25 and 50
multiplicity of infection (MOI) in the presence of 5 µg/ml
Polybrene. Following 72 h of incubation, the supernatant was
removed and serum-free culture medium containing 5 mg/ml MTT was
added to each well. Following 4 h of incubation with MTT, the
supernatant was discarded and 150 µl dimethyl sulfoxide
(Sigma-Aldrich; Merck Millipore) was added for 10 min. The optical
density (OD) of each well was measured at 570 nm using a Bio-Rad
2550 microplate reader (Bio-Rad Laboratories, Inc., Hercules, CA,
USA). All experiments were performed in triplicate.
Antitumor effects in vivo
The antitumor effects of retroviral vectors
containing Tum-5 gene were examined in vivo using a H22
mouse HCC xenograft implanted in Kunming (KM) female mice (55–70
days old; weight, 15–20 g) that were obtained from Jilin University
(Changchun, China). The mice were implanted subcutaneously with
1×106 H22 cells in 0.1 ml serum-free medium to produce a
subcutaneous tumor xenograft. The mice were housed in sterile
prebedded plastic cages and maintained at 20°C with a 12 h light/12
h dark cycle and had free access to mouse food and water. When the
tumor size reached 30–70 mm3, 15 xenograft-bearing mice
were randomly divided into three groups: Saline (n=5), pLXSN (empty
virus; n=5) and pLXSN-Tum-5 (n=5). Injections of saline, pLXSN and
pLXSN-Tum-5 were administered on days 0, 2, 4, 6 and 8 into tumor
tissues at a MOI of 5 per mouse. The tumor size and body weight of
each mouse were recorded every other day. The antitumor effects
were determined by measuring the tumor dimensions via vernier
caliper to the nearest 0.1 mm, and calculating the volume using the
following equation: V=ab2/2, where a and b represent the
length and width of tumor, respectively. After 10 days, mice under
pentobarbital anesthesia (80 mg/kg body weight; Sigma-Aldrich) were
sacrificed by cervical dislocation, and tumor tissues were
carefully excised from the body and weighed. All animal experiments
were performed in compliance with the NIH guidelines for the care
and use of laboratory animals. The animal experiments in this study
were approved by the Animal Ethics Committee of Beihua University
(Jilin City, China).
Immunohistochemical staining for
CD31
Tumor tissues from the H22 tumor-bearing mice
(saline, pLXSN and pLXSN-Tum-5 groups) were fixed in 10% formalin
at room temperature for 24 h, embedded in paraffin and cut into
4-µm consecutive sections. Following deparaffinization and antigen
retrieval, immunohistochemical staining was performed using the
Ready-to-Use Immunohistochemistry Hypersensitivity UltraSensitive™
S-P kit according to the manufacturer's instructions. Sections were
treated with 3% hydrogen peroxide for 10 min at room temperature to
block the activity of endogenous peroxidase. The sections were
washed with phosphate-buffered saline (PBS) for 5 min and blocked
with normal goat serum (provided with the kit) for 10 min at room
temperature. The sections were subsequently incubated with a 1:100
dilution of the monoclonal antibody for CD31 at 4°C overnight. The
sections were then washed with PBS and treated with biotinylated
secondary antibody (provided with the kit) for 10 min, followed by
further incubation with streptavidin-horseradish peroxidase
complex. Following additional washing, diaminobenzidine was used as
a chromogen and counterstaining was performed using hematoxylin.
Sections were dehydrated, cleared and mounted with resin.
Microvessel density (MVD)
From the CD31-stained sections, the MVD was
determined at the hot spot through light microscopy examination
(BX43F; Olympus Corporation, Tokyo, Japan). For each section,
positively stained microvessels were counted from 5 high-power
fields (HPF; magnification, ×400). The average count was regarded
as the MVD per HPF.
Statistical analysis
Statistical analysis was performed with the
Statistical Package for Social Sciences version 17.0 (SPSS, Inc.,
Chicago, IL, USA). All experiments were performed at least three
times and all values were expressed as the mean ± standard
deviation. Student's t-test was used to compare values between two
groups and one-way analysis of variance (ANOVA) was used for more
than two groups. For all analyses, P<0.05 was considered to
indicate a statistically significant difference.
Results
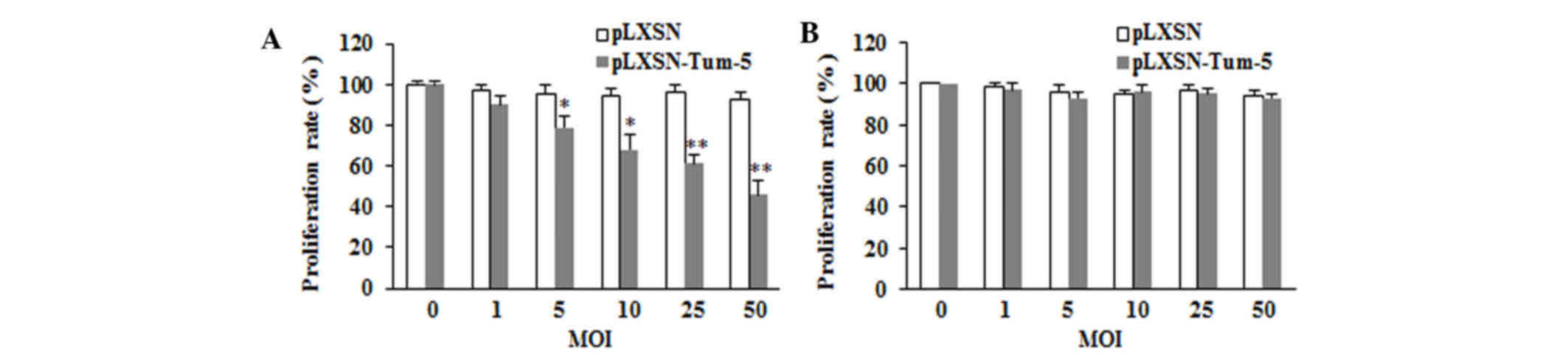
Effect of Tum-5 transfection on HUVEC
growth in vitro
To examine the role of Tum-5 in tumor angiogenesis,
HUVECs were used to mimic the tumor environment. HUVECs were
transfected with pLXSN-Tum-5 or pLXSN virus at different MOIs of 0,
1, 5, 10, 25 and 50. The proliferation rate was measured using the
MTT assay at 72 h. The results demonstrated that cells in the
pLXSN-Tum-5 group exhibited a significantly lower proliferation
rate compared with the pLXSN group (P<0.05). Tum-5 significantly
inhibited the proliferation of HUVECs in a titer-dependent manner
(Fig. 1A).
Effect of Tum-5 transfection on HepG2
cell growth in vitro
Based on the anti-angiogenesis activity of Tum-5 in
HUVECs, it was investigated whether Tum-5 exerts antitumor activity
in tumor cells in vitro. HepG2 cells were transfected with
Tum-5 virus at different MOIs of 0, 1, 5, 10, 25 and 50 for 72 h.
The results demonstrated that the growth of the pLXSN-Tum-5 group
was not significantly different from that of the pLXSN group
(P>0.05; Fig. 1B). This result
indicated that the introduction of Tum-5 into HepG2 cells did not
affect HepG2 cell proliferation.
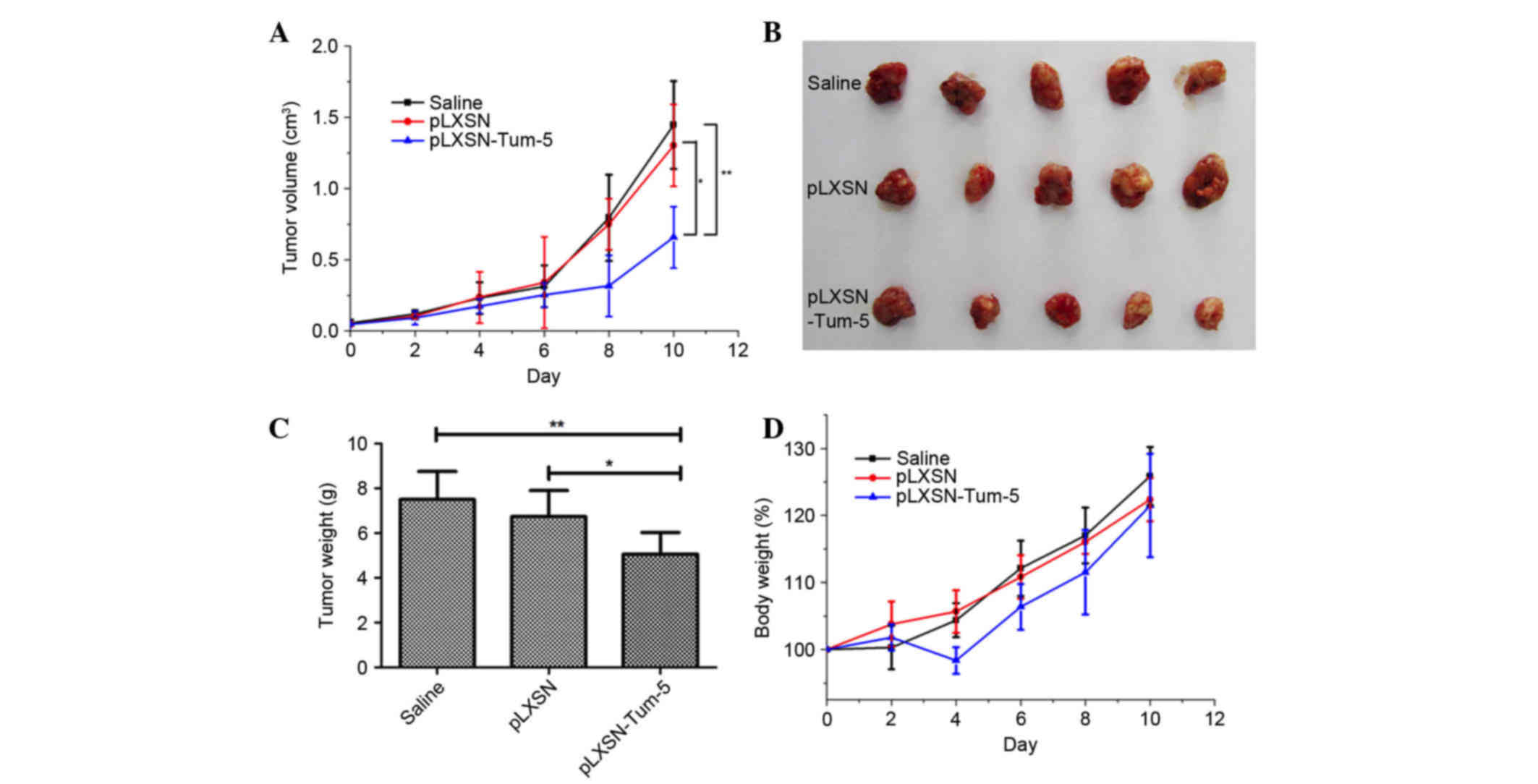
Antitumor effect of Tum-5 in vivo
To test if the retrovirus containing the Tum-5 gene
was able to inhibit tumor growth in vivo, a H22 mouse HCC
xenograft model was employed. When the tumor size reached 30–70
mm3, the 15 mice were randomly divided into three equal
groups. Tumors were injected with pLXSN or pLXSN-Tum-5 retroviral
vectors (MOI of 5 per mouse) on days 0, 2, 4, 6 and 8. The results
indicated that, compared with saline (P<0.01) and empty
retroviral vectors (P<0.05), pLXSN-Tum-5 significantly inhibited
tumor growth on day 10, after the administration of 5 injections
(Fig. 2A). Considering that pLXSN
had no effect on tumor growth, it was concluded that tumor growth
was inhibited by the Tum-5 gene carried by the retroviral vector.
Tumor tissues were excised on the 10th day and weighed. The size
and weight of tumors from mice in the pLXSN-Tum-5 group were
significantly lower than those of mice in the saline (P<0.01)
and pLXSN groups (P<0.05) (Fig. 2B
and C). Changes in body weight were used to evaluate the
toxicity of the retroviral vectors. The results demonstrated no
significant weight loss in mice in the pLXSN-Tum5 group compared
with mice from the other groups following 10 days of treatment,
suggesting that retroviral vectors containing the Tum-5 gene
exhibit low toxicity (Fig. 2D). The
notable weight loss of the pLXSN-Tum-5 group at day 4 may be
attributed to the inhibited tumor growth caused by Tum-5.
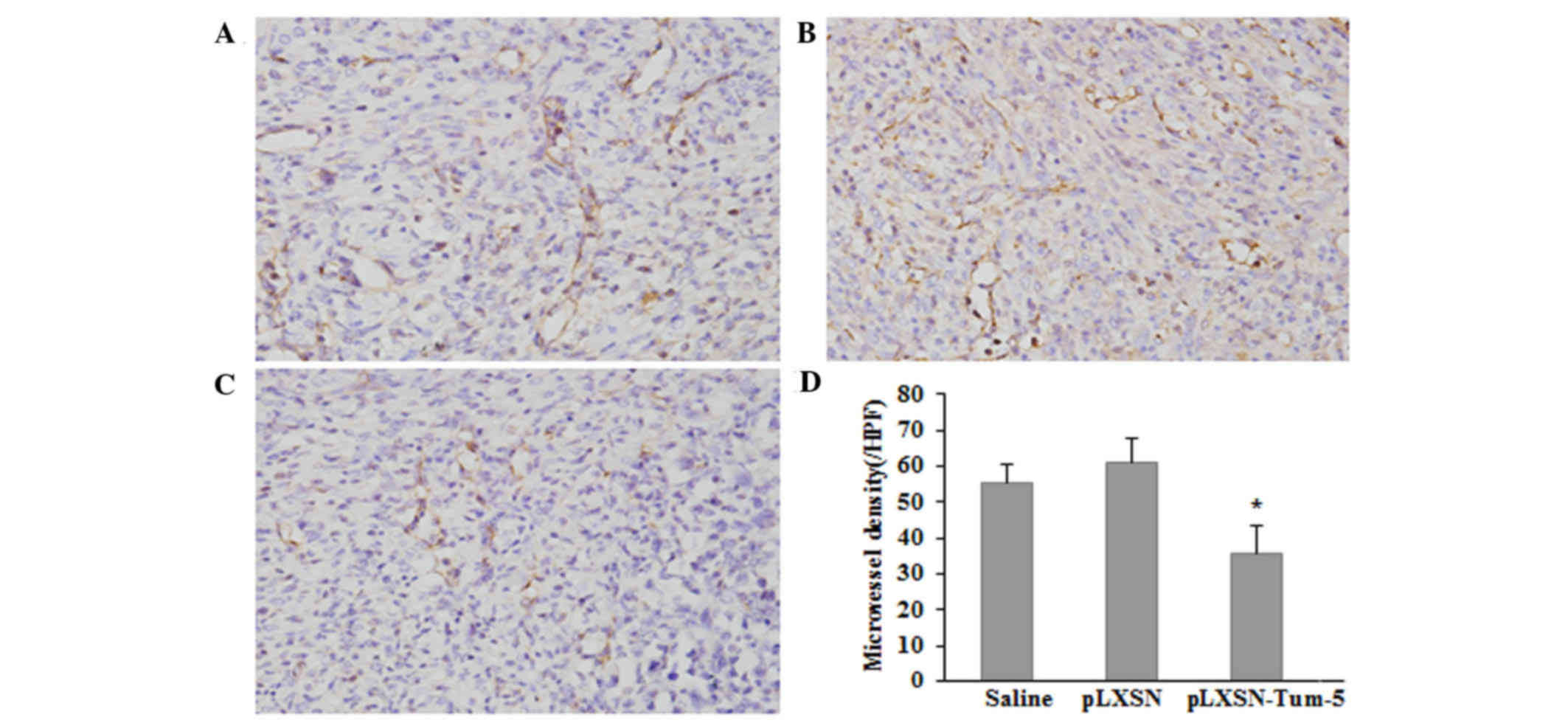
Expression of CD31 in tumor
tissue
As no direct antitumor effect of Tum-5 was detected
in vitro, it was speculated that the antitumor effect of
Tum-5 observed in vivo was ascribed to the failure of
angiogenesis in H22 tumor-bearing mice. Therefore, the endothelial
cell marker CD31, was used to analyze tumor angiogenesis. Tumor
tissues from H22 tumor-bearing mice were immunohistochemically
stained with an antibody against CD31. The average MVD of
CD31-stained sections in the pLXSN-Tum-5 group was significantly
lower than that in the saline and pLXSN groups (P<0.05; Fig. 3). These results suggested that Tum-5
may decrease HCC tumor growth by inhibiting angiogenesis in
vivo.
Discussion
Tumor angiogenesis is a key process for the majority
of solid tumors, since the growth and metastasis of malignant cells
require the formation of new blood vessels (5–7,15,16).
Tumstatin specifically inhibits endothelial cell proliferation and
induces apoptosis by interacting with αVβ3
integrin (12,17). Deletion mutagenesis analysis has
revealed that the anti-angiogenic activity of tumstatin is
localized to the 54–132 amino acid region (Tum-5) (11). It is easier to transfer smaller
fragments into tissue or cells, and in the present study, Tum-5
cDNA was therefore transfected into the retrovirus plasmid pLXSN to
construct pLXSN-Tum-5 (13), a
carrier which can effectively transfect host cells to stably
express Tum-5 (18). To determine
whether Tum-5 exhibits anti-angiogenic activity, HUVECs were
transfected with pLXSN-Tum-5 virus in vitro. The results of
the MTT assay indicated that the transfection of pLXSN-Tum-5 virus
significantly decreased HUVEC proliferation in a titer-dependent
manner and that the Tum-5 fragment, like full-length tumstatin,
exhibits proliferation-inhibitory activity.
It has been demonstrated that tumstatin inhibits
tumor growth in a number of different types of cancer, including
human renal carcinoma, prostate carcinoma, lung carcinoma and
glioma (11,12,19–22). As
a gene fragment encoding the region of tumstatin with
anti-angiogenic activity, Tum-5 has been identified to exhibit
antitumor activity by exerting anti-angiogenic activity, but does
not act directly on tumor cells (10–12).
However, it has been reported that the Tum-5 gene may significantly
inhibit gastric cancer cell proliferation and promote cell
apoptosis in vitro (23).
Therefore, the present study evaluated the antitumor effects of
Tum-5 on HepG2 cells in vitro. The results showed that the
proliferation of HepG2 cells transfected with Tum-5 did not
significantly differ from that of cells transfected with retroviral
vector, suggesting that Tum-5 had no direct antitumor activity on
HepG2 cells. It was considered that Tum-5 may have different
effects on gastric cancer and HCC cells. However, Tum-5 did not
exert an antitumor effect on H22 cells in vitro in our
prelimary experiment. In blood vessels formed during tumor
angiogenesis, vascular endothelial cells may serve important roles
in several steps of tumor cell activation, proliferation,
migration, invasion and tubule formation (24,25).
Considering the rich blood vessels observed in HCC, specific
angiogenesis-targeting interventions provide a possible treatment
for HCC by inhibiting the abovementioned processes (26–28).
Therefore, the inhibition of angiogenesis represents a potential
therapeutic target for HCC that may reduce the mortality of
patients with HCC. The present study investigated the inhibitory
effects of Tum-5 on HCC growth in vivo by injecting
pLXSN-Tum-5 virus into KM mice bearing H22 tumors, and a
significant antitumor effect was observed. The size and weight of
tumors from mice in the pLXSN-Tum-5 group were significantly lower
than those from the pLXSN and wild-type H22 HCC groups, suggesting
that Tum-5 is effective at inhibiting HCC tumor growth.
The rate of angiogenesis is typically estimated by
measuring the MVD of fixed tissue immunostained for endothelial
markers, including CD31, CD34 and factor VIII. CD31 is a
pan-endothelial marker for small and large vessels (29). In order to assess the MVD, CD31 and
associated antibodies are used to mark tumor vascular endothelial
cells and the capillary number per unit area is counted (30). In the present study,
immunohistochemical staining for CD31 revealed that the average MVD
in the pLXSN-Tum-5 group was significantly lower compared with that
in the pLXSN and wild-type H22 HCC groups. The results indicated
that Tum-5 may have a significant anti-angiogenic effect on the
neovascular endothelial cells of HCC.
In conclusion, the present study determined that
Tum-5 specifically inhibited HUVEC proliferation, but did not have
any direct effect on HCC cell growth in vitro. The in
vivo study demonstrated that Tum-5 exerted stronger antitumor
activity through suppression of vascular endothelial cells compared
with direct action on HCC cells. Taken together, the results of the
present study suggested that the gene fragment of Tum-5 may be an
effective angiogenesis inhibitor and may be developed as novel
therapeutic strategy to treat patients with HCC.
Acknowledgements
The present study was supported by the Science and
Technology Department of Jilin province (grant nos. 20110728 and
20150101128JC) and by the Health Department of Jilin Province
(grant no. 20122120).
References
|
1
|
Yuan MM, Xu YY, Chen L, Li XY, Qin J and
Shen Y: TLR3 expression correlates with apoptosis, proliferation
and angiogenesis in hepatocellular carcinoma and predicts
prognosis. BMC Cancer. 15:2452015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lin W, Zhao J, Cao Z, Zhuang Q, Zheng L,
Zeng J, Hong Z and Peng J: Livistona chinensis seeds inhibit
hepatocellular carcinoma angiogenesis in vivo via suppression of
the Notch pathway. Oncol Rep. 31:1723–1728. 2014.PubMed/NCBI
|
|
3
|
Wang W, Li GY, Zhu JY, Huang DB, Zhou HC,
Zhong W and Ji CS: Overexpression of AGGF1 is correlated with
angiogenesis and poor prognosis of hepatocellular carcinoma. Med
Oncol. 32:1312015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lewis CE, Leek R, Harris A and McGee JO:
Cytokine regulation of angiogenesis in breast cancer: The role of
tumor-associated macrophages. J Leukoc Biol. 57:747–751.
1995.PubMed/NCBI
|
|
5
|
Folkman J: Tumor angiogenesis: Therapeutic
implications. N Engl J Med. 285:1182–1186. 1971. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Folkman J: Angiogenesis in cancer,
vascular, rheumatoid and other disease. Nat Med. 1:27–31. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bielenberg DR and Zetter BR: The
contribution of angiogenesis to the process of metastasis. Cancer
J. 21:267–273. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hamano Y, Zeisberg M, Sugimoto H, Lively
JC, Maeshima Y, Yang C, Hynes RO, Werb Z, Sudhakar A and Kalluri R:
Physiological levels of tumstatin, a fragment of collagen IV alpha3
chain, are generated by MMP-9 proteolysis and suppress angiogenesis
via alphaV beta3 integrin. Cancer Cell. 3:589–601. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sudhakar A and Boosani CS: Inhibition of
tumor angiogenesis by tumstatin: Insights into signaling mechanisms
and implications in cancer regression. Pharm Res. 25:2731–2739.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Maeshima Y, Manfredi M, Reimer C, Holthaus
KA, Hopfer H, Chandamuri BR, Kharbanda S and Kalluri R:
Identification of the anti-angiogenic site within vascular basement
membrane-derived tumstatin. J Biol Chem. 276:15240–15248. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Maeshima Y, Colorado PC, Torre A, Holthaus
KA, Grunkemeyer JA, Ericksen MB, Hopfer H, Xiao Y, Stillman IE and
Kalluri R: Distinct antitumor properties of a type IV collagen
domain derived from basement membrane. J Biol Chem.
275:21340–21348. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Maeshima Y, Colorado PC and Kalluri R: Two
RGD-indepentdent alpha vbeta3 integrin binding sites on tumstatin
regulate distinct anti-tumor properties. J Biol Chem.
275:23745–23750. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gai XD, Luo H, Li C and Feng K: The
construction of recombined retroviral plasmid with human Tum-5 gene
and packing cell line. Zhouguo Lao Nian Xue Zazhi. 29:1194–1196.
2009.(In Chinese).
|
|
14
|
Bodine DM, McDonagh KT, Brandt SJ, Ney PA,
Agricola B, Byrne E and Nienhuis AW: Development of a high-titer
retrovirus producer cell line capable of gene transfer into rhesus
monkey hematopoietic stem cells. Proc Natl Acad Sci USA.
87:3738–3742. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bisacchi D, Benelli R, Vanzetto C, Ferrari
N, Tosetti F and Albini A: Anti-angiogenesis and angioprevention:
Mechanisms, problems and perspectives. Cancer Detect Prev.
27:229–238. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chen Y, Gou X, Ke X, Cui H and Chen Z:
Human tumor cells induce angiogenesis through positive feedback
between CD147 and insulin-like growth factor-I. PLoS One.
7:e409652012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Pedchenko V, Zent R and Hudson BG:
Alpha(v)beta3 and alpha(v)beta5 integrins bind both the proximal
RGD site and non-RGD motifs within noncollagenous (NC1) domain of
the alpha3 chain of type IV collagen: Implication for the mechanism
of endothelia cell adhesion. J Biol Chem. 279:2772–2780. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
McTaggart S and Al-Rubeai M: Retroviral
vectors for human gene delivery. Biotechnol Adv. 20:1–31. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Maeshima Y, Yerramalla UL, Dhanabal M,
Holthaus KA, Barbashov S, Kharbanda S, Reimer C, Manfredi M,
Dickerson WM and Kalluri R: Extracellular matrix-derived peptide
binds to alpha(v)beta(3) integrin and inhibits angiogenesis. J Biol
Chem. 276:31959–31968. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sund M, Hamano Y, Sugimoto H, Sudhakar A,
Soubasakos M, Yerramalla U, Benjamin LE, Lawler J, Kieran M, Shah A
and Kalluri R: Function of endogenous inhibitors of angiogenesis as
endothelium-specific tumor suppressors. Proc Natl Acad Sci USA.
102:2934–2939. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ye HX, Yao Y, Jiang XJ and Yuan XR:
Tumstatin transfected into human glioma cell line U251 represses
tumor growth by inhibiting angiogenesis. Chin Med J (Engl).
126:1720–1725. 2013.PubMed/NCBI
|
|
22
|
You Y, Xue X, Li M, Qin X, Zhang C, Wang
W, Giang C, Wu S, Liu Y, Zhu W, et al: Inhibition effect of
pcDNA-tum-5 on the growth of S180 tumor. Cytotechnology. 56:97–104.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang J: A study on the influence of
proliferation and apoptosis for Tum-5 gene to human gastric
carcinoma cell. Masters thesisFujian Medical University Fuzhou,
China: 2010, (In Chinese).
|
|
24
|
Jahroudi N and Greenberger JS: The role of
endothelial cells in tumor invasion and metastasis. J Neurooncol.
23:99–108. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang YH, Dong YY, Wang WM, Xie XY, Wang
ZM, Chen RX, Chen J, Gao DM, Cui JF and Ren ZG: Vascular
endothelial cells facilitated HCC invasion and metastasis through
the Akt and NF-κB pathways induced by paracrine cytokines. J Exp
Clin Cancer Res. 32:512013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang ZL, Zhang JF, Yuan YF, He YM, Liu
QY, Mao XW, Ai YB and Liu ZS: Suppression of angiogenesis and tumor
growth in vitroin vivo using an anti-angiopoietin-2 single-chain
antibody. Exp Ther Med. 7:543–552. 2014.PubMed/NCBI
|
|
27
|
Grothey A and Galanis E: Targeting
angiogenesis: Progress with anti-VEGF treatment with large
molecules. Nat Rev Clin Oncol. 6:507–518. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sugimachi K, Tanaka S, Taguchi K, Aishima
S, Shimada M and Tsuneyoshi M: Angiopoietin switching regulates
angiogenesis and progression of human hepatocellular carcinoma. J
Clin Pathol. 56:854–860. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hasan J, Byers R and Jayson GC:
Intra-tumoural microvessel density in human solid tumours. Br J
Cancer. 86:1566–1577. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Cârţână T, Săftoiu A, Gruionu LG, Gheonea
DI, Pirici D, Georgescu CV, Ciocâlteu A and Gruionu G: Confocal
laser endomicroscopy for the morphometric evaluation of
microvessels in human colorectal cancer using targeted anti-CD31
antibodies. PLoS One. 7:e528152012. View Article : Google Scholar : PubMed/NCBI
|