Introduction
Diabetic gastroparesis is a common and debilitating
disease affecting millions of patients with diabetes mellitus
worldwide (1). Up to 50% of patients
with type 1 or type 2 diabetes may suffer from gastroparesis, which
can occur as a result of nutritional deficiency, poor glycemic
control and psychological distress. Gastroparesis affects the
quality of life of the patient and the management of diabetes
(1–3). Current pharmacological therapies
available for the treatment of gastroparesis have low effectiveness
in drug resistant and non-responding patients.
In previous years, electroacupuncture (EA) has been
widely used to treat patients with symptoms suggestive of
gastroparesis (4–8). EA has potential benefits for the
control of disorders of gastrointestinal motility in a more
systematic way compared with other alternative therapies (9–12). Three
small, randomized pilot studies demonstrated that EA effectively
alleviated the symptoms of diabetic gastroparesis; however, the
overall efficiency was not satisfactory (4,6,7). The possible cause of the contrasting
results may be a result of a failure to categorize the patients
according to the severity of their symptoms (4,6–8). A study by Wang (5) showed that the effect of acupuncture was
superior to that of drug treatment (with respect to domperidone) in
terms of symptom alleviation, with a total efficacy rate of 94%.
However, the abovementioned study did not analyze the association
between the severity of symptoms and the efficiency of the
treatment. At present, detailed information regarding the treatment
with EA has yet to be provided. The present study investigated
whether EA is effective in patients with severe symptoms suggestive
of gastroparesis and evaluated the effects of combination therapy
of EA and mosapride.
Materials and methods
Patients
A total of 56 patients with type 2 diabetes mellitus
who had symptoms suggestive of gastroparesis for >3 months, and
had not received treatment with prokinetics or other medications
that may affect gastrointestinal motility in the previous 3 weeks,
were enrolled in the present study. All the patients were recruited
from clinical departments or the endocrinopathy and
gastroenteropathy ward of Qianfoshan Hospital Affiliated to
Shandong University (Jinan, China) from January 2011 to May 2012.
Insulin was administered to patients if required. Endoscopy or
abdominal X-ray examination was performed to exclude patients with
primary gastrointestinal diseases if necessary. None of the
patients had previously undergone surgical treatment on the
gastrointestinal tract. The patients whose symptoms were severe
enough for the clinician to consider nutritional support or other
strategy, and those with severe complications (end-stage renal
disease, coronary artery disease, stroke, cancer or lung disease)
were also excluded from the study. Patient safety was evaluated by
a daily assessment of adverse events. All patients received a daily
physical examination from the commencement of the trial, and were
continuously monitored by telephone interview for 7 days after
treatment. The study protocol was approved by the Ethics Review
Committee of Qianfoshan Hospital Affiliated to Shandong University.
Written informed consent was obtained from all patients or their
families.
Gastroparesis Cardinal Symptom Index
(GCSI) assessment
GCSI, a validated questionnaire consisting of 3
subscales, was used to assess the predominant symptoms associated
with gastroparesis, including: Postprandial fullness/early satiety,
nausea/vomiting and bloating. GCSI total scores ranged between 0
and 5, with a higher score indicating greater symptom severity.
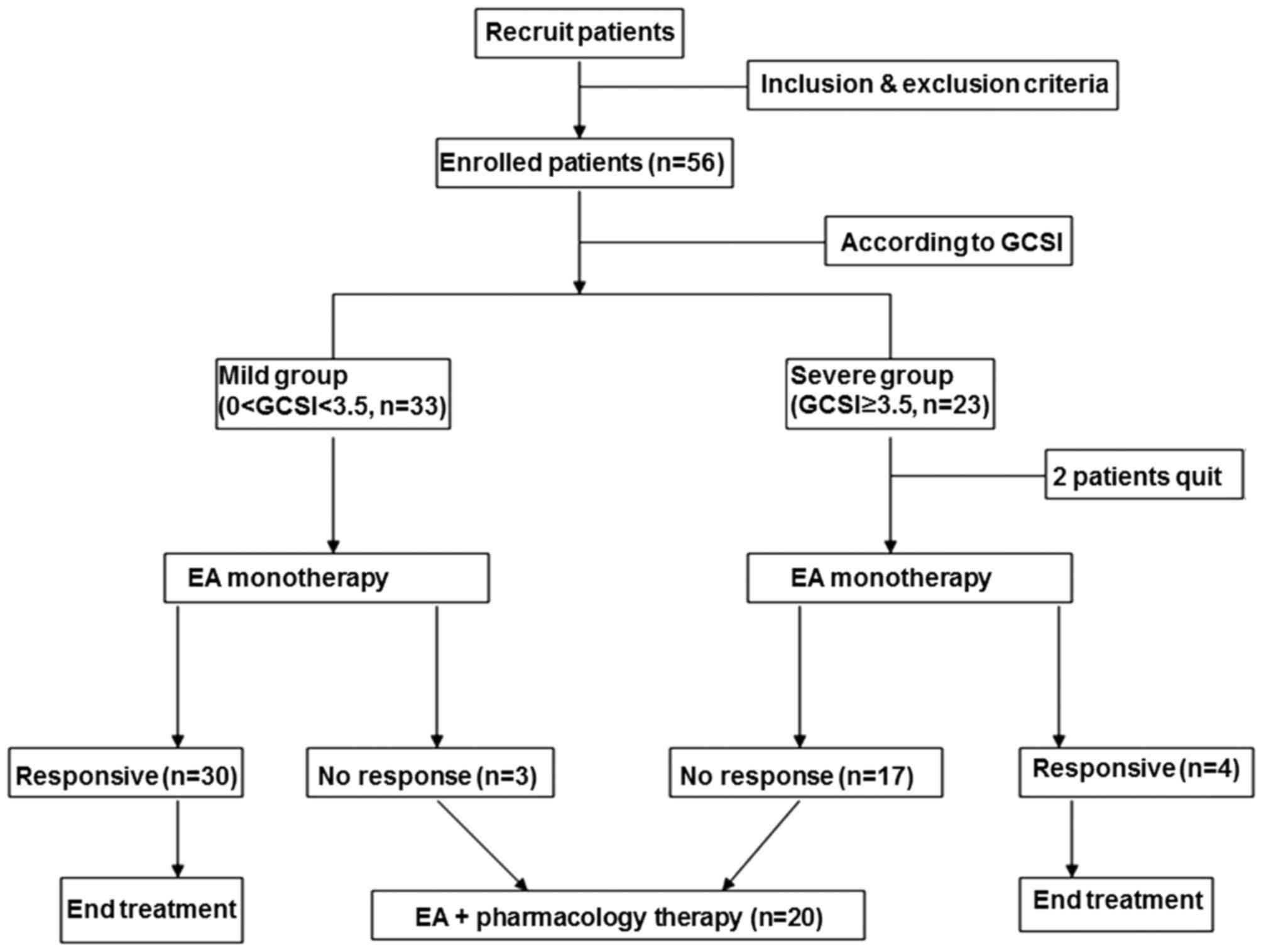
Symptom severity was classified as mild (GCSI score <3.5) or
severe (GCSI score ≥3.5) (13,14). The
patients were divided into two groups according to their GCSI
scores as follows: i) Mild group (n=33) and ii) severe group
(n=23). However, 2 patients from the severe group dropped out of
the study during the initial treatment session; therefore, only 21
patients from the severe group completed the study. In the first
phase of the study, all patients received EA treatment once a day
for 14 consecutive days. Individual and total GCSI scores were
recorded each day in the morning prior to treatment. At the end of
the 14-day treatment period, patients were considered to be
non-responsive if the severity score was not reduced by >25%
from the baseline, and were then transferred to the second phase
for combination therapy (EA and mosapride tablet) for a further 14
days (Fig. 1). The average frequency
of vomiting of every patient on each day during each treatment
session was also evaluated.
EA and mosapride administration
All acupuncture technicians had clinical and
educational experience of >10 years, and had attained the
corresponding certifications. Zusanli (ST36) and Neiguan (PC6) were
selected as acupuncture points, as they have been reported to be
effective stimulating points for improving gastroparesis (9–12). The
acupuncture needle was inserted into the points, and stimulation
was performed by acupuncture needles using an electroacupuncture
apparatus (model G6805-2A; Shanghai Huayi Medical Instrument
Factory, Shanghai, China) at a frequency of 25 Hz and an amplitude
of 10–30 mA for a tolerable stimulation. Each daily treatment
session lasted for 30 min. Mosapride, a drug shown to effectively
reduce the symptoms of diabetic gastroparesis, was selected for the
combination therapy (15). Mosapride
(5 mg per tablet; Sumitomo Pharmaceutical Suzhou Co., Suzhou,
China) was orally administered at a dose of 5 mg 3 times a day for
2 consecutive weeks.
13C-octanoic acid breath
test
Gastric emptying was assessed by the
13C-octanoic acid breath test (16,17). All
patients fasted overnight (≥8 h) and consumed a standard test meal,
consisting of a raw egg mixed with 100 mg 13C-octanoic
acid (99% 13C) fried into an omelet and served with 2
pieces of white bread (420 kcal) and 150 ml plain water. Each test
meal was consumed within 10 min. The 13C concentration
was measured by isotope ratio mass spectrometry (Breath MAT plus;
Finnigan GmbH, Bremen, Germany) prior to the test meal and every 15
min for 4 h after the meal. Changes in 13C
concentrations at each time-point from baseline were analyzed to
assess the gastric half-emptying time.
Statistical analysis
All evaluations were performed using SPSS
statistical software (version 17.0; SPSS, Inc., Chicago, IL, USA).
Non-parametric tests were performed by means of the Mann-Whitney
U-test. Parametric tests used included the Student's t-test. All
values are presented as the mean ± standard deviation. P≤0.05 was
considered to indicate a statistically significant difference. All
analyses pertaining to efficacy were based on two-sided tests.
Results
Patient demographics and gastric
half-emptying
The patient demographics of all 56 patients included
in the present study and their baseline characteristics were
summarized in Table I. With the
exception of the severity of symptoms at baseline (referring to
GCSI total and individual scores), there were no differences
between the two groups in terms of patient demographics (age,
gender and body mass index) and clinical characteristics (diabetes
duration and glycated hemoglobin). The gastric half-emptying time
of patients was not significantly different between the mild and
severe groups. No adverse events were observed during or after the
trial.
 | Table I.Patient demographics prior to
treatment. |
Table I.
Patient demographics prior to
treatment.
| Characteristics | Mild group
(n=33) | Severe group
(n=23) | P-value |
|---|
| Age (years) | 53.6±11.2 | 60.5±9.2 | >0.05 |
| Gender
(male/female) | 11/10 | 11/8 | >0.05 |
| Diabetes history
(years) | 9.7±3.1 | 11.9±7.7 | >0.05 |
| Mean body mass index
(kg/m2) | 27.9±5.6 | 27.7±6.1 | >0.05 |
| HbA1c (%) | 6.5±0.5 | 6.7±1.0 | >0.05 |
| Gastric half-emptying
time (min) | 126.3±28.2 | 140.3±19.2 | >0.05 |
EA or mosapride monotherapy alleviates
symptoms and significantly reduces GCSI total scores in mild
diabetic gastroparesis, while combination therapy is necessary for
patients with severe symptoms
To assess the efficacy of EA monotherapy and
combination therapy on diabetic gastroparesis, a GCSI assessment
was performed. A total of 54 patients completed the study,
including all 33 patients in the mild group and 21 patients in the
severe group. Two patients in the severe group were dropped of the
study due to severe vomiting that required medication during the
first treatment session. These patients were aged 68 and 72 years,
respectively, which was greatly above the average age of the
treatment group. In addition, the diabetic duration of these
patients was much longer than the average course. In the mild
group, EA treatment resulted in significantly reduced GCSI total
scores at the end of week 2 (P=0.016). Furthermore, the average
reduction of the GCSI total score was >25%. In addition,
analysis of the severe group showed that EA treatment failed to
alleviate the symptoms of gastroparesis. There were 20 patients
with no response to EA treatment (3 from the mild subgroup and 17
from the severe subgroup), and these patients achieved
significantly improved GCSI total scores after combination therapy
(P=0.013; Table II). By analysis of
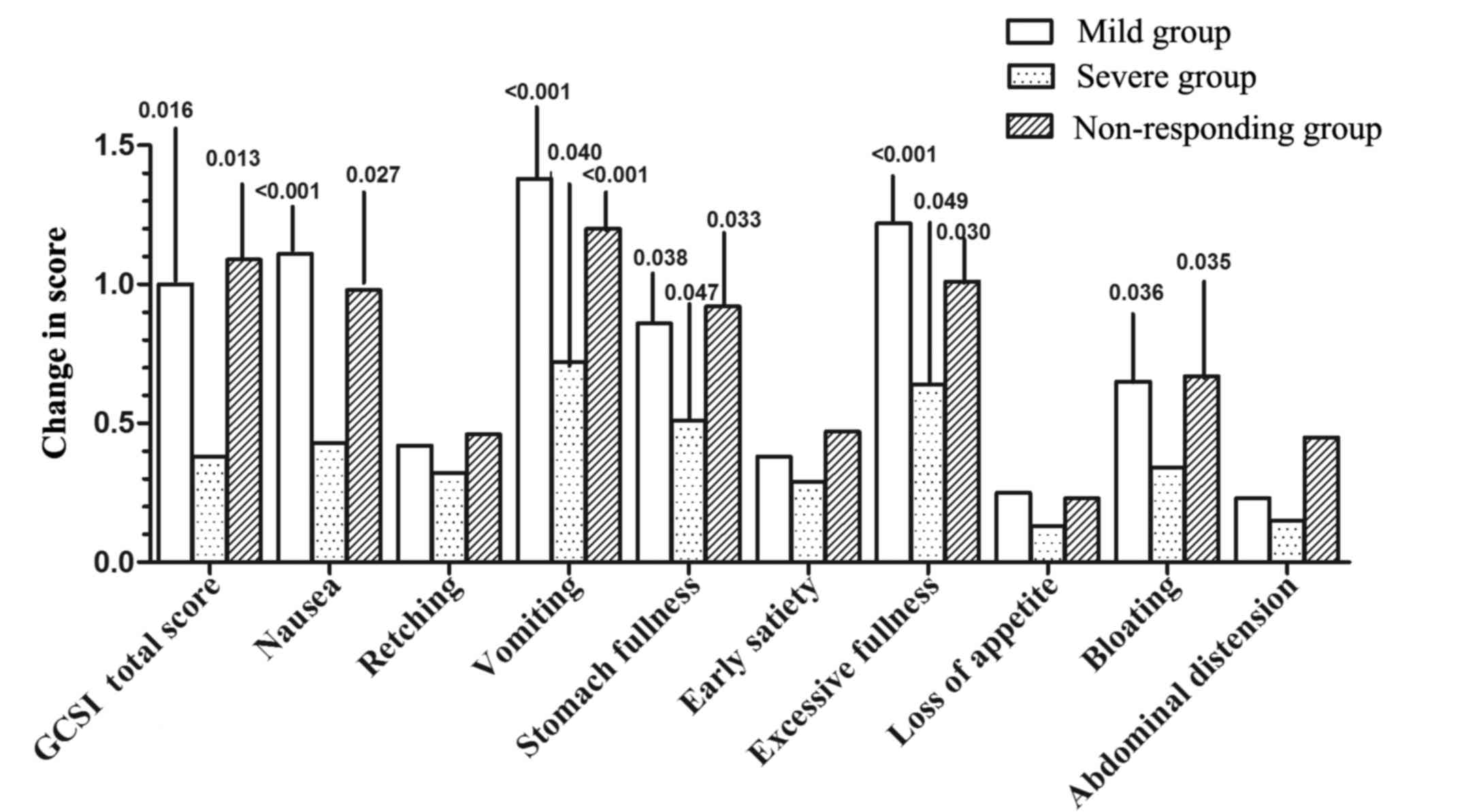
categorical outcomes, numerical improvements were obtained for all
individual scores; however, statistical significance was only
observed in individual symptom scores for nausea, vomiting, stomach
fullness, excessive fullness and bloating (Fig. 2). Although EA treatment did not
alleviate all symptoms experienced by the severe group, it was
beneficial to individual symptom scores of vomiting, stomach
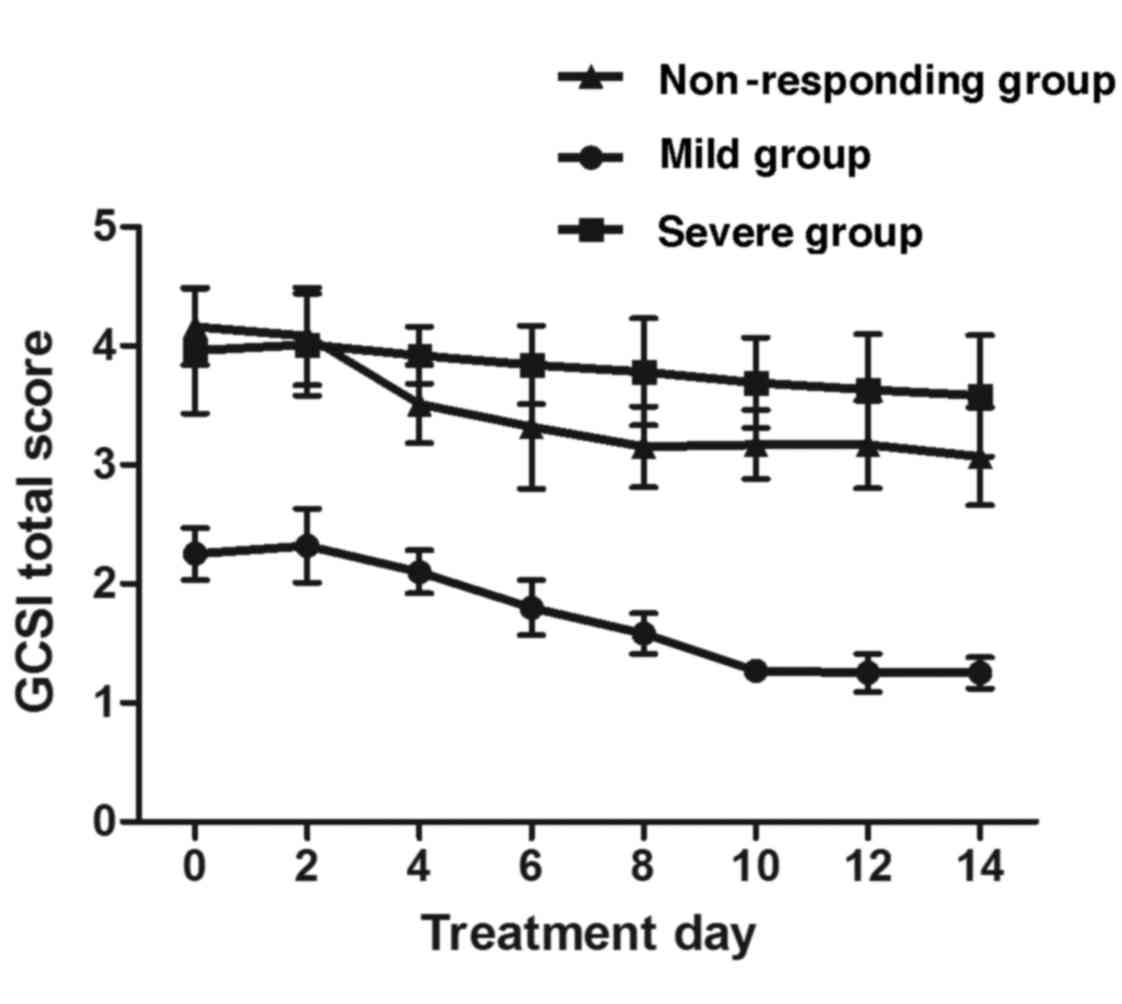
fullness and excessive fullness. After 6 days of treatment, in the
mild group the GCSI total scores were observed to be reduced, and
the highest efficacy was achieved on day 10. For non-responding
patients, the scores began to improve on day 4 and plateaued on day
8 after the initiation of the combination therapy (Fig. 3). These results indicated that EA
monotherapy alleviated symptoms and resulted in significantly
reduced GCSI total scores in patients with mild diabetic
gastroparesis, while combination therapy was necessary for patients
with severe symptoms.
 | Table II.Parameters prior to and after
treatment. |
Table II.
Parameters prior to and after
treatment.
|
| Mild subgroup
(n=33) | Severe subgroup
(n=21) | Non-responding
subgroupa (n=20) |
|---|
|
|
|
|
|
|---|
| Parameter | Baseline | At 2 weeks | P-value | Baseline | At 2 weeks | P-value | At 2 weeks | At 4 weeks | P-value |
|---|
| GCSI score |
| Total | 2.25±0.54 | 1.25±0.19 | 0.016 | 3.96±0.60 | 3.58±0.52 | >0.05 | 4.16±0.36 | 3.07±0.49 | 0.013 |
| Nausea | 2.53±0.69 | 1.42±0.34 | <0.001 | 4.01±0.39 | 3.58±0.23 | >0.05 | 4.21±0.56 | 3.23±0.12 | 0.027 |
| Retching | 2.63±0.64 | 2.21±0.32 | >0.05 | 4.03±0.54 | 3.71±0.23 | >0.05 | 3.98±0.34 | 3.52±0.14 | >0.05 |
| Vomiting | 2.01±0.62 | 0.63±0.34 | <0.001 | 3.71±0.62 | 2.99±0.18 | 0.040 | 3.64±0.12 | 2.44±0.33 | <0.001 |
| Stomach
fullness | 2.75±0.63 | 1.89±0.41 | 0.038 | 4.56±0.54 | 4.05±0.58 | 0.047 | 4.62±0.34 | 3.70±0.64 | 0.033 |
| Early satiety | 2.75±0.54 | 2.37±0.26 | >0.05 | 3.78±0.79 | 3.49±0.68 | >0.05 | 4.08±0.32 | 3.61±0.23 | >0.05 |
| Excessive
fullness | 2.55±0.76 | 1.33±0.66 | <0.001 | 3.85±0.45 | 3.21±0.32 | 0.049 | 4.05±0.42 | 3.04±0.54 | 0.030 |
| Loss of
appetite | 2.08±0.86 | 1.83±0.76 | >0.05 | 3.92±0.44 | 3.79±0.56 | >0.05 | 4.10±0.33 | 3.87±0.45 | >0.05 |
| Bloating | 2.05±0.63 | 1.40±0.41 | 0.036 | 4.12±0.72 | 3.78±0.88 | >0.05 | 3.99±0.15 | 3.32±0.34 | 0.035 |
| Abdominal
distension | 1.21±0.98 | 0.98±0.72 | >0.05 | 3.56±0.46 | 3.41±0.65 | >0.05 | 3.69±0.12 | 3.24±0.15 | >0.05 |
| Gastric half
emptying time (min) | 126.3±28.2 | 113.8±27.3 | 0.078 | 140.3±19.2 | 133.8±13.2 | 0.096 | 128.7±30.1 | 98.8.0±12.4 | 0.018 |
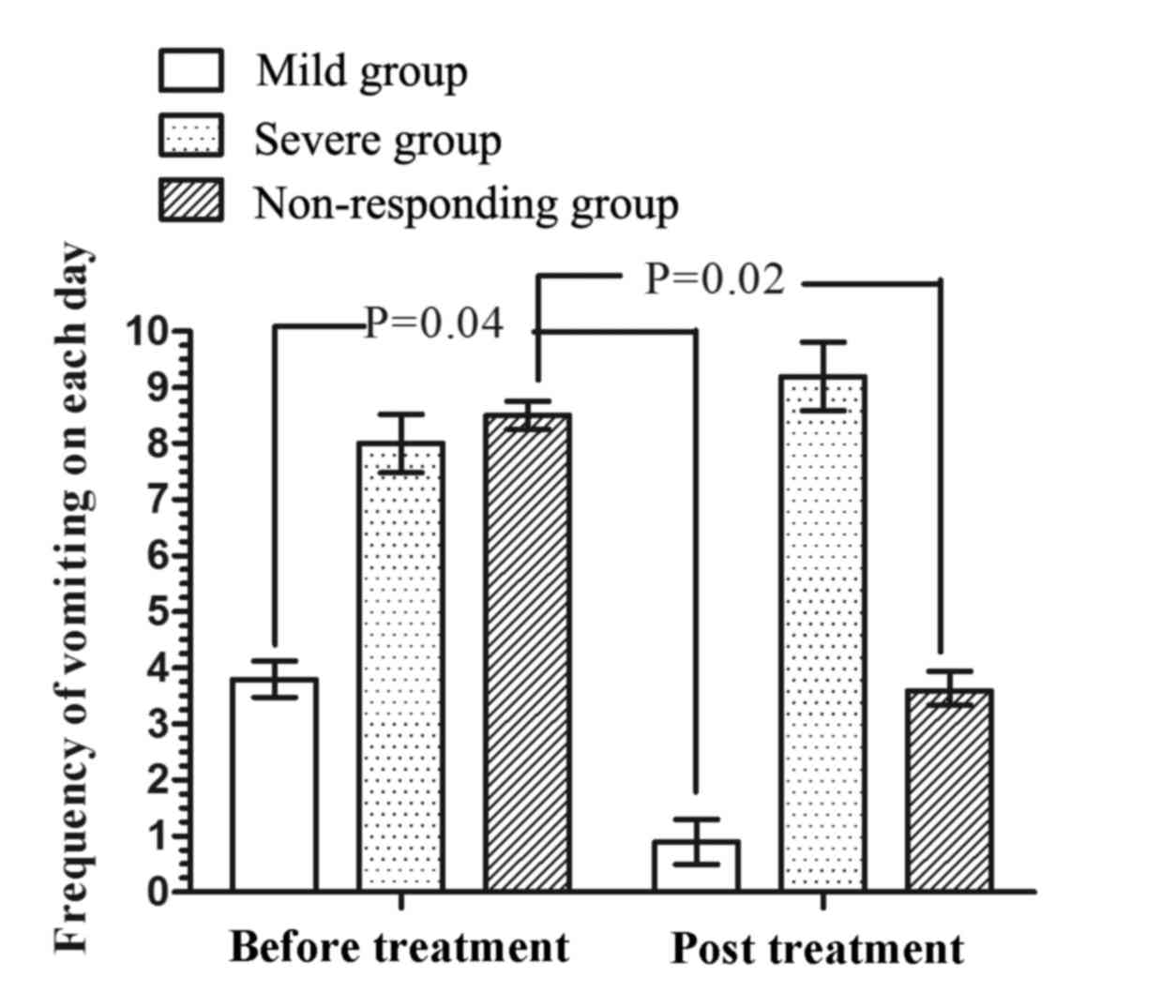
EA treatment and combination with mosapride reduces
the frequency of vomiting in patients with mild and severe diabetic
gastroparesis, respectively. To determine the effect of EA and
mosapride on vomiting, the frequency of vomiting was assessed
during the EA and combination treatment periods. The frequency of
vomiting on each day experienced by mild and non-responding
patients after treatment were significantly reduced compared with
those prior to treatment (P=0.04 and P=0.02, respectively; Fig. 4). These results suggested that EA
mono-treatment reduced the frequency of vomiting in patients with
mild diabetic gastroparesis, while combined EA and mosapride
treatment reduced the frequency of vomiting in patients not
responding, which mainly comprised those with severe diabetic
gastroparesis.
EA and mosapride combination treatment
significantly reduces gastric half-emptying time
To evaluate gastric emptying time, the
13C-octanoic acid breath test was employed. The gastric
half-emptying time in the mild group was slightly but not
significantly decreased after EA (126.3±28.2 vs. 113.8±27.3 min;
P=0.078). In the severe group, the gastric half-emptying time was
not altered after 2 weeks of EA therapy (140.3±19.2 vs. 133.8±13.2
min; P=0.096); however, it was significantly decreased in the
non-responding group following combination therapy (128.7±30.1 vs.
98.8±12.4 min; P=0.018). These results indicated that the gastric
half-emptying time was significantly reduced by the combination
treatment.
Discussion
Patients with diabetic gastroparesis may have mild
or severe symptoms. EA or prokinetic agents are typically
administered to alleviate the symptoms; however, patients may show
different responses to the treatments. The findings of the present
study suggested that EA significantly alleviates mild symptoms of
gastroparesis and the results are consistent with those of previous
studies (4,5,7). Among
the 34 patients with early responses to EA treatment, 30 had mild
symptoms, while only 3 patients had severe symptoms. However, EA
failed to relieve the symptoms in the severe group. The results
indicated that a higher total GCSI score was associated with an
unfavorable response to EA. However, non-responding patients,
either from the mild or severe subgroup, showed significant
alleviation of symptoms at the final evaluation after combination
treatment. The 3 non-responding patients in the mild symptom group
had a longer duration of upper gastrointestinal symptoms and a
higher age compared with the remaining patients. However, whether
the differences in diabetes history and age had a clinically
significant effect cannot be concluded from the present study.
Therefore, the treatment modality, namely combined treatment rather
than EA therapy alone, should be selected based on the severity of
the symptoms. Combination treatment involving EA and mosapride is
an optimal treatment option for patients with diabetic
gastroparesis with severe symptoms.
EA has been reported to improve or normalize gastric
dysrhythmia via the vagal pathway, and to increase plasma
pancreatic polypeptide levels in humans (4,7).
Acupuncture restores the balance of parasympathetic and sympathetic
nerves, the disturbance of which is considered to be the sole cause
of gastrointestinal dysmotility in diabetes mellitus (18,19). In
addition, EA has been demonstrated to modulate gastric motility
through regulating the expression of transient receptor potential
vanilloid 1, a Ca2+-permeable non-selective cation
channel that has important physiological functions in peripheral
and central nervous systems (20,21). In
recent decades, numerous studies on patients and animals have been
performed to elucidate the effects of acupuncture on gastric
myoelectrical activity as well as the underlying mechanisms
(4–12,18–24).
However, the exact mechanisms by which acupuncture improves gastric
motility and dyspeptic symptoms have yet to be elucidated.
Although acupuncture has been reported to accelerate
gastric emptying in animals and patients (6,7,10,25,26), the
data of the present study are not fully consistent with this.
Gastroparesis symptoms may be incited by various etiologies,
including functional dyspepsia and chronic gastritis. Delayed
gastric half-emptying has been observed in ~35% of patients with
functional dyspepsia (27,28). In the present study, a cohort of
patients was selected based on clinical symptoms suggestive of
gastroparesis. Therefore, the patients enrolled in the present
study cannot be assumed to have delayed gastric half-emptying
times. Previous studies have demonstrated an insignificant
correlation between the gastric emptying rate and upper
gastrointestinal symptom severity or symptom alleviation in
patients with diabetes (29,30), and the gastric emptying rate is
reportedly also affected by plasma glucose levels and gender
(31). In the present study, no
statistically significant difference was detected in terms of
gastric emptying rate prior to and following EA mono-treatment in
all patients, which may be due to the small sample size of the
study and the aforementioned factors.
Patients with gastroparesis display symptoms
including nausea, vomiting, excessive fullness and bloating. In
order to investigate the different effects of EA on specific
symptoms, GCSI individual scores were evaluated prior to and after
EA treatment in the present study. In the present study,
significant alleviation was observed in nausea, vomiting, stomach
fullness, excessive fullness and bloating after EA treatment in
early-responding patients, while other symptoms were only slightly
alleviated. Although patients in the severe group failed to respond
to EA according to GCSI individual scores, they experienced
significant alleviation in levels of vomiting, stomach fullness and
excessive fullness. Previous reviews also showed evidence of the
efficacy of acupuncture in reducing nausea and vomiting (32,33).
In addition, the present study found that EA did not
have any effect after 1 day of treatment, and significant
alleviation of symptoms appeared to occur between days 7 and 9.
This indicated that repeated treatment with EA may be required to
achieve a satisfactory effect in clinical practice. By contrast, an
extended treatment time may not be beneficial. Between days 7 and
9, the effects reached a peak and fluctuated slightly between days
9 and 14. Therefore, excessively long EA treatment is not
necessary. Of note, in EA and mosapride combination therapy, the
onset and peak time were advanced. This indirectly suggests that EA
may enhance the effects of mosapride. Therefore, it can be
speculated that EA may enable a reduction in the dose of
prokinetics in clinical practice. A previous study demonstrated the
association between the use of another prokinetic, domperidone, and
sudden cardiac death at doses of >30 mg/day (34).
In conclusion, the present study suggested that EA
combined with mosapride may be an optimal treatment for patients
with severe diabetic gastroparesis symptoms, although EA alone is
effective for alleviating mild symptoms. However, the sample size
in the present study was not of sufficient size and further studies
are required to investigate its optional use in clinical
practice.
Acknowledgements
The authors thank their colleagues in various
departments of Qianfoshan Hospital Affiliated to Shandong
University and Shandong College of Traditional Chinese Medicine
(Jinan, China) for their cooperation and assistance.
References
|
1
|
Maleki D, GR III Locke, Camilleri M,
Zinsmeister AR, Yawn BP, Leibson C and LJ III Melton:
Gastrointestinal tract symptoms among persons with diabetes
mellitus in the community. Arch Intern Med. 160:2808–2816. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bytzer P, Talley NJ, Leemon M, Young LJ,
Jones MP and Horowitz M: Prevalence of gastrointestinal symptoms
associated with diabetes mellitus: A population-based survey of
15,000 adults. Arch Intern Med. 161:1989–1996. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Camilleri M, Parkman HP, Shafi MA, Abell
TL and Gerson L; American College of Gastroenterology, : Clinical
guideline: Management of gastroparesis. Am J Gastroenterol.
108:18–37; quiz 38. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chang CS, Ko CW, Wu CY and Chen GH: Effect
of electrical stimulation on acupuncture points in diabetic
patients with gastric dysrhythmia: A pilot study. Digestion.
64:184–190. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wang L: Clinical observation on
acupuncture treatment in 35 cases of diabetic gastroparesis. J
Tradit Chin Med. 24:163–165. 2004.PubMed/NCBI
|
|
6
|
Xu S, Hou X, Zha H, Gao Z, Zhang Y and
Chen JD: Electroacupuncture accelerates solid gastric emptying and
improves dyspeptic symptoms in patients with functional dyspepsia.
Dig Dis Sci. 51:2154–2159. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang CP, Kao CH, Chen WK, Lo WY and Hsieh
CL: A single-blinded, randomized pilot study evaluating effects of
electroacupuncture in diabetic patients with symptoms suggestive of
gastroparesis. J Altern Complement Med. 14:833–839. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liu S, Peng S, Hou X, Ke M and Chen JD:
Transcutaneous electroacupuncture improves dyspeptic symptoms and
increases high frequency heart rate variability in patients with
functional dyspepsia. Neurogastroenterol Motil. 20:1204–1211. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lin X, Liang J, Ren J, Mu F, Zhang M and
Chen JD: Electrical stimulation of acupuncture points enhances
gastric myoelectrical activity in humans. Am J Gastroenterol.
92:1527–1530. 1997.PubMed/NCBI
|
|
10
|
Ouyang H, Yin J, Wang Z, Pasricha PJ and
Chen JD: Electroacupuncture accelerates gastric emptying in
association with changes in vagal activity. Am J Physiol
Gastrointest Liver Physiol. 282:G390–G396. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gao X, Qiao Y, Jia B, Jing X, Cheng B, Wen
L, Tan Q, Zhou Y, Zhu B and Qiao H: NMDA Receotor-Dependent
synaptic activity in dorsal motor nucleus of vagus mediates the
enhancement of gastric motility by stimulating ST36. Evid Based
Complement Alternat Med. 2012:4384602012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lee CH, Kim DK, Yook TH, Sasaki M and
Kitamura N: Effectiveness of electroacupuncture at Zusanli(ST36) on
the immunohistochemical density of enteroendocrine cells related to
gastrointestinal function. J Acupunct Meridian Stud. 5:63–71. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Revicki DA, Rentz AM, Dubois D, Kahrilas
P, Stanghellini V, Talley NJ and Tack J: Development and validation
of a patient-assessed gastroparesis symptom severity measure: The
Gastroparesis Cardinal Symptom Index. Aliment Pharmacol Ther.
18:141–150. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Revicki DA, Rentz AM, Dubois D, Kahrilas
P, Stanghellini V, Talley NJ and Tack J: Gastroparesis Cardinal
Symptom Index (GCSI): Development and validation of a patient
reported assessment of severity of gastroparesis symptoms. Qual
Life Res. 13:833–844. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Talley NJ: Diabetic gastropathy and
prokinetics. Am J Gastroenterol. 98:264–271. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mansi C, Mekga P and Savarino V: Gastric
emptying evaluation by 13C-octanoic acid breath test. Diabetes Nutr
Metab. 17:43–46. 2004.PubMed/NCBI
|
|
17
|
Satta P Usai, Scarpa M, Oppia F and Loriga
F: 13C-octanoic acid breath test in function and organic disease:
Critical review of literature. Eur Rev Med Pharmacol Sci. 9 5 Suppl
1:S9–S13. 2005.
|
|
18
|
Takahashi T: Mechanism of acupuncture on
neuromodulation in the gut: A review. Neuromodulation. 14:8–12.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Takahashi T: Effect and mechanism of
acupuncture on gastrointestinal diseases. Int Rev Neurobiol.
111:273–294. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Abraham TS, Chen ML and Ma SX: TRPV1
expression in acupuncture points: Response to electroacupuncture
stimulation. J Chem Neuroanat. 41:129–136. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang SJ, Yang HY and Xu GS: Acupuncture
alleviates colorectal hypersensitivity and correlates with the
regulatory mechanism of TrpV1 and p-ERK. Evid Based Complement
Alternat Med. 2012:4831232012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Schneider A, Steitberger K and Joos S:
Acupunture treatment in gastrointestinal disease: A systematic
review. World J Gastroenterol. 13:3417–3424. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yin J and Chen JD: Gastrointestinal
motility disorders and acupuncture. Auton Neurosci. 157:31–37.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Fukuta H, Koshita M, Nakamura E, Nakamura
H, Yamada A, Kawase Y, Ishigami T, Kurono Y, Iino S and Suzuki H:
Acupuncture modulates mechanical responses of smooth muscle
produced by transmural nerve stimulation in gastric antrum of
genetically hyperglycemic rats. J Smooth Muscle Res. 45:167–185.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Iwa M, Nakade Y, Pappas TN and Takahashi
T: Electroacupuncture elicits dual effects: Stimulation of delayed
gastric emptying and inhibition of accelerated colonic transit
induced by restraint stress in rats. Dig Dis Sci. 51:1493–1500.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tabosa A, Yamamura Y, Forno ER and Mello
LE: A comparative study of the effects of electroacupuncture and
moxibustion in the gastrointestinal motility of the rat. Dig Dis
Sci. 49:602–610. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lorena SL, Tinois E, Brunetto SQ, Camargo
EE and Mesquita MA: Gastric emptying and intragastric distribution
of a solid meal in functional dyspepsia: Influence of gender and
anxiety. J Clin Gastroenterol. 38:230–236. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Stanghellini V, De Giorgio R, Barbara G,
Cogliandro R, Tosetti C, De Ponti F and Corinaldesi R: Delayed
gastric emptying in functional dyspepsia. Curr Treat Options
Gastroenterol. 7:259–264. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Samsom M, Vermeijden JR, Smout AJ, Van
Doorn E, Roelofs J, Van Dam PS, Martens EP, Eelkman-Rooda SJ and
Van Berge-Henegouwen GP: Prevalence of delayed gastric emptying in
diabetic patients and relationship to dyspeptic symptoms: A
prospective study in unselected diabetic patients. Diabet Care.
26:3116–3122. 2003. View Article : Google Scholar
|
|
30
|
Pasricha PJ, Colvin R, Yates K, Hasler WL,
Abell TL, Unalp-Arida A, Nguyen L, Farrugia G, Koch KL, Parkman HP,
et al: Characteristics of patients with chronic unexplained nausea
and vomiting and normal gastric emptying. Clin Gastroenterol
Hepatol. 9:567–576.e1-4. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Parkman HP, Yates K, Hasler WL, Nguyen L,
Pasricha PJ, Snape WJ, Farrugia G, Koch KL, Abell TL, McCallum RW,
et al: Clinical features of idiopathic gastroparesis vary with sex,
body mass, symptom onset, delay in gastric emptying, and
gastroparesis severity. Gastroenterology. 140:101–115. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Schneider A, Löwe B and Streitberger K:
Perception of bodily sensation as a predictor of treatment response
to acupuncture for postoperative nausea and vomiting prophylaxis. J
Altern Complement Med. 11:119–125. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lee A and Done ML: The use of
nonpharmacologic techniques to prevent postoperative nausea and
vomiting: A meta-analysis. Anesth Analg. 88:1362–1369. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Janssen P, Harris MS, Jones M, Masaoka T,
Farré R, Törnblom H, Van Oudenhove L, Simrén M and Tack J: The
relation between symptom improvement and gastric emptying in the
treatment of diabetic and idiopathic gastroparesis. Am J
Gastroenterol. 108:1382–1391. 2013. View Article : Google Scholar : PubMed/NCBI
|