Introduction
Pre-diabetes (‘intermediate hyperglycaemia’) is
based on glycaemic parameters above normal but below diabetes
thresholds considered to be a high-risk state for diabetes with an
annualized conversion rate of 5–10%, with a similar proportion
converting back to normoglycaemia (1). Multifactorial risk scores may optimize
the estimation of diabetes risk using non-invasive parameters and
blood-based metabolic features in addition to glycaemic values. For
prediabetic individuals, lifestyle modification is the cornerstone
of diabetes prevention.
At present, the prevalence of diabetes mellitus (DM)
and type 2 DM (T2DM) in children has significantly increased
(2). To the best of our knowledge,
few reports have focused on early intervention for children with DM
and even literature based on pre-diabetic children intervention
with regard to lifestyle is limited. The present study was
conducted to evaluate the effect of glucagon-like peptide-1 (GLP-1)
analogues for reversal of normal blood glucose in patients with
pre-diabetes.
Materials and methods
Subjects
In total, 42 children diagnosed with pre-diabetes
visited the outpatients of the Department of Endocrinology of
Weifang People's Hospital. All the samples were selected from the
period of April 2015 to April 2016, and patients were aged 6–18
years. Out of 42 children, 29 subjects were male and 13 subjects
were female. Ethical clearance for the study was obtained from the
Institutional Ethics Board of Weifang People's Hospital. Informed
patient consent was also obtained from all the subjects.
Exclusion criteria
Any children with genetic metabolism, endocrine
disease, kidney disease high blood pressure and high blood lipid
profile were excluded from the study.
General information of both groups of patients is
shown in Table I and the diagnostic
criteria for the early stage of DM and dyslipidemia are shown in
Table II.
 | Table I.General information of both the groups
of patients. |
Table I.
General information of both the groups
of patients.
| Group | Cases, no. | Gender,
male/female | Age | BMI,
kg/m2 | HbA1c, % |
|---|
| Control | 21 | 15/6 | 11.12±2.10 | 30.89±5.02 | 5.85±1.33 |
| Observation | 21 | 14/7 | 11.19±2.27 | 30.98±4.97 | 5.87±1.35 |
 | Table II.Diagnostic criteria of DM in the early
stage and dyslipidemia. |
Table II.
Diagnostic criteria of DM in the early
stage and dyslipidemia.
| DM and DM
pre-diagnostic criteria, mmol/l | Diagnostic criteria
for dyslipidemia, mmol/l |
|---|
|
|
|---|
| IFG | IGT | DM | High TG | High TC | Low HDL-C |
|---|
| FPG: (5.6–6.9), while
OGTT | OGTT 2 h blood
glucose | FPG ≥7.0 or OGTT 2
h | ≥1.7 | ≥5.2 | ≤1.03 |
| 2 h blood glucose
<7.8 | (7.8–11.0), while FPG
<5.6 | blood glucose
>11.1 |
|
|
|
The children were randomly divided into the
intervention group (control group) and the lifestyle
intervention+GLP-1 analogues liraglutide group (observation
group).
In the lifestyle intervention group, all the
children followed a unified diet exercise prescription with regular
telephonic conversation, outpatient follow-up, and education of the
parents and children at the same time.
For the observation group, Liraglutide Injection (18
mg/piece; Novo Nordisk, Copenhagen, Denmark) treatment was
administered. The initial dose was 0.6 mg/day subcutaneous
injection, administered daily. After 1 week it was adjusted to 1.2
mg/day subcutaneous injection, daily, followed by 1.2 mg/day
maintenance therapy for ≤3 months.
Determination of fasting height and weight of
children was obtained manually. After 10 h of fasting, the next
morning, venous blood indexes including fasting blood glucose
(FPG), hemoglobin A1c (HbA1C), total cholesterol (TC), triglyceride
(TG), low-density lipoprotein cholesterol (LDL-C), high-density
lipoprotein cholesterol (HDL-C), fasting insulin (FINS), 2 h
postprandial blood glucose (2hPG) were measured and oral glucose
tolerance test was taken as a diagnostic test for DM. After 10 h of
fasting, oral glucose was taken as 1.75 g/kg, ≤75 g/time. All other
diagnostic methods of DM including TC, enzymatic measurement,
LDL-C, HDL-C, direct method of measurement, FPG, measurement by the
sugar kinase method, body mass index (BMI), homeostasis model
assessment for β-cell function index (HOMA-β) and insulin
resistance index (HOMA-IR) were calculated.
BMI was calculated as weight kg/height
m2. HOMA-β was calculated as = 20 × FINS/(FPG-3.5). IR
was calculated by homeostasis model assessment (HOMA), and HOMA was
calcaluted as HOMA-IR = (FPG mmol/l × FINS mU/l/22.5). A HOMA-IR
value of <4 indicated prompted high insulin sensitivity, whereas
if the HOMA-IR value was >4, it indicated prompted low insulin
sensitivity and high IR (3).
Statistical analysis
SPSS1 8.0 software (SPSS, Inc., Chicago, IL, USA)
was used for statistics and analysis of data. Data of normal
distribution were recorded as mean ± standard deviation. HOMA-β was
transformed into normal data for analysis. Measurement data between
the two groups were tested by t-test. The analysis before and after
treatment was performed using paired t-test. P<0.05 was
considered to indicate a statistically significant difference.
Results
Analysis of blood glucose metabolism
of the two groups before and after the intervention
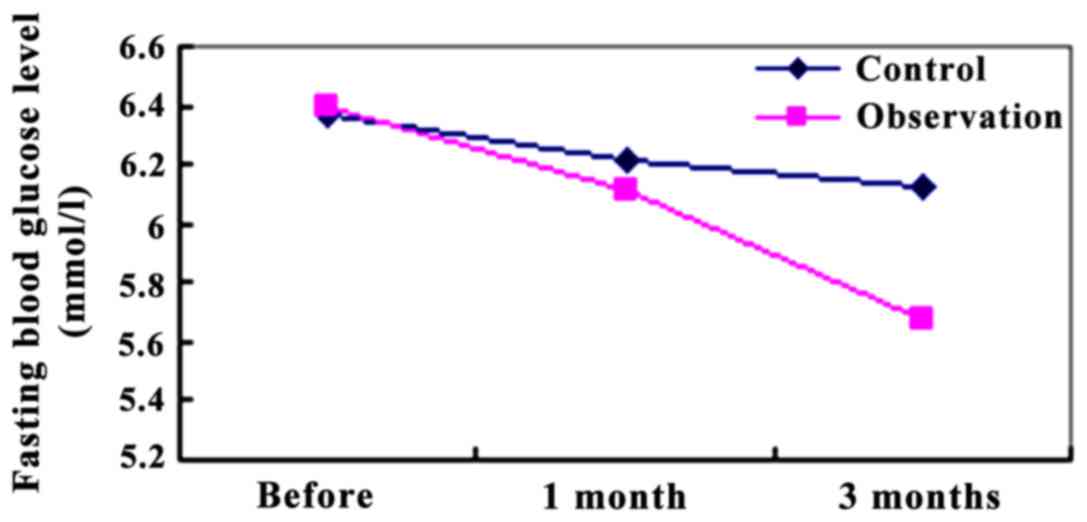
FPG levels of the two groups were compared before
the intervention and after the 1 and 3 months of the intervention.
After 1 month of intervention, the FPG in the observation group was
6.11±0.78 mmol/l, which was lower than that in the control group
(6.21±1.21 mmol/l) (P<0.05). After 3 months of intervention, the
FPG (0.01±0.66 mmol/l) in the observation group was significantly
lower than that in the control group (6.12±1.08 mmol/l). The
difference was statistically significant P<0.01 (Fig. 1).
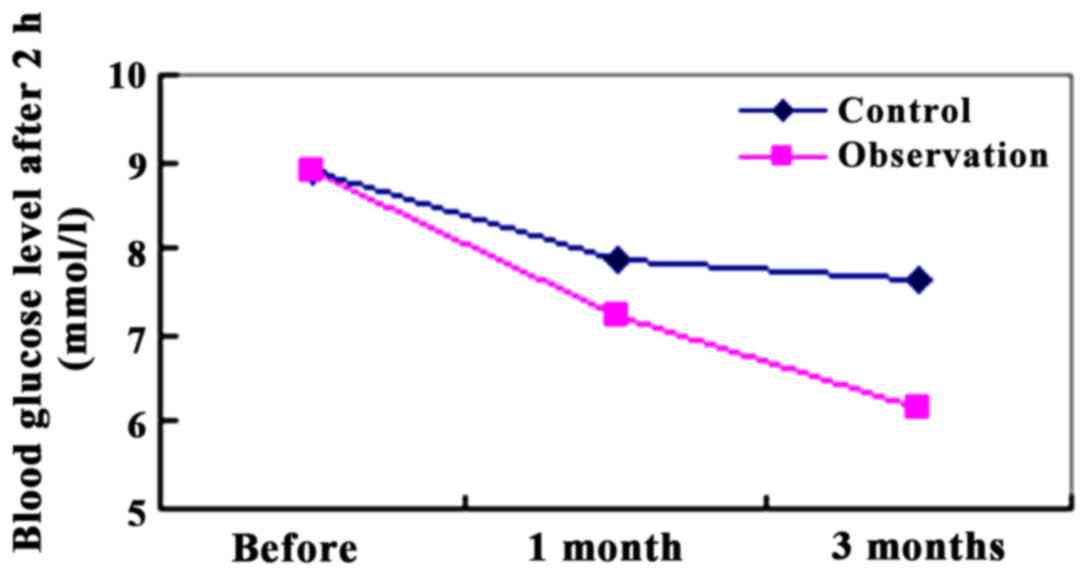
Glucose levels after the 2-h glucose
loading
We compared the blood glucose levels after 2 h of
glucose loading between the two groups. After 1 month of
intervention with 2 h of glucose loading, the blood glucose
(7.22±2.01 mmol/l) in the observation group was lower than that in
the control group (7.89±1.97 mmol/l). The difference was not
significant (P>0.05). After 3 months of intervention with after
2 h of glucose loading, the blood glucose (6.15±1.67 mmol/l) in the
observation group was significantly lower than that of the control
group (7.63±1.82 mmol/l). The difference was significant
(P<0.01) (Fig. 2).
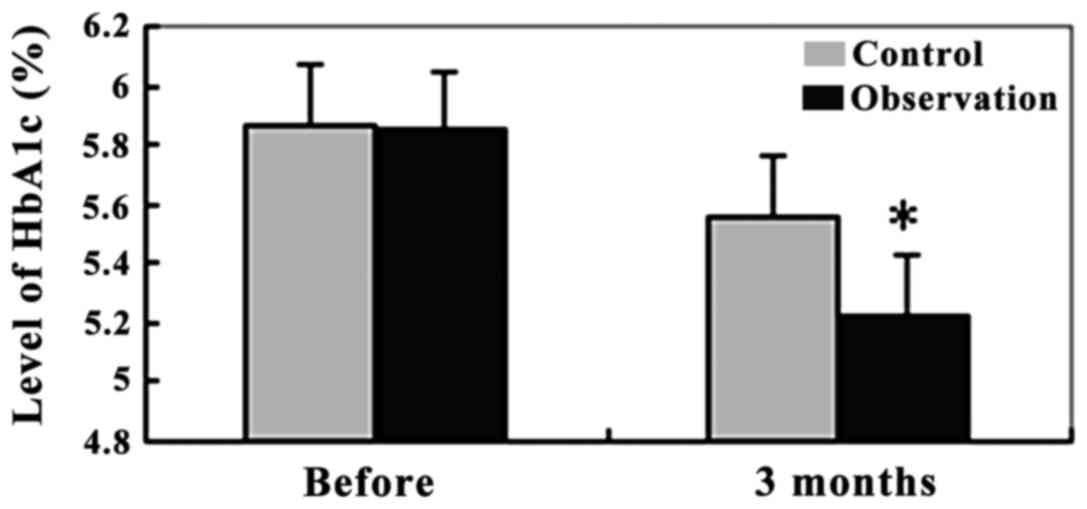
HbA1C levels in the two groups
At the HbA1C level, the observation group was
5.87±1.35% and the control group was 5.85±1.33%. No significant
difference in HbA1C levels between the two groups was observed
(P>0.05). After 3 months of intervention, the HbA1C level in the
observation group was 5.23±1.01%, which was significantly lower
than that in the control group at 5.56±1.14% (P<0.01) (Fig. 3).
The serum lipid profile and BMI levels were analyzed
in the two groups before and after the intervention. BMI, TC, TG,
LDL-C, HDL-C, and FINS of the observation group revealed
statistically significant results, when compared with the control
group (P<0.05). Referring to the Chinese BMI criteria (7–18
years of age) and CDC 2000 criteria of the United States (6 years
of age), children in the group were mostly overweight and obese,
with the cumulative incidence at ≤83.33% (35/42 cases) (Table III).
 | Table III.Blood lipids and fasting insulin were
compared between the two groups. |
Table III.
Blood lipids and fasting insulin were
compared between the two groups.
|
| Control group | Observation
group |
|---|
|
|
|
|
|---|
| Index | Enrollment | Intervention for 3
months | Enrollment | Intervention for 3
months |
|---|
| BMI,
kg/m2 | 30.89±5.02 |
29.88±5.32a | 30.98±4.97 |
28.68±5.19a,b |
| FINS, µU/ml | 31.21±8.01 |
28.82±9.64a | 31.19±8.11 |
26.79±9.69a,b |
| TG, mmol/l | 1.13±0.72 | 1.10±0.39 | 1.14±0.62 |
1.04±0.37a,b |
| TC, mmol/l | 4.19±1.26 |
3.95±1.03a | 4.17±1.27 |
3.80±1.01a,b |
| HDL, mmol/l | 1.18±0.31 | 1.20±0.19 | 1.19±0.24 |
1.33±0.25a,b |
| LDL, mmol/l | 2.45±1.00 | 2.39±0.66 | 2.47±0.96 |
2.21±0.88a,b |
Comparison of the level of HOMA-IR and
HOMA-β
The levels of HOMA-IR and HOMA-β were analyzed
before and after intervention in the two groups. The decreased IR
index of the observation group was higher than that of the control
group, and the difference was statistically significant
(P<0.05). Islet function index of the β-cell of the observation
group was significantly higher than that of the control group, and
the difference was statistically significant (P<0.05) (Table IV).
 | Table IV.Comparison of the changes of HOMA-IR
and HOMA-β in the two groups after 3 months of intervention. |
Table IV.
Comparison of the changes of HOMA-IR
and HOMA-β in the two groups after 3 months of intervention.
| Index | Cases, no. | Control group | Observation
group |
|---|
| HOMA-IR of
enrollment | 21 | 7.04±2.73 | 7.06±2.68 |
| HOMA-IR after 3
months | 21 |
6.52±3.51a |
6.02±3.11a,b |
| HOMA-β of
enrollment | 21 | 2.64±0.38 | 2.65±0.32 |
| HOMA-IR after 3
months | 21 | 2.56±0.37 |
2.46±0.31a,b |
Discussion
The results of the present study showed a total of
≤83.33% (35/42 cases) of obese and overweight children in the early
stage of DM. Lifestyle changes reduced physical activity and
unhealthy eating habits have caused an increased obesity incidence.
In fact, approximately 110 million of the world's children are
overweight or obese (5). Related
findings also showed that compared with normal children, overweight
and obese children had higher average blood glucose levels, blood
glucose abnormalities, impaired glucose tolerance (IGT) and
diabetes incidence (6). BMI in
children with T2DM was elevated, while BMI and IR in childhood
appeared simultaneously (7). The
sensitivity of insulin was negatively correlated with BMI and
obesity. Children with increased BMI revealed decreased insulin
sensitivity and increased blood glucose level that resulted in
impaired glucose metabolism. The period of normal glucose tolerance
and hyperlipidemia are considered to be the early stage of DM
(8). There are three stages of
glucose metabolism in children and adolescents: Hyperinsulinemia,
pre-DM and DM stage. The early stage of diabetes refers to the
impaired glucose regulation between normal glucose metabolism and
DM, including impaired fasting glucose (IFG), IGT, and IFG/IGT.
Previous results obtained from China show 148 million individuals
in the early stage of diabetes, thereby increasing the incidence
rate to ≤15.5% (9). Approximately
70% of the patients of the early stage of diabetes eventually
progress into diabetes, which is harmful for the population.
Overweight, obesity and decreased physical activity of children
with diabetes means they are at greater risk of developing
diabetes. Over the past 40 years, there have been 8 large-scale
clinical trials showing that 25–60% of pre-diabetes patients can
prevent the development of diabetes by merely lifestyle
intervention (9). In order to
prevent the development of obesity in children, the key is how to
prevent the development of obesity through early control. By
changing the lifestyle such as introducing a proper diet plan and
adequate exercise therapy, the conversion of pre-diabetes into
diabetes may be preveted. Change of lifestyle is considered to be
the cornerstone in the treatment of pre-diabetes. However, it is
generally believed that the long-term effectiveness of lifestyle
intervention is not good, and it is easy to rebound. Even if the
body mass is reduced, there is a 40–50% likelihood that the IGT
damages may cause T2DM (10).
Therefore, lifestyle intervention alone cannot completely prevent
the occurrence and development of diabetes. Few reports are
available regarding the long-term effectiveness of only lifestyle
intervention for the obese children. The practicability of only
lifestyle intervention is poor, and is widely questioned by doctors
and patients. In view of the particularity of school age children,
due to the lack of self-discipline in children, the simple
lifestyle intervention may be difficult in achieving individual
weight loss because of its inability to achieve the amount of
physical activity. On the other hand, considering the children's
stage, calorie-restricted intake affects their growth and
development, leading to the fact that the only simple lifestyle
intervention cannot be implemented to reduce obesity. Consequently,
early drug intervention programmes are correctly becoming more
popular.
GLP-1 belongs to the gut peptide hormone secreted by
intestinal epithelial L cells. GLP-1, the incretin hormone
regulates appetite, delaying gastric emptying, stimulating islet
β-cell proliferation, suppressing apoptosis, promoting insulin
secretion, improving insulin sensitivity, fat mobilization, and
restoring the function of islet β-cells. Due to its main role in a
number of metabolic activities, GLP-1 peptide has become a hot
topic in the study of obesity and diabetes prevention (11,12).
Natural GLP-1 has a half-life of only 1–2 min and can be rapidly
degraded by DPP-4 in vivo. After the degradation it became
inactive, further hindering its clinical application. The GLP-1
analogues have a long half-life, which is not easily degraded and
has the physiological function of GLP-1 to be applied in clinical
practice (repeated). The representative drugs of GLP-1 peptide are
exenatide and liraglutide. The peptide is the first GLP-1 analogue
used for the treatment of T2DM. Previous studies revealed that the
twice a day dose of exenatide subcutaneous injection and daily one
dose of liraglutide subcutaneous injection, plays an important role
in the regulation of blood sugar, reduction in weight, protection
of islet β-cells and the prevention of cardiovascular disease
(13–19). LEAD results showed that liraglutide
administered alone or in combination with other antidiabetic drugs,
effectively improved the blood glucose level, and controlled the
pancreatic β-cell function as well as weight loss with less risk of
hypoglycemia (20–25). When the glucose concentration was
lower than 4.5 mmol/l, GLP-1 lost its hypoglycemic effect (21).
As the early application of GLP-1 analogues may
reverse the early development of diabetes, this study used a
prospective randomized controlled trial to compare the blood
glucose, lipid profile, body weight, IR and β-cell function of
pre-diabetic children after the intervention of 3 months in simple
lifestyle intervention group (control group) and lifestyle
intervention+GLP-1 analogue liraglutide treatment group
(observation group). After 1 month of intervention, 2hPG and FPG in
the observation group were lower than those in the control group
(P<0.05). After 3 months, FPG and 2hPG of the observation group
were significantly lower than those of the control group
(P<0.01). After the 3 months of intervention, HbA1C, TC, TG,
LDL-C, HDL-C, and BMI of the observation group were statistically
different compared with the control group (P<0.05). The
decreased IR index of the observation group was significantly
higher than that of the control group (P<0.05). The islet
function index of the β-cell of the observation group was
significantly higher than that of the control group (P<0.05).
GLP-1 analogues can control FPG, 2hPG blood glucose in early
stages, improve blood lipid profile, improve BMI and IR, and
improve the function of islet β-cell, while exploring new ways of
early childhood intervention. In view of the current domestic and
international use of GLP-1 powder injection of the long-acting
analogue of chemical synthesis, with complex production process,
high cost, expensive market price, storage and transportation
difficulties, and inconvenience of subcutaneous administration,
thus, clinical feasibility is not ideal. Therefore, we need to
improve the production mode of GLP-1 similar peptide, change the
way of using GLP-1 similar peptide, and develop GLP-1 similar
peptide of high efficiency, that is cost effective and easy to use,
in order to obtain good social benefits.
References
|
1
|
American Diabetes Association, . Standards
of medical care in diabetes - 2010. Diabetes Care. 33 Suppl
1:S11–S61. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Amed S, Daneman D, Mahmud FH and Hamilton
J: Type 2 diabetes in children and adolescents. Expert Rev
Cardiovasc Ther. 8:393–406. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
American Diabetes Association, . Diagnosis
and classification of diabetes mellitus. Diabetes Care. 35 Suppl
1:S64–S71. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Matthews DR, Hosker JP, Rudenski AS,
Naylor BA, Treacher DF and Turner RC: Homeostasis model assessment:
Insulin resistance and beta-cell function from fasting plasma
glucose and insulin concentrations in man. Diabetologia.
28:412–419. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Haslam DW and James WP: Obesity. Lancet.
366:1197–1209. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rush EC, Plank LD, Mitchelson E and Laulu
MS: Central obesity and risk for type 2 diabetes in Maori, Pacific,
and European young men in New Zealand. Food Nutr Bull. 23 Suppl
3:82–86. 2002.PubMed/NCBI
|
|
7
|
Brosnan CA, Upchurch S and Schreiner B:
Type 2 diabetes in children and adolescents: An emerging disease. J
Pediatr Health Care. 15:187–193. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Craig ME, Hattersley A and Donaghue KC:
Definition, epidemiology and classification of diabetes in children
and adolescents. Pediatr Diabetes. 10 Suppl 12:3–12. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yang W, Lu J, Weng J, Jia W, Ji L, Xiao J,
Shan Z, Liu J, Tian H, Ji Q, et al: China National Diabetes and
Metabolic Disorders Study Group: Prevalence of diabetes among men
and women in China. N Engl J Med. 25:1090–1101. 2010. View Article : Google Scholar
|
|
10
|
Weerakiet S, Srisombut C, Bunnag P,
Sangtong S, Chuangsoongnoen N and Rojanasakul A: Prevalence of type
2 diabetes mellitus and impaired glucose tolerance in Asian women
with polycystic ovary syndrome. Int J Gynaecol Obstet. 75:177–184.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
D'Alessio DA and Vahl TP: Glucagon-like
peptide 1 Evolution of an incretin into a treatment for diabetes.
Am J Physiol Endocrinol Metab. 286:E882–E890. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Theodorakis MJ, Carlson O, Michopoulos S,
Doyle ME, Juhaszova M, Petraki K and Egan JM: Human duodenal
enteroendocrine cells: Source of both incretin peptides, GLP-1 and
GIP. Am J Physiol Endocrinol Metab. 290:E550–E559. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Deacon CF, Nauck MA, Toft-Nielsen M,
Pridal L, Willms B and Holst JJ: Both subcutaneously and
intravenously administered glucagon-like peptide I are rapidly
degraded from the NH2-terminus in type II diabetic patients and in
healthy subjects. Diabetes. 44:1126–1131. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Buse JB, Henry RR, Han J, Kim DD, Fineman
MS and Baron AD; Exenatide-113 Clinical Study Group, : Effects of
exenatide (exendin-4) on glycemic control over 30 weeks in
sulfonylurea-treated patients with type 2 diabetes. Diabetes Care.
27:2628–2635. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
DeFronzo RA, Ratner RE, Han J, Kim DD,
Fineman MS and Baron AD: Effects of exenatide (exendin-4) on
glycemic control and weight over 30 weeks in metformin-treated
patients with type 2 diabetes. Diabetes Care. 28:1092–1100. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kendall DM, Riddle MC, Rosenstock J,
Zhuang D, Kim DD, Fineman MS and Baron AD: Effects of exenatide
(exendin-4) on glycemic control over 30 weeks in patients with type
2 diabetes treated with metformin and a sulfonylurea. Diabetes
Care. 28:1083–1091. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bunck MC, Diamant M, Cornér A, Eliasson B,
Malloy JL, Shaginian RM, Deng W, Kendall DM, Taskinen MR, Smith U,
et al: One-year treatment with exenatide improves beta-cell
function, compared with insulin glargine, in metformin-treated type
2 diabetic patients: A randomized, controlled trial. Diabetes Care.
32:762–768. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Russell-Jones D: Molecular,
pharmacological and clinical aspects of liraglutide, a once-daily
human GLP-1 analogue. Mol Cell Endocrinol. 15:137–140. 2009.
View Article : Google Scholar
|
|
19
|
Astrup A, Carraro R, Finer N, Harper A,
Kunesova M, Lean ME, Niskanen L, Rasmussen MF, Rissanen A, Rössner
S, et al: NN8022-1807 Investigators: Safety, tolerability and
sustained weight loss over 2 years with the once-daily human GLP-1
analog, liraglutide. Int J Obes (Lond). 36:843–854. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Marre M, Shaw J, Brändle M, Bebakar WM,
Kamaruddin NA, Strand J, Zdravkovic M, Le Thi TD and S; LEAD-1 SU
study group Colagiuri: Liraglutide, a once-daily human GLP-1
analogue, added to a sulphonylurea over 26 weeks produces greater
improvements in glycaemic and weight control compared with adding
rosiglitazone or placebo in subjects with type 2 diabetes (LEAD-1
SU). Diabet Med. 26:268–278. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Nauck M, Frid A, Hermansen K, Shah NS,
Tankova T, Mitha IH, Zdravkovic M, Düring M and Matthews DR; LEAD-2
Study Group, : Efficacy and safety comparison of liraglutide,
glimepiride, and placebo, all in combination with metformin, in
type 2 diabetes: The LEAD (liraglutide effect and action in
diabetes)-2 study. Diabetes Care. 32:84–90. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Garber A, Henry R, Ratner R,
Garcia-Hernandez PA, Rodriguez-Pattzi H, Olvera-Alvarez I, Hale PM,
Zdravkovic M and Bode B; LEAD-3 (Mono) Study Group, : Liraglutide
versus glimepiride monotherapy for type 2 diabetes (LEAD-3 Mono): a
randomised, 52-week, phase III, double-blind, parallel-treatment
trial. Lancet. 373:473–481. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zinman B, Gerich J, Buse JB, Lewin A,
Schwartz S, Raskin P, Hale PM, Zdravkovic M and Blonde L: LEAD-4
Study Investigators: Efficacy and safety of the human glucagon-like
peptide-1 analog liraglutide in combination with metformin and
thiazolidinedione in patients with type 2 diabetes (LEAD-4
Met+TZD). Diabetes Care. 32:1224–1230. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Russell-Jones D, Vaag A, Schmitz O, Sethi
BK, Lalic N, Antic S, Zdravkovic M, Ravn GM and Simó R; Liraglutide
Effect and Action in Diabetes 5 (LEAD-5) met+SU Study Group:
Liraglutide vs insulin glargine and placebo in combination with
metformin and sulfonylurea therapy in type 2 diabetes mellitus
(LEAD-5 met+SU): A randomised controlled trial. Diabetologia.
52:2046–2055. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Buse JB, Rosenstock J, Sesti G, Schmidt
WE, Montanya E, Brett JH, Zychma M and Blonde L; LEAD-6 Study
Group, : Liraglutide once a day versus exenatide twice a day for
type 2 diabetes: a 26-week randomised, parallel-group,
multinational, open-label trial (LEAD-6). Lancet. 374:39–47. 2009.
View Article : Google Scholar : PubMed/NCBI
|