Introduction
Osteoporosis (OP) is a metabolic bone disease that
results from an imbalance between bone resorption and bone
formation. OP is associated with decreased bone mass and damage to
bone tissue microstructure that leads to an increased risk of
fractures (1). As the world
population ages, osteoporosis is becoming a global health
problem.
Several drugs with a demonstrated anti-fracturative
effects, achieved by inhibiting bone resorption or stimulating bone
formation, are currently available for the treatment of OP
(2). Their use, however, is not free
from limitations or side effects. Compounds extracted from
traditional Chinese medicines have been found to be safe and
effective for the treatment of OP (3).
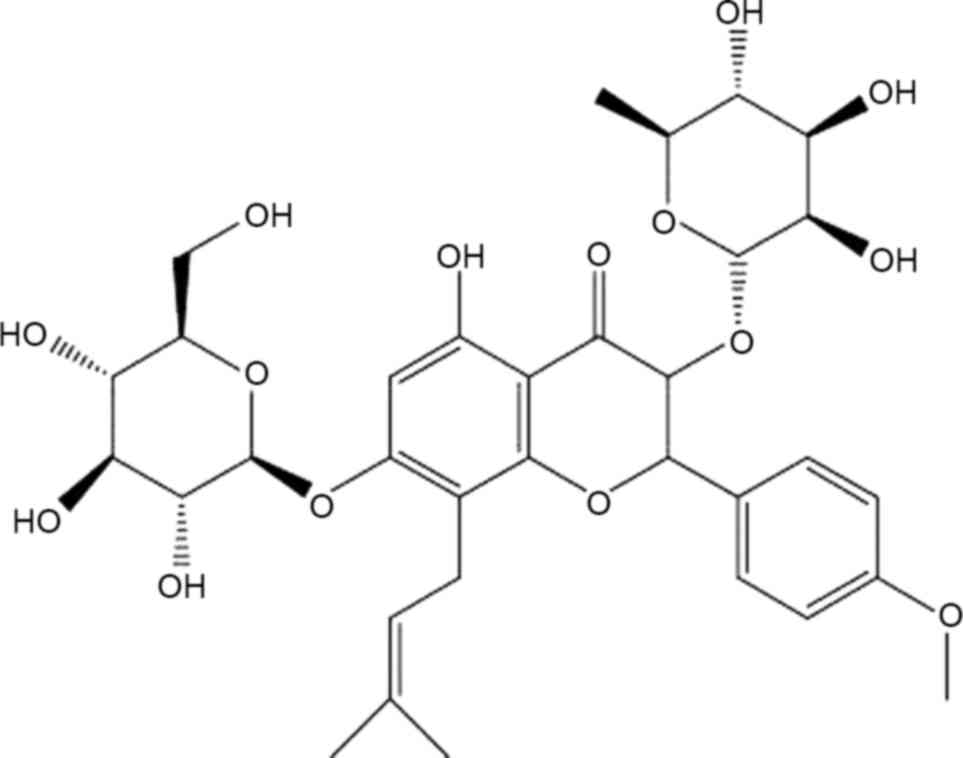
Icariin (ICA), a pharmacologically active prenylated
flavonoid glycoside (Fig. 1), is one
of the most abundant constituent in Herba Epimedii, a
medicinal plant that has been used to treat OP in traditional
Chinese medicine for thousands of years and is still currently used
(4). Recent studies have shown that
ICA exerts a variety of pharmacological activities, including
antioxidant activity (5),
immunoregulatory activity (6),
antitumor activity (7,8) and estrogen-like activities (9,10). The
promotion of osteogenesis (11) by
ICA may be associated with its estrogen-like structure (12,13); in
addition, ICA suppresses bone resorption and osteoclastogenesis
(14–16).
Osseous tissue originates from mesenchymal stem
cells (MSCs), which can differentiate into adipocytes or
osteoblasts (OB) (17). The Notch
signaling pathway is an important regulatory pathway that serves a
key role in bone metabolism. The Notch signaling pathway mediates
signaling between bone cells and is involved in the proliferation
differentiation processes of bone cells (18). Notch proteins directly enhance
osteogenic differentiation (19,20) and
indirectly suppress osteogenic differentiation by promoting
adipogenic differentiation (21,22),
resulting in a two-directional regulatory effect on the
differentiation of MSCs (21,22). To
date, however, little effort has been made to understand the effect
of ICA on the Notch signaling pathway. In the present study, the
effect of ICA on proteins in the Notch signaling pathway, and its
effect on target genes, is investigated in an ovariectomized (OVX)
rat model of OP.
Materials and methods
Animals
Specific pathogen-free (SPF) female Sprague-Dawley
rats (aged 3 months and weighing 250±20 g) were provided by
Guangdong Medical Laboratory Animal Center (Guangzhou, China). The
study was approved by the Laboratory Animal Ethics Committee of
Jinan University (ethical approval certificate no. SCXK
2013–0002).
Experimental medication
ICA (molecular weight, 676 g/mol; cat. no.
EPE-120215) was provided by the Changsha Green Vine Biological
Technology Co., Ltd. (Changsha, China). A reference sample (20 mg)
of ICA (cat. no. 110737–200415) was purchased from the Guangdong
Institute for Drug Control (Guangzhou, China). The ICA samples,
which are insoluble in water, were stored in brown bottles. The ICA
provided by the Changsha Green Vine Biological Technology Co., Ltd.
was 98% pure, as compared with the reference sample. The positive
control drug, Fosamax (cat. no. 130124), was purchased from MSD
Pharmaceutical Co., Ltd. (Hangzhou, China).
Reagents
An alkaline phosphatase assay kit (cat. no. A059-2)
was purchased from Nanjing Jiancheng Bioengineering Institute
(Nanjing, China). Notch1 intracellular domain (N1ICD) and Jagged1
polyclonal antibodies were purchased from Abcam (Cambridge, MA,
USA). Rabbit anti-rat GAPDH polyclonal antibody was purchased from
Cell Signaling Technology, Inc. (Danvers, MA, USA; cat. no. 2118).
Jagged2 polyclonal antibody was purchased from Merck Millipore
(Darmstadt, Germany; cat. no. NRG1764426). Bovine serum albumin was
purchased from Roche Diagnostics (Basel, Switzerland). PrimeScript
RT Reagent kit with gDNE Eraser (cat. no. AK3501) and RT-PCR
SYBR® (cat. no. AK4401) kits were purchased from TaKaRa
Biotechnology Co., Ltd. (Dalian, China). Phenylmethanesulfonyl
fluoride was purchased from Sigma-Aldrich (Merck Millipore). A
bicinchoninic acid (BCA) protein assay kit was purchased from
KeyGen Biotech. Co., Ltd. (Nanjing, China). An SDS-PAGE gel
preparation kit, SDS-PAGE protein loading buffer (5X) and SDS-PAGE
electrophoresis liquid were purchased from the Beyotime Institute
of Biotechnology (Haimen, China). Goat anti-rabbit secondary
antibody was purchased from EarthOx (Wuhan, China). Other
commercially available reagents and chemicals used in the study
were of analytical grade.
Instrumentation
The following equipment was used in the study: A
dual X-ray absorptiometer (DEXA; Lunar iDXA; GE Healthcare
Bio-Sciences, Pittsburgh, PA, USA), a microplate absorbance reader
(model 680; Bio-Rad Laboratories, Inc., Hercules, CA, USA), a micro
CT system (u-CT80; SCANCO Medical AG, Brüttisellen, Switzerland), a
multi-function biomechanics tester (MTS model 858; Bionix, Toledo,
OH, USA), an inverted phase contrast microscope (model CKX41;
Olympus Corporation, Tokyo, Japan), a fluorescence
spectrophotometer (NanoDrop 1000; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA), a G-Storm Gradient PCR thermal cycler (Veriti
96-Well; Applied Biosystems; Thermo Fisher Scientific, Inc.), a
quantitative fluorescence PCR system (Light Cycler® 480;
Roche Diagnostics) and a gel imaging system (Bio-Rad Laboratories,
Inc.).
Animal husbandry
SPF rats were reared by the Jinan University Medical
Laboratory Animal Center and had ad libitum access to water
and standard laboratory chow (1.01% Ca2+, 0.78%
P3+).
Experimental design
Eighty-four rats were randomly divided into an
ovariectomized (OVX) group (n=70) that would develop OP and a
sham-operated group (n=14). After 12 weeks, rats underwent a
dual-energy X-ray bone mineral density (BMD) test. Once the OP
model was successfully established as previously described
(23,24), the OVX group was randomly divided
into the following five groups of 14 rats: A no treatment group
(OVX-NT), a low-dose ICA group (ICA-L), a medium-dose ICA group
(ICA-M), a high-dose ICA group (ICA-H) and a Fosamax-treated
positive control group (FOS). The rats underwent treatment for 12
weeks.
ICA was dissolved in sodium carboxymethyl cellulose
and administered by oral gavage. Fosamax was dissolved in distilled
water and administered by oral gavage. The treatment regimens were
as follows (Table I): i)
Sham-operated, administered water (Sham group); ii) OVX,
administered water (OVX-NT group); iii) OVX, administered 125
mg/kg/day ICA (ICA-L group); iv) OVX, administered 250 mg/kg/day
ICA (ICA-M group); v) OVX, administered 500 mg/kg/day ICA (ICA-H
group); and vi) OVX, administered 0.514 mg/kg/day Fosamax (FOS
group).
 | Table I.Treatment groups. |
Table I.
Treatment groups.
| Group | Model | Drug | Dose
(mg/kg/day) | Dosing period
(weeks) | Administration |
|---|
| Sham | Sham | Water | – | 12 | Oral gavage |
| OVX-NT | OVX | Water | – | 12 | Oral gavage |
| ICA-L | OVX | ICA | 125 | 12 | Oral gavage |
| ICA-M | OVX | ICA | 250 | 12 | Oral gavage |
| ICA-H | OVX | ICA | 500 | 12 | Oral gavage |
| FOS | OVX | Fosamax | 0.514 | 12 | Oral gavage |
Dual-energy x-ray absorptiometry
(BMD)
After 12 weeks, BMD was tested by dual X-ray scans.
The rats were then anesthetized using pentobarbital sodium (0.15
ml/100 g; Vortech Pharmaceuticals, Ltd., Dearborn, MI, USA) and
whole body scans were conducted. Bone mineral densities of the
whole body, femora, tibias, and fourth and fifth lumbar vertebrae
(LV4, 5) were measured following sacrifice using an overdose of
pentobarbital sodium (0.4 ml/100 g).
Micro-CT
Tibia, femora and LV4, 5 were separated, dissected
free of soft tissues and stored at −80°C for subsequent analysis.
The microarchitecture of trabecular bone in the right proximal
femora and LV4 was analyzed by micro CT (60 KV, 50 W). The same
specimen was scanned to obtain different section images and the
distal femoral stem epiphyseal and vertebral scans were performed
in three spatial dimensions. Micro View software (version 4.1;
Scanco Medical AG, Wangen-Bruttisellen, Switzerland) was used to
calculate the following parameters: Trabecular thickness,
trabecular number and trabecular separation.
Immunohistochemical staining
One third of the right distal femur was fixed with
4% paraformaldehyde and decalcified using the Aojiang
decalcification method as follows: The femur was fixed in 20% EDTA
for decalcification at 4°C, dehydrated and embedded in paraffin.
Five micron paraffin sections were prepared for immunohistochemical
staining. Slices were dewaxed using xylene and hydrated with
gradient alcohol. Endogenous peroxidase activity was quenched
(using 3% hydrogen peroxide) and the slices were then incubated
with Jagged1 primary antibody (1:1,000) at 37°C for 1 h. The slices
were then washed three times with phosphate buffer, incubated with
streptavidin-horseradish peroxidase (HRP)-conjugated anti-rabbit
secondary antibody (1:2,000; cat. no. 7074S; Cell Signaling
Technology, Inc.) at 37°C for 10 min and washed another three times
with phosphate buffer. Diaminobenzidine-colored slices were then
stained with hematoxylin and dehydrated using alcohol. Xylene was
added and the slices were sealed using neutral gum. A light
microscope was used to view the stains.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Chopped rat femora were treated with liquid nitrogen
and ground to a powder. Total RNA was extracted from the femora by
triturating several times with TRIzol reagent (Thermo Fisher
Scientific, Inc.) and allowed to stand at room temperature for 5
min. Chloroform (1/5 of the volume of Trizol) was added and the
sample was blended in a vortex mixer. After standing at room
temperature for 5 min, the mixture was centrifuged (12,000 ×
g) for 5 min at 4°C. The top 70% of the aqueous phase (0.5
ml) was transferred to an Eppendorf tube and shaken with isopropyl
alcohol (0.25 ml), 0.8 M aqueous sodium citrate solution (0.125 ml)
and 0.125 M aqueous NaCl solution (0.8 ml). After standing at room
temperature for 10 min, the mixture was centrifuged (12,000 ×
g) for 15 min at 4°C. After washing twice with 75% ethanol,
the sediment was dissolved in diethylpyrocarbonate (DEPC)-treated
water (20 µl). Avoiding bubbles, RNA samples (1 µl) were placed in
the spectrophotometer and the ratio (A260/A280) of absorbance at
260 nm (A260) and 280 nm (A280) was determined to provide an
assessment of purity. Total RNA (1 µg) was reverse transcribed into
cDNA. Template DNA was used in gene-specific PCR for PPARγ, C/EBPα,
FABP4, Notch2 and GAPDH mRNA. Details of the primers are listed in
Table II. qPCR for gene expression
was performed in 96-well plates with a total reaction volume of 20
µl per well, comprising 2x SYBR green master mix, diluted gene
primers (10 µl), cDNA (2 µl), forward primer (0.8 µl) or reverse
primer (0.8 µl), and DEPC-treated water (6.4 µl). Quantitative
analysis was performed using a Roche LightCycler 480 Sequence
Detection System. Operating conditions were 95°C for 30 sec, 95°C
for 5 sec and 60°C for 30 sec, with a total of 40 cycles. The
fluorescence signal was collected at the end of the second step of
each cycle. Each sample was analyzed in triplicate and the average
Cq was calculated. Gene expression was analyzed using the
2−ΔΔCq quantification approach (25).
 | Table II.Primer sequences for quantitative
fluorescence polymerase chain reaction. |
Table II.
Primer sequences for quantitative
fluorescence polymerase chain reaction.
| Gene name | Primer sequence
(5′-'3) |
|---|
| PPARγ-sense |
ACCCTTTACCACGGTTGATTTCTC |
|
PPARγ-antisense |
CAGGCTCTACTTTGATCGCACTTT |
| C/EBPα-sense |
GCGCAAGAGCCGAGATAAAG |
|
C/EBPα-antisense |
CGTGTCCAGTTCACGGCTCA |
| FABP4-sense |
ACATGAAAGAAGTGGGAGTTGGC |
|
FABP4-antisense |
AAGTACTCTCTGACCGGATGACG |
| Notch2-sense |
AGTGGTATGGACTGTGAGGAGG |
|
Notch2-antisense |
CAGGAGAAGGTGTTCACTTTGTC |
| GAPDH-sense |
CAACGGGAAACCCATCACCA |
|
GAPDH-antisense |
ACGCCAGTAGACTCCACGACAT |
Western blotting analysis
Rat femora were subjected to cell lysis to extract
proteins. The concentration of total protein was determined using a
BCA protein assay kit. Proteins (30 µg) were separated by 12%
SDS-PAGE and transferred onto polyvinylidene difluoride membranes.
Membranes were blocked with a buffer containing 0.05% Tween-20 and
5% defatted milk and reacted sequentially with primary antibodies
against GAPDH and N1ICD (1:1,000) for 10 h at 4°C and
HRP-conjugated anti-rabbit secondary antibody (1:3,000) for 1 h at
25°C. The membranes were washed and rinsed with enhanced
chemiluminescence (ECL) detection reagents (EMD Millipore,
Billerica, MA, USA). The band images were photographed using ECL.
Immunoreactive bands were visualized using ECL substrates and an
X-ray film processor. Protein expression was calculated using
Quantity One® software (version 6.0; Media Cybernetics,
Inc., Rockville, MD, USA).
Statistical analysis
Values are expressed as the mean ± standard
deviation. The significance of the difference between two
experimental groups was estimated by one-way analysis of variance.
P<0.05 was considered to indicate a statistically significant
difference. All statistical evaluations were performed using SPSS
version 19.0 (IBM SPSS, Amronk, NY, USA).
Results
Establishment of the OP model
Twelve weeks after OVX, BMD of the LV4, 5, right
femur and left femur was significantly reduced in the OVX-NT group
compared with the sham-operated group (P<0.05), demonstrating
that the OP model had been established successfully (Table III).
 | Table III.Comparisons with the sham-operated
group. |
Table III.
Comparisons with the sham-operated
group.
| Group | LV4, 5 | Right femur | Left femur |
|---|
| Sham | 0.285±0.009 | 0.340±0.020 | 0.310±0.013 |
| OVX-NT |
0.212±0.006a |
0.262±0.006a | 0.247±
0.005a |
ICA treatment increases BMD
BMD was measured using dual-energy X-ray
absorptiometry. BMD was significantly lower (P<0.05) in the
right distal femora, left primal femora and whole body bone regions
in the OVX-NT group compared with the sham-operated group. In
addition, BMD in the lumbar spine and right proximal femora were
significantly reduced compared with the sham-operated group
(P<0.01). BMD of the lumbar spine, the left proximal femora and
right proximal femora in the FOS and ICA groups were significantly
increased compared with the OVX-NT group (P<0.05 and P<0.01),
except the ICA-H group in the lumbar spine. BMD in the ICA-M group
showed the most significant difference compared with the OVX-NT
group (P<0.01) (Fig. 2).
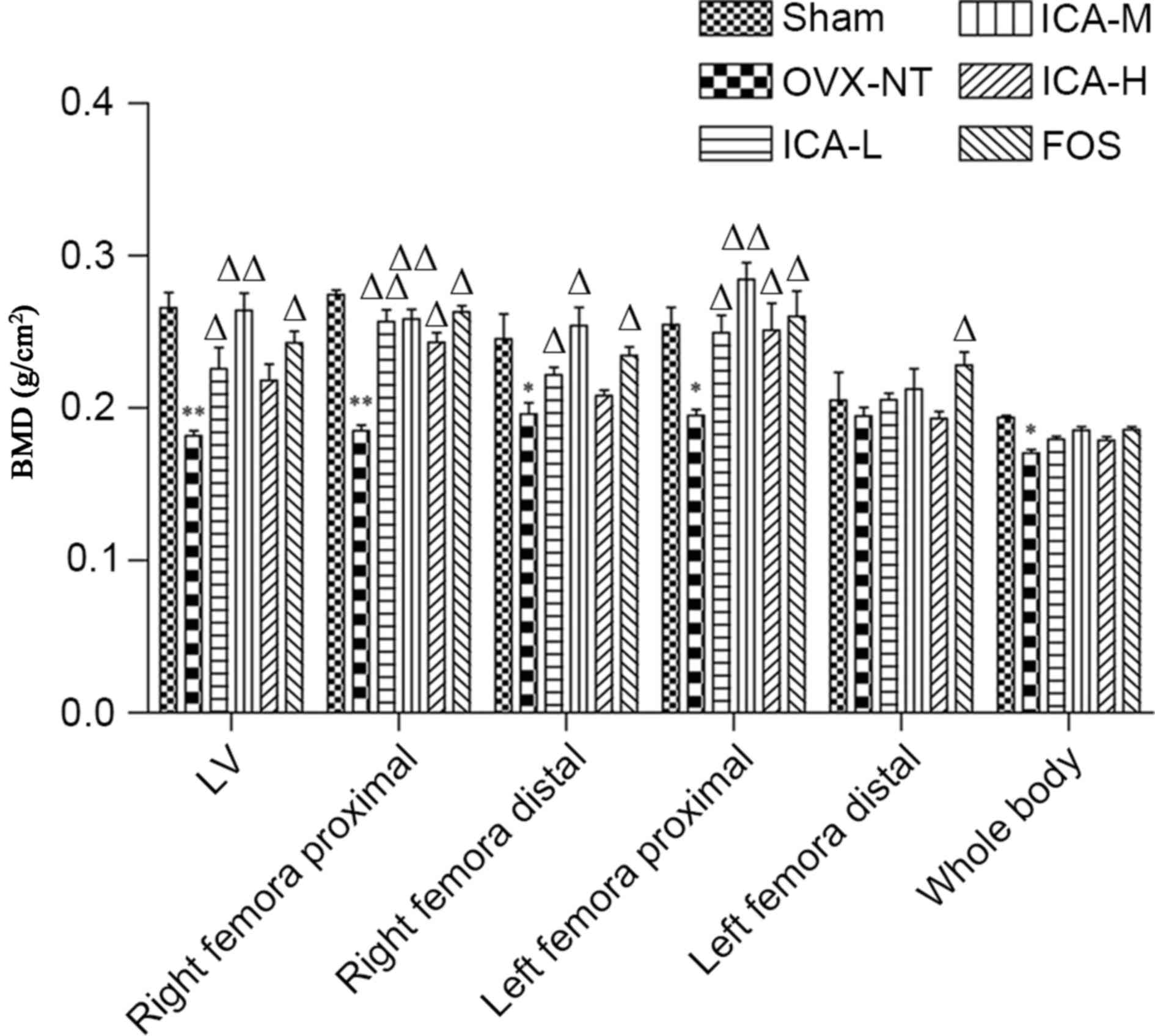 | Figure 2.ICA treatment increases BMD. After 12
weeks of treatment, mice were anesthetized and whole body scans
were conducted. BMD of the whole body, femora and tibias, as well
as the fourth and fifth lumbar vertebrae, were measured.
*P<0.05, **P<0.01 vs. the sham-operated group;
∆P<0.05, ∆∆P<0.01 vs. the OVX-NT group.
BMD, bone mineral density; OVX-NT, ovariectomized-no treatment
group; ICA-L, low-dose icariin group; ICA-M, medium-dose icariin
group; ICA-H, high-dose icarrin group; FOS, Fosamax-treated
positive control group; LV, lumbar vertebrae. |
ICA treatment improves bone
trabeculae
Micro CT showed that the bone trabecular number in
the right distal femora and LV4 was higher, and that the bone
trabecular separation degree was smaller, in the sham-operated
group compared with the OVX-NT group. In the OVX-NT group, the bone
trabeculae were rod-shaped, thinner and fractured, and the bone
trabecular separation was increased. Compared with the OVX-NT
group, bone trabecular number was higher in the treated groups,
particularly in the ICA-M and FOS groups. In the treated groups,
bone trabecular thickness tended towards that in the sham-operated
group. Although bone trabecular separation was reduced, some
trabecular bone was missing. Bone trabecular separation increased
in ICA-L group, but there displayed some improvement compared with
the OVX-NT group (Fig. 3).
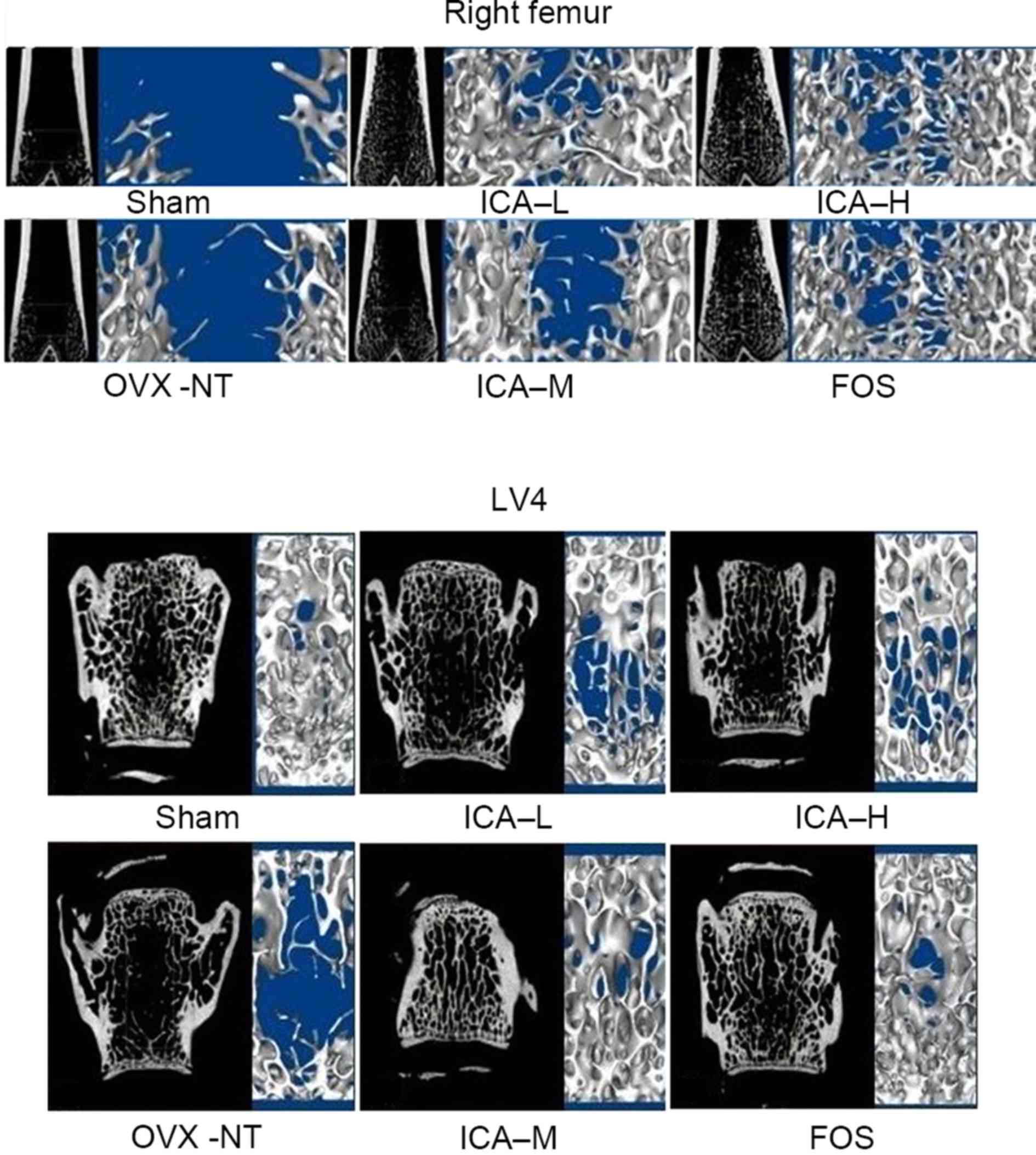 | Figure 3.ICA treatment improves bone
trabeculae. Microarchitecture of trabecular bone was analyzed using
micro computed tomography. Bone trabecular number in the right
distal femora and LV4 increased, and the degree of bone trabecular
separation became smaller in the sham-operated group. Bone
trabeculae were rod-shaped, thinner, and fractured and bone
trabecular separation increased in the OVX-NT group. Compared with
the OVX-NT group, bone trabecular number increased in the ICA-L,
ICA-M, ICA-H and FOS groups, particularly in the ICA-M and FOS
groups, where bone trabecular thickness tended towards that of the
sham-operated group. Although bone trabecular separation was
reduced, some trabecular bone was missing; bone trabecular
separation increased in the ICA-L group, but there was some
improvement compared with the OVX-NT group. OVX, ovariectomized
group; OVX-NT, OVX-no treatment group; ICA-L, low-dose icariin
group; ICA-M, medium-dose icariin group; ICA-H, high-dose icarrin
group; FOS, Fosamax-treated positive control group; LV4, fourth
lumar vertebra. |
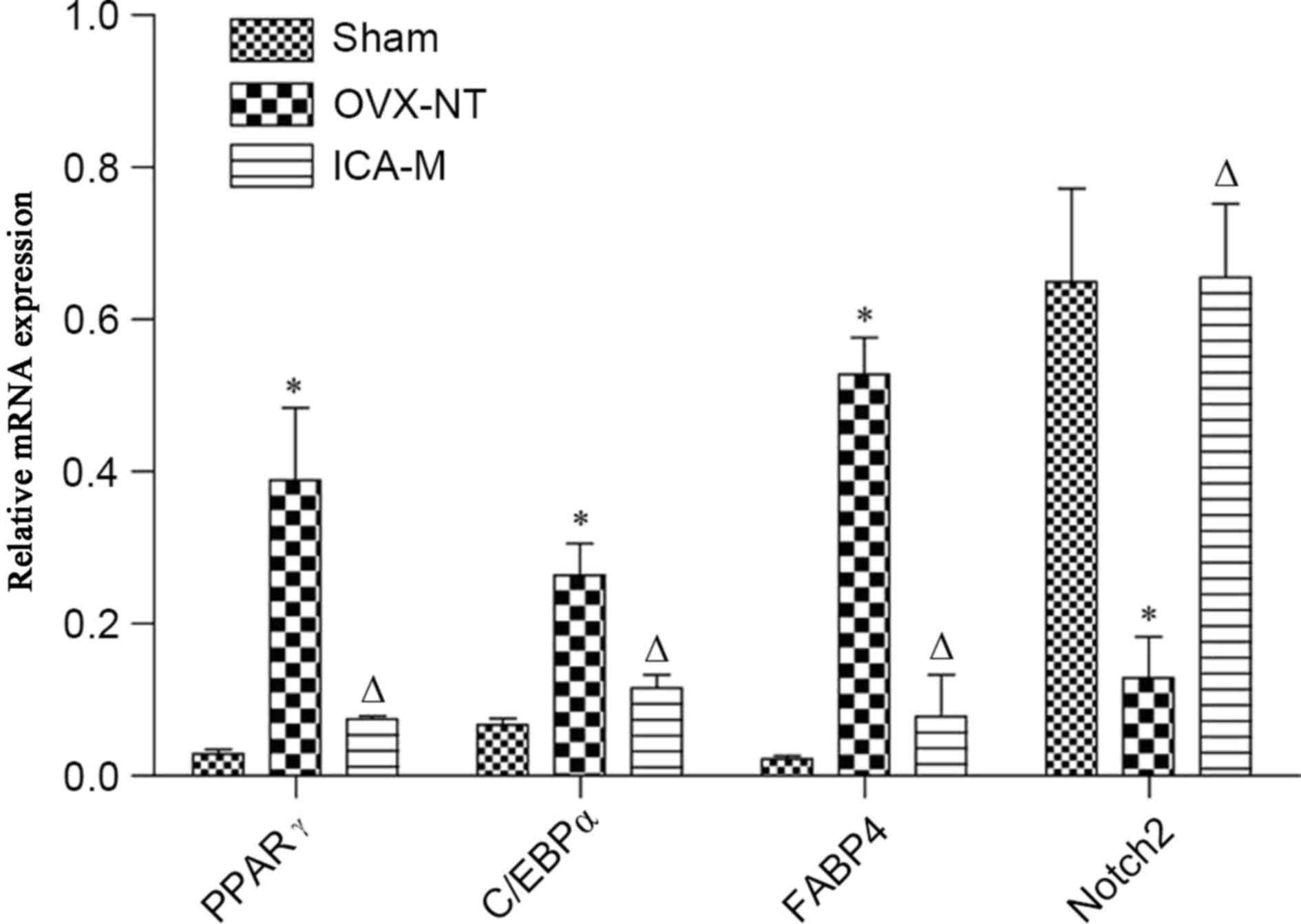
ICA inhibits the expression of PPARγ,
C/EBPα, and FABP4 mRNA and increases Notch2 mRNA
Compared with the sham-operated group, the
expression of PPARγ, C/EBPα and FABP4 mRNA was significantly
increased (P<0.05) and the expression of Notch2 mRNA was
decreased in the OVX-NT group (P<0.05). Compared with the OVX-NT
group, the expression of PPARγ, C/EBPα and FABP4 mRNA expression
was significantly decreased (P<0.05) and Notch2 mRNA expression
was significantly increased in the ICA-M group (P<0.05)
(Fig. 4).
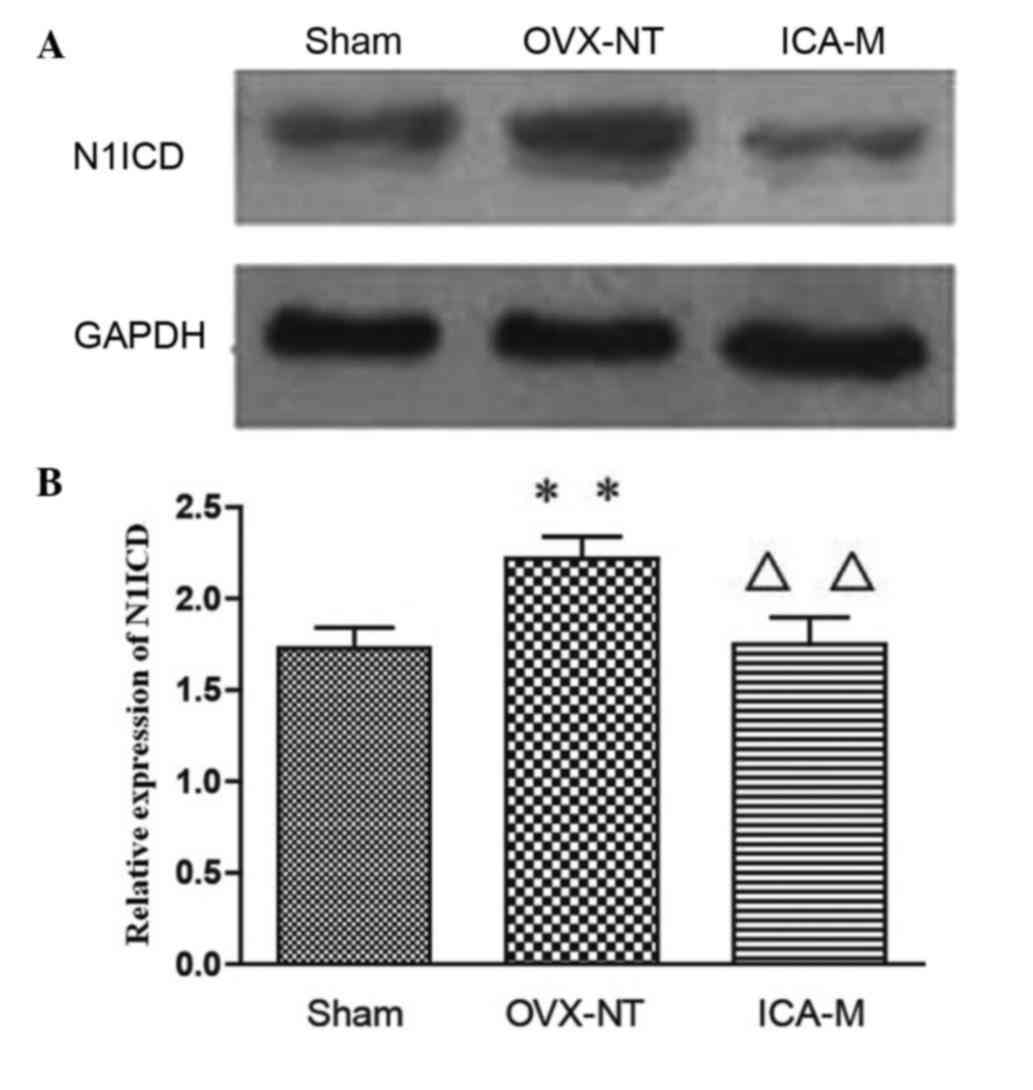
ICA inhibits the expression of N1ICD
protein
Western blotting showed that, compared with the
sham-operated group, expression of N1ICD was significantly
increased (P<0.05) in the OVX-NT group. Compared with the OVX-NT
group, expression of N1ICD decreased in the ICA-M group (P<0.01)
(Fig. 5).
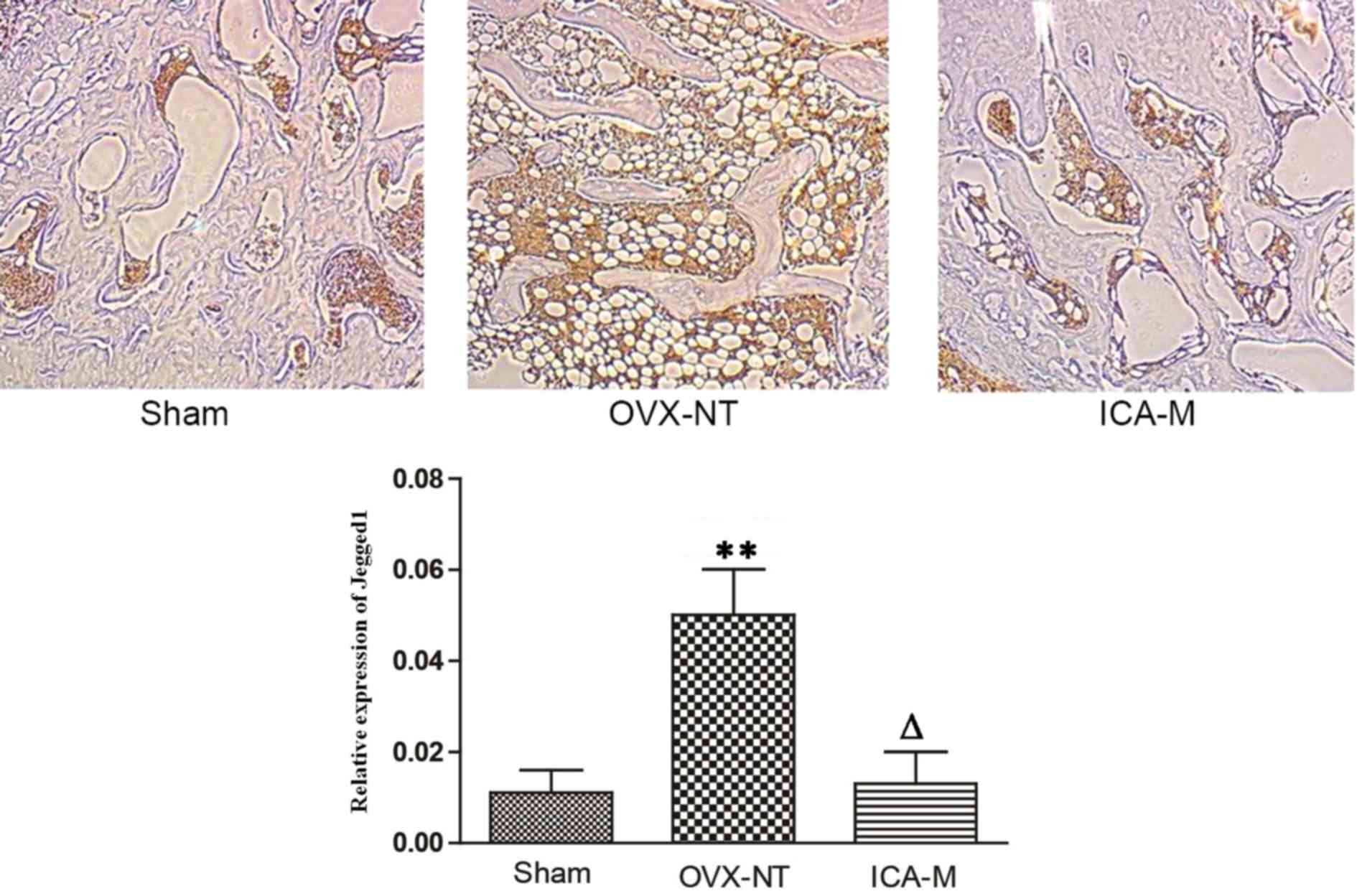
ICA inhibits the expression of Jagged1
protein
Immunohistochemistry showed that Jagged1 was
distributed in the bone marrow cavity on fat cell membranes.
Compared with the sham-operated group, the expression of Jagged1
protein increased in the OVX-NT group and, compared with the OVX-NT
group, the expression of Jagged1 protein decreased in the ICA-M
group (Fig. 6). The average density
was determined using Image-Pro Plus version 6.0 image analysis
software (Media Cybernetics, Inc.). Compared with the sham-operated
group, the expression of Jagged1 protein increased significantly in
the OVX-NT group (P<0.01) and, compared with the OVX-NT group,
expression of Jagged1 protein decreased in the ICA-M group.
Discussion
Despite the lack of a clearly defined
pharmacological mechanism of action, previous studies have
demonstrated that ICA, extracted from the dried leaves of the
medicinal plant Herba Epimedii, stimulates osteogenic
differentiation in vitro and prevents bone loss in
vivo (26–31). Because of its low toxicity and
favorable side effect profile, ICA would be an attractive and
promising candidate for the treatment and prevention of OP
(30). The present study helps to
explain the pharmacological mechanism of action of ICA in
preventing bone loss in OVX rats.
Fosamax was chosen as the positive control since it
is known to increase bone mineral density (31). The present study shows that ICA
effectively reduces bone mass loss in OVX rats, increases bone
trabecular number and thickness, reduces the degree of separation
of trabecular bone, and improves its morphological structure. ICA-M
showed the most pronounced effect on these indices, indicating that
ICA-M has the greatest therapeutic effect in osteoporosis and,
perhaps, suggesting a bell-shaped dose-response curve.
A reduced capacity of MSCs for osteogenesis and an
increased capacity for adipogenesis, which results in an imbalance
between bone resorption and bone formation, serves an important
role in the pathogenesis of OP (32). These biological processes are
partially regulated by the activation of the Notch signaling
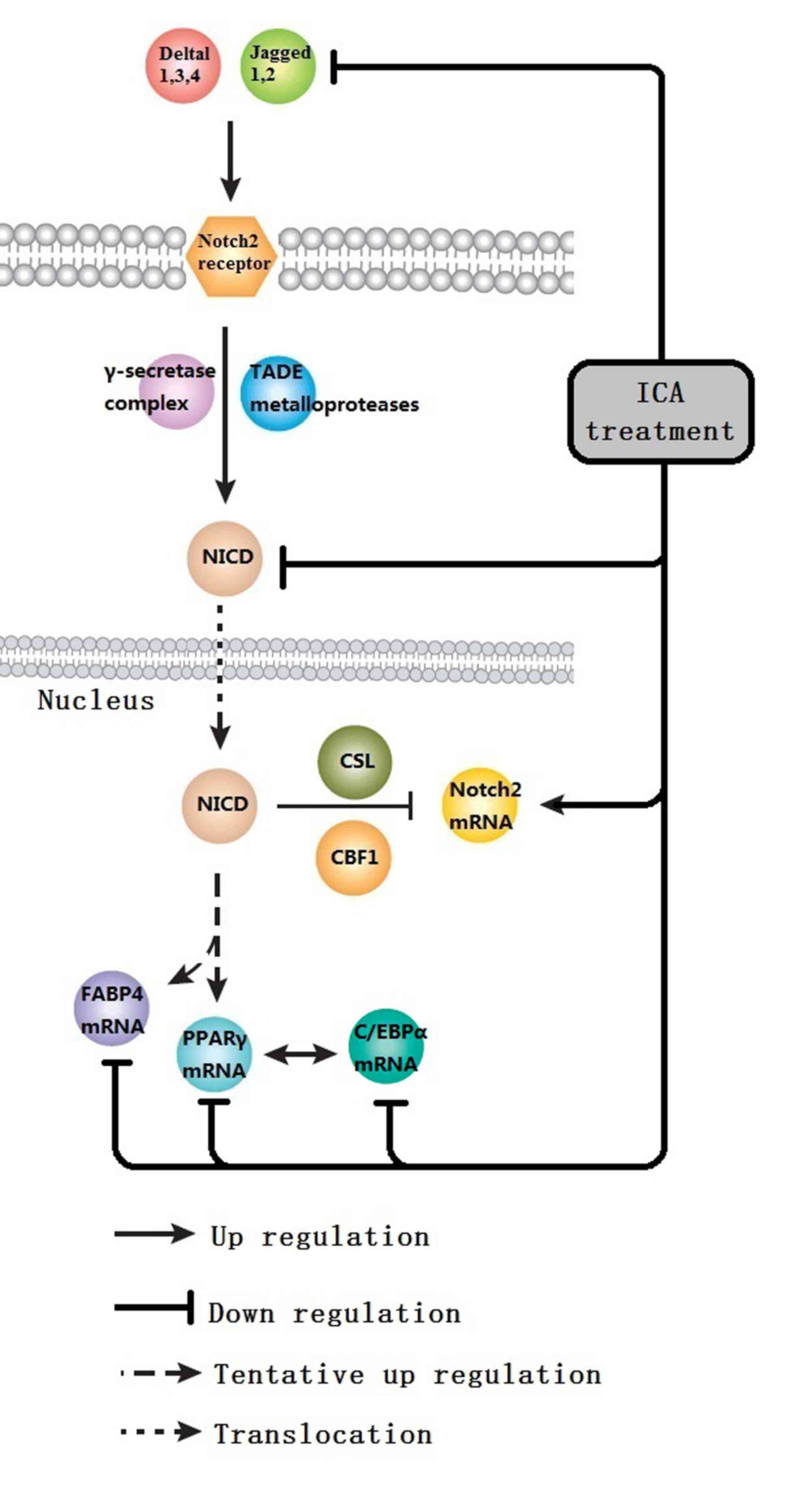
pathway, the primary focus of the present study.
The Notch receptor family consists of four
receptors: Notch 1, 2, 3 and 4. Notch ligands are transmembrane
proteins with conserved structures. In mammals, there are five
known Notch ligands: Delta 1, 3 and 4 and Jagged1 and 2. Ligand
binding to receptors results in successive proteolytic cleavage
mediated by TADE metalloproteases and a γ-secretase complex.
Cleavage of the Notch receptor results in the release of a
constitutively active Notch intracellular domain (NICD) that
translocates to the nucleus, where it binds with the transcription
complex CSL/CBF1. NICD switches the CSL/CBF1 complex from a
repressed to an activated state, which promotes cell
differentiation, proliferation and apoptosis. Campa et al
(33) showed that the Notch1-Jagged1
pathway is active in MSCs during OB differentiation, indicating a
regulatory role for Notch signaling in OB differentiation. Jagged1
is an essential ligand for activation of Notch in the early stages
of chondrogenesis, but expression of Jagged1 is downregulated at
later stages of the process (34).
NICD is one of the nuclear signaling molecules that suppresses
differentiation of OB. Transfection with Jagged1 and Delta1 genes
enhances the activity of alkaline phosphatase and increases
mineralization (19). This improves
differentiation of mouse embryo MSCs through osteoblast induction,
and suppresses the expression of lipogenic genes (such as FABP4 and
PPARγ) in MSCs through promotion of adipogenesis (35). Bai et al (36) identified that knockout of Notch1,
Notch2 and Notch3 in bone macrophagocytes increases the
differentiation of mononuclear macrophages into OC, and that
deficiency of Notch1 reduces the release of osteoprotegerin.
Activation of Notch signaling thus enhances the differentiation of
MSCs into adipocytes and suppresses their differentiation into OB
(21,22).
The present study shows that ICA treatment
suppresses the expression of N1ICD and Jagged1 proteins, and
promotes the expression of Notch2 mRNA. N1ICD is the active
intracellular form of the Notch1 receptor, which serves an
important role within the cell by regulating downstream target
genes (37,38). This suggests that the beneficial
effect of ICA on OP may be associated with the regulation of Notch
signaling, which increases the expression of adipocyte
differentiation transcription factors. The results of the present
study agree with those of Zanotti et al (39), which showed that an increase in N1ICD
reduces the expression of Notch2 mRNA. It is proposed, therefore,
that Notch2 depresses Notch signaling, through negative feedback of
Notch1.
PPARγ and C/EBP family proteins are two of the
primary transcription factors that directly affect preadipocyte
proliferation and differentiation. Ugarte et al (40) identified that the enhancement of
Notch signaling suppresses the differentiation of MSCs into
adipocytes by inhibition of PPARγ and FABP4 gene expression. The
inhibition of osteogenesis is possibly associated with PPARγ, one
of the important factors controlling adipogenic differentiation.
Once activated, PPARγ can spontaneously initiate the process of
adipogenic differentiation (19).
C/EBPα was the first transcription factor proven to serve an
important role in the process of adipocyte differentiation
(41). Additionally, there is a
synergistic interaction between C/EBPα and PPARγ. PPARγ activates
C/EBPα expression, and C/EBPα has a positive feedback effect on
PPARγ. The combination of C/EBPα and PPARγ activates the expression
of genes associated with differentiation. The expression of PPARγ
mRNA and C/EBPα mRNA directly reflects the adipocyte
differentiation status of MSCs.
In the present study, the expression of PPARγ,
C/EBPα and FABP4 mRNA were significantly reduced following
treatment with ICA (250 mg/kg/day). This is in agreement with a
study by Lewis et al (42),
which showed that PPARγ, C/EBPα and FABP4 mRNA are significantly
increased in animals with OP compared with normal animals. The
pathogenesis of OP was thus shown to be closely associated with
enhanced differentiation of MSCs into adipocytes and suppressed
differentiation of MSCs into OB. The results of the current study
suggest that ICA has a beneficial effect on OP by suppressing
differentiation of MSCs into adipocytes through reduced expression
of mRNA for adipogenesis correlation factors, PPARγ, C/EBPα and
FABP4.
In conclusion, the current study demonstrated that
ICA has a beneficial effect on OP rats, with 250 mg/kg/day being
the most effective of the doses examined. With its good safety
profile, ICA could be a promising candidate for further development
as a way of treating and preventing OP. ICA is, however, likely to
inhibit differentiation of MSCs into adipocytes by suppressing the
expression of PPARγ, C/EBPα and FABP4 mRNA. ICA may also inhibit
Notch2 mRNA expression through the inhibition of N1ICD expression.
Further preclinical investigations will be required to better
define the pharmacological targets of ICA and to dissect the
associations between the different signaling pathways involved in
the treatment of OP (Fig. 7).
Acknowledgements
The authors thank the International Science Editing
Compuscript, Ltd. (Shannon, Ireland) for critically reading and
checking the first draft. The Lunar Prodigy dual-energy X-ray
absorptiometer used in this study was a generous gift from Dr Jian
Gong of the Overseas Hospital and The First Affiliated Hospital of
Jinan University (Guangzhong, China). The present study was
supported by the National Natural Science Foundation of China
(grant nos. 81173619 and 81473509) and Natural Science Foundation
of Guangdong Province, China (grant no. S2012040007531).
References
|
1
|
United States Food and Drug Association,
Division of Metabolism and Endocrine Drug Products, . Guidelines
for Preclinical and Clinical Evaluation of Agents Used in the
Prevention or Treatment of Postmenopausal Osteoporosis. Food and
Drug Administration, USA. 1997.
|
|
2
|
Cairoli E, Zhukouskaya VV, Eller-Vainicher
C and Chiodini I: Perspectives on osteoporosis therapies. J
Endocrinol Invest. 38:303–311. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
An J, Yang H, Zhang Q, Liu C, Zhao J,
Zhang L and Chen B: Natural products for treatment of osteoporosis:
The effects and mechanisms on promoting osteoblast-mediated bone
formation. Life Sci. 147:46–58. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Indran IR, Liang RL, Min TE and Yong EL:
Preclinical studies and clinical evaluation of compounds from the
genus Epimedium for osteoporosis and bone health. Pharmacol Ther.
162:188–205. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sze SC, Tong Y, Ng TB, Cheng CL and Cheung
HP: Herba Epimedii: Anti-oxidative properties and its medical
implications. Molecules. 15:7861–7870. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Guo J, Li F, Wu Q, Gong Q, Lu Y and Shi J:
Protective effects of icariin on brain dysfunction induced by
lipopolysaccharide in rats. Phytomedicine. 17:950–955. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li S, Dong P, Wang J, Zhang J, Gu J, Wu X,
Wu W, Fei X, Zhang Z, Wang Y, et al: Icariin, a natural flavonol
glycoside, induces apoptosis in human hepatoma SMMC-7721 cells via
a ROS/JNK-dependent mitochondrial pathway. Cancer Lett.
298:222–230. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhou J, Wu J, Chen X, Fortenbery N,
Eksioglu E, Kodumudi KN, Pk EB, Dong J, Djeu JY and Wei S: Icariin
and its derivative, ICT, exert anti-inflammatory, anti-tumor
effects, and modulate myeloid derived suppressive cells (MDSCs)
functions. Int Immunopharmacol. 11:890–898. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang Z, Zhang X, Wang H, Qi L and Lou Y:
Neuroprotective effects of icaritin against beta amyloid-induced
neurotoxicity in primary cultured rat neuronal cells via
estrogen-dependent pathway. Neuroscience. 145:911–922. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yang L, Lu D, Guo J, Meng X, Zhang G and
Wang F: Icariin from Epimedium brevicornum Maxim promotes the
biosynthesis of estrogen by aromatase (CYP19). J Ethnopharmacol.
145:715–721. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Qin L, Han T, Zhang Q, Cao D, Nian H,
Rahman K and Zheng H: Antiosteoporotic chemical constituents from
Er-Xian Decoction, a traditional Chinese herbal formula. J
Ethnopharmacol. 118:271–279. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang G, Qin L, Sheng H, Yeung KW, Yeung
HY, Cheung WH, Griffith J, Chan CW, Lee KM and Leung KS:
Epimedium-derived phytoestrogen exert beneficial effect on
preventing steroid-associated osteonecrosis in rabbits with
inhibition of both thrombosis and lipid-deposition. Bone.
40:685–692. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ye HY and Lou YJ: Estrogenic effects of
two derivatives of icariin on human breast cancer MCF-7 cells.
Phytomedicine. 12:735–741. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yamaguchi M and Gao YH: Inhibitory effect
of genistein on bone resorption in tissue culture. Biochem
Pharmacol. 55:71–76. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen KM, Ge BF, Liu XY, Ma PH, Lu MB, Bai
MH and Wang Y: Icariin inhibits the osteoclast formation induced by
RANKL and macrophage-colony stimulating factor in mouse bone marrow
culture. Pharmazie. 62:388–391. 2007.PubMed/NCBI
|
|
16
|
Huang J, Yuan L, Wang X, Zhang TL and Wang
K: Icaritin and its glycosides enhance osteoblastic, but suppress
osteoclastic, differentiation and activity in vitro. Life Sci.
81:832–840. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Titorencu I, Pruna V, Jinga VV and
Simionescu M: Osteoblast ontogeny and implications for bone
pathology: An overview. Cell Tissue Res. 355:23–33. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Grogan SP, Olee T, Hiraoka K and Lotz MK:
Repression of chondrogenesis through binding of notch signaling
proteins HES-1 and HEY-1 to N-box domains in the COL2A1 enhancer
site. Arthritis Rheum. 58:2754–2763. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Nobta M, Tsukazaki T, Shibata Y, Xin C,
Moriishi T, Sakano S, Shindo H and Yamaguchi A: Critical regulation
of bone morphogenetic protein-induced osteoblastic differentiation
by Delta1/Jagged1 activated Notch1 signaling. J Biol Chem.
280:15842–15848. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Garcés C, Ruiz-Hidalgo MJ, de Mora J Font,
Park C, Miele L, Goldstein J, Bonvini E, Porrás A and Laborda J:
Notch-1 controls the expression of fatty acid-activated
transcription factors and is required for adipogenesis. J Biol
Chem. 272:29729–29734. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Akune T, Ohba S, Kamekura S, Yamaguchi M,
Chung UI, Kubota N, Terauchi Y, Harada Y, Azuma Y, Nakamura K, et
al: PPARgamma insufficiency enhances osteogenesis through
osteoblast formation from bone marrow progenitors. J Clin Invest.
113:846–855. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Teitelbaum SL: Osteoclasts: What do they
do and how do they do it? Am J Pathol. 170:427–435. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Devlin H and Ferguson MW: Compositional
changes in rat femur following ovariectomy. Acta Anat (Baslel).
136:38–41. 1989. View Article : Google Scholar
|
|
24
|
Kalu DN: Evaluation of the pathogenesis of
skeletal changes in ovariectomized rats. Endocrinology.
115:507–512. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-tie quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Mok SK, Chen WF, Lai WP, Leung PC, Wang
XL, Yao XS and Wong MS: Icariin protects against bone loss induced
by oestrogen deficiency and activates oestrogen receptor-dependent
osteoblastic functions in UMR 106 cells. Br J Pharmacol.
159:939–949. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Nan H, Ma MH, Nan SS and Xu LL:
Antiosteoporotic activity of icariin in ovariectomized rats.
Phytomedicine. 16:320–326. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Feng R, Feng L, Yuan Z, Wang D, Wang F,
Tan B, Han S, Li T, Li D and Han Y: Icariin protects against
glucocorticoid-induced osteoporosis in vitro and prevents
glucocorticoid-induced osteocyte apoptosis in vivo. Cell Biochem
Biophys. 67:189–197. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ma HP, Ming LG, Ge BF, Zhai YK, Song P,
Xian CJ and Chen KM: Icarrin is more potent than genistein in
promoting osteoblast differentiation and mineralization in vitro. J
Cell Biochem. 112:916–923. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhao J, Ohba S, Shinkai M, Chung UI and
Nagamune T: Icariin induces osteogenic differentiation in vitro in
a BMP- and Runx2-dependent manner. Biochem Biophys Res Commun.
369:444–448. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Vladyslav V Povoroznyuk, Nikonenko Pavel I
and Grygoryeva Natalija V: Bone. 42:1:S83. 2008. View Article : Google Scholar
|
|
32
|
Peng S, Zhang G, He Y, Wang X, Leung P,
Leung K and Qin L: Epimedium-derived flavonoids promote
osteoblastogenesis and suppress adipogenesis in bone marrow stromal
cells while exerting an anabolic effect on osteoporotic bone. Bone.
45:534–544. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Campa VM, Gutiérrez-Lanza R, Cerignoli F,
Díaz-Trelles R, Nelson B, Tsuji T, Barcova M, Jiang W and Mercola
M: Notch activates cell cycle reentry and progression in quiescent
cardiomyocytes. J Cell Biol. 183:129–141. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Augello A and De Bari C: The regulation of
differentiation in mesenchymal stem cells. Hum Gene Ther.
21:1226–1238. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Huang Y, Yang X, Wu Y, Jing W, Cai X, Tang
W, Liu L, Liu Y, Grotkau BE and Lin Y: Gamma-secretase inhibitor
induces adipogenesis of adipose-derived stem cells by regulation of
Notch and PPAR-gamma. Cell Prolif. 43:147–156. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Bai S, Kopan R, Zou W, Hilton MJ, Ong CT,
Long F, Ross FP and Teitelbaum SL: NOTCH1 regulates
osteoclastogenesis directly in osteoclast precursors and indirectly
via osteoblast lineage cells. J Biol Chem. 283:6509–6518. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Fiúza UM and Arias AM: Cell and molecular
biology of Notch. J Endocrinol. 194:459–474. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Weinmaster G: Notch signal transduction: A
real rip and more. Curr Opin Genet Dev. 10:363–369. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zanotti S, Smerdel-Ramoya A, Stadmeyer L,
Durant D, Radtke F and Canalis E: Notch inhibits osteoblast
differentiation and causes osteopenia. Endocrinology.
149:3890–3899. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ugarte F, Ryser M, Thieme S, Fierro FA,
Navratiel K, Bornhäuser M and Brenner S: Notch signaling enhances
osteogenic differentiation while inhibiting adipogenesis in primary
human bone marrow stromal cells. Exp Hematol. 37:867–875.e1. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kim HL, Sim JE, Choi HM, Choi IY, Jeong
MY, Park J, Jung Y, Youn DH, Cho JH, Kim JH, et al: The AMPK
pathway mediates an anti-adipogenic effect of fruits of Hovenia
dulcis Thunb. Food Funct. 5:2961–2968. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Lewis J, Hanisch A and Holder M: Notch
signaling, the segmentation clock, and the patterning of vertebrate
somites. J Biol. 8:442009. View Article : Google Scholar : PubMed/NCBI
|