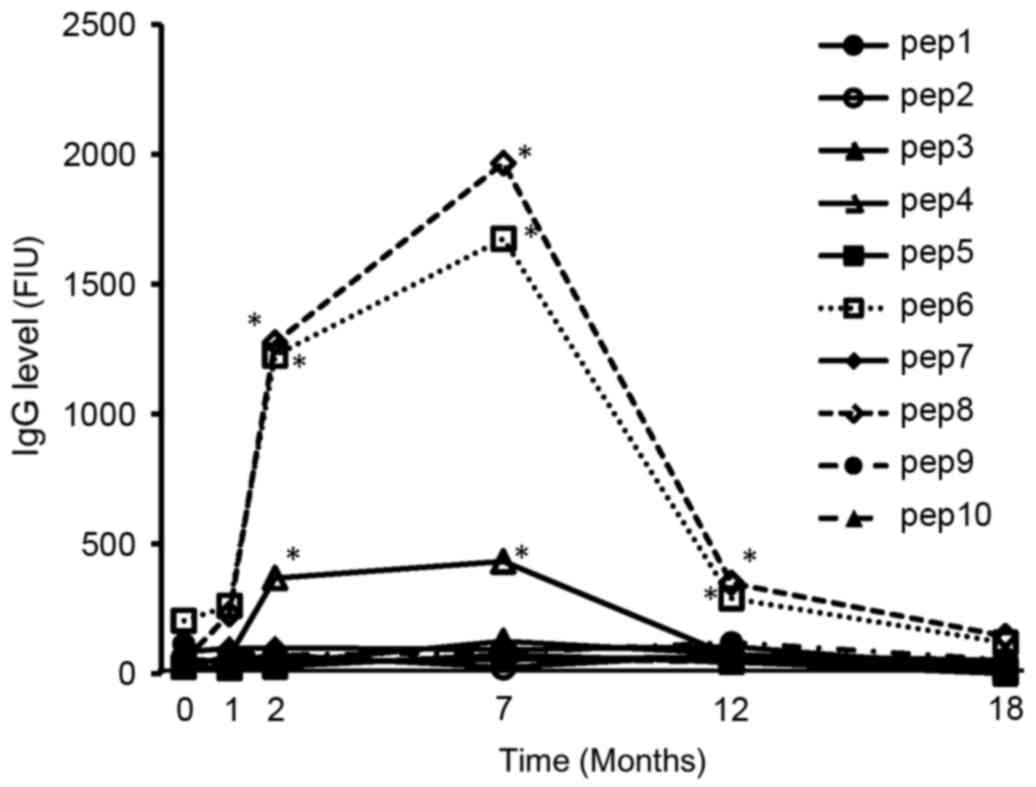
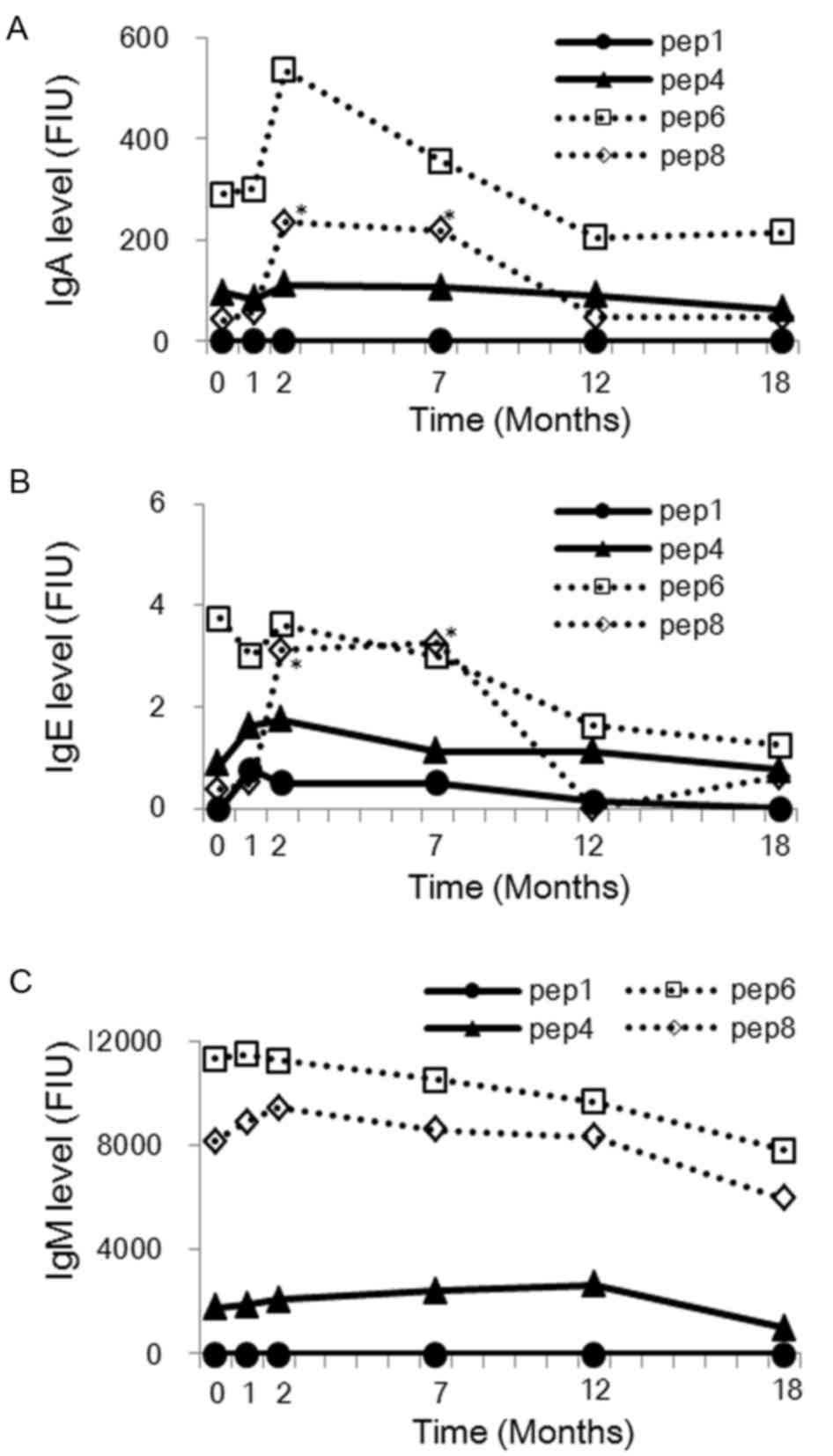
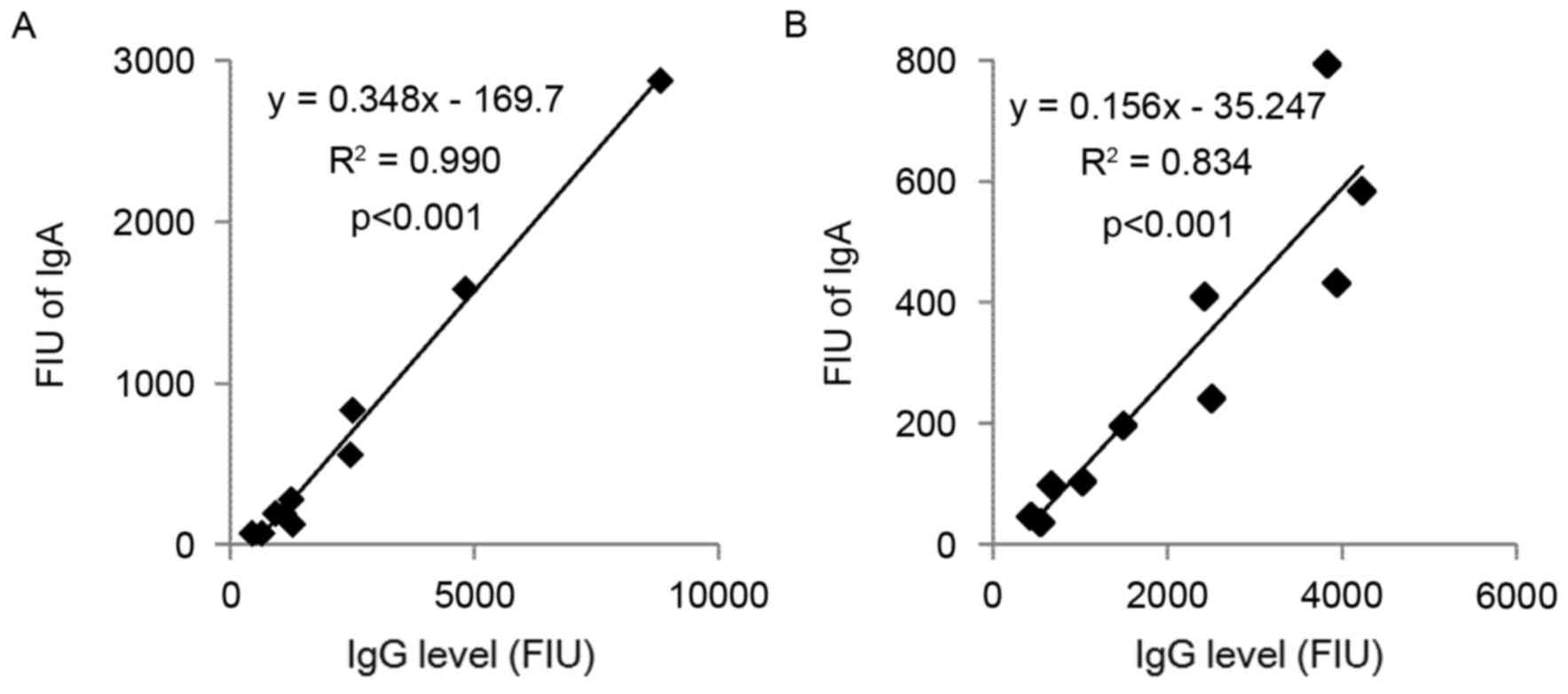
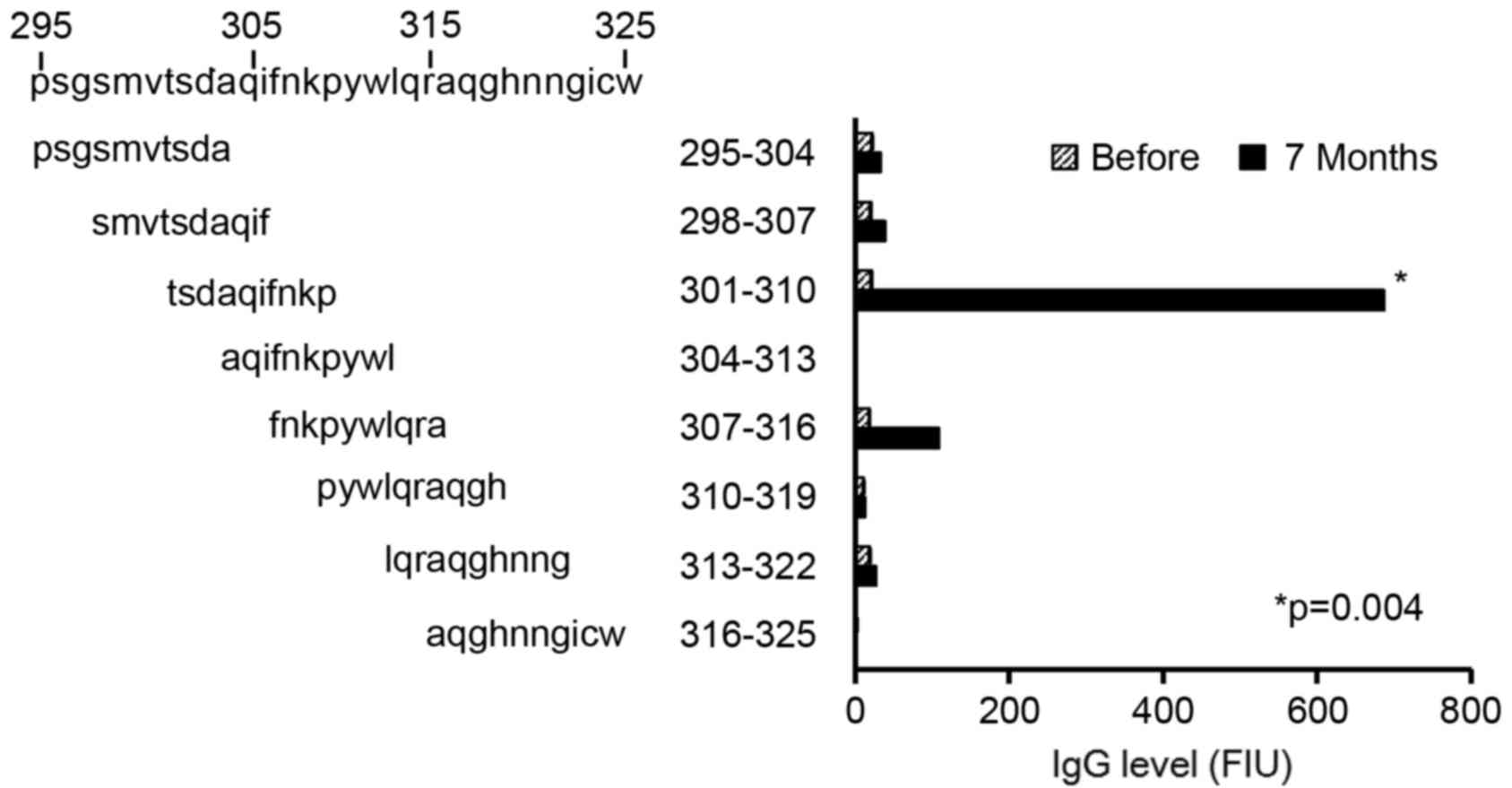
|
1
|
International Agency for Research on
Cancer, . 2012 Globocan 2012: Estimated cancer incidence, mortality
and prevalence worldwide in 2012. http://globocan.iarc.fr/Pages/fact_sheets_cancer.aspx27–November;2016.
|
|
2
|
Muñoz N, Bosch FX, de Sanjosé S, Herrero
R, Castellsagué X, Shah KV, Snijders PJ and Meijer CJ:
International agency for research on cancer multicenter cervical
cancer study group. Epidemiologic classification of human
papillomavirus types associated with cervical cancer. N Engl J Med.
348:518–527. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Li N, Franceschi S, Howell-Jones R,
Snijders PJ and Clifford GM: Human papillomavirus type distribution
in 30,848 invasive cervical cancers worldwide: Variation by
geographical region, histological type and year of publication. Int
J Cancer. 128:927–935. 2010. View Article : Google Scholar
|
|
4
|
Wingo PA, Cardinez CJ, Landis SH, Greenlee
RT, Ries LA, Anderson RN and Thun MJ: Long-term trends in cancer
mortality in the United States, 1930–1998. Cancer. 97:3133–3275.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Villa LL, Costa RL, Petta CA, Andrade RP,
Ault KA, Giuliano AR, Wheeler CM, Koutsky LA, Malm C, Lehtinen M,
et al: Prophylactic quadrivalent human papillomavirus (types 6, 11,
16, and 18) L1 virus-like particle vaccine in young women: A
randomised double-blind placebo-controlled multicentre phase II
efficacy trial. Lancet Oncol. 6:271–278. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Harper DM, Franco EL, Wheeler C, Ferris
DG, Jenkins D, Schuind A, Zahaf T, Innis B, Naud P, De Carvalho NS,
et al: Efficacy of a bivalent L1 virus-like particle vaccine in
prevention of infection with human papillomavirus types 16 and 18
in young women: A randomised controlled trial. Lancet.
364:1757–1765. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Garland SM, Hernandez-Avila M, Wheeler CM,
Perez G, Harper DM, Leodolter S, Tang GW, Ferris DG, Steben M,
Bryan J, et al: Quadrivalent vaccine against human papillomavirus
to prevent anogenital diseases. N Engl J Med. 356:1928–1943. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Paavonen J, Naud P, Salmerón J, Wheeler
CM, Chow SN, Apter D, Kitchener H, Castellsague X, Teixeira JC,
Skinner SR, et al: Efficacy of human papillomavirus (HPV)-16/18
AS04-adjuvanted vaccine against cervical infection and precancer
caused by oncogenic HPV types (PATRICIA): Final analysis of a
double-blind, randomised study in young women. Lancet. 374:301–314.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Roteli-Martins CM, Naud P, De Borba P,
Teixeira JC, De Carvalho NS, Zahaf T, Sanchez N, Geeraerts B and
Descamps D: Sustained immunogenicity and efficacy of the HPV-16/18
AS04-adjuvanted vaccine: Up to 8.4 years of follow-up. Hum Vaccin
Immunother. 8:390–397. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rowhani-Rahbar A, Alvarez FB, Bryan JT,
Hughes JP, Hawes SE, Weiss NS and Koutsky LA: Evidence of immune
memory 8.5 years following administration of a prophylactic human
papillomavirus type 16 vaccine. J Clin Virol. 53:239–243. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Joura EA, Kjaer SK, Wheeler CM, Sigurdsson
K, Iverson OE, Hernandez-Ailta M, Perez G, Brown DR, Koutsky LA,
Tay EH, et al: HPV antibody levels and clinical efficacy following
administration of a prophylactic quadrivalent HPV vaccine. Vaccine.
26:6844–6851. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ryding J, Dahlberg L, Wallen-Ohman M and
Dilner J: Deletion of a major epitope of human papillomavirus
tyle16 virus-like particles. J Gen Virol. 88:792–802. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kirnbauer R, Hubbert NL, Wheeler CM,
Becker TM, Lowy DR and Schiller JT: A virus-like particle
enzyme-linked immunosorbent assay detects serum antibodies in a
majority of women infected with human papillomavirus type 16. J
Natl Cancer Inst. 86:494–499. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Pastrana DV, Buck CB, Pang YY, Thompson
CD, Castle PE, FitzGerald PC, Kjaer S Kruger, Lowy DR and Schiller
JT: Reactivity of human sera in a sensitive, high-throughput
pseudovirus-based papillomavirus neutralization assay for HPV16 and
HPV18. Virology. 321:205–216. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Dias D, Van Doren JV, Schlottmann S, Kelly
S, Puchalski D, Ruiz W, Boerckel P, Kessler J, Antonello JM, Green
T, et al: Optimization and validation of a multiplexed luminex
assay to quantify antibodies to neutralizing epitopes on human
papillomaviruses 6, 11, 16, and 18. Clin Diagn Lab Immunol.
12:959–969. 2005.PubMed/NCBI
|
|
16
|
Waterboer T, Sehr P, Michael KM,
Franceschi S, Nieland JD, Joos TO, Templin MF and Pawlita M:
Multiplex human papillomavirus serology based on in situ-purified
glutathione S-transferase fusion proteins. Clin Chem. 51:1845–1853.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fukui A, Matsueda S, Kawano K, Tsuda N,
Komatsu N, Shichijo S, Sasada T, Hattori S, Ushijima K, Itoh K and
Kamura T: Identification of B cell epitopes reactive to human
papillomavirus type-16 L1-derived peptides. Virol J. 9:1992012.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Komatsu N, Shichijo S, Nakagawa M and Itoh
K: New multiplexed flow cytometric assay to measure anti-peptide
antibody: A novel tool for monitoring immune responses to peptides
used for immunization. Scand J Clin Lab Invest. 64:535–546. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Avogadri F, Merghoub T, Maughan MF,
Hirschhorn-Cymerman D, Morris J, Ritter E, Olmsted R, Houghton AN
and Wolchok JD: Alphavirus replicon particles expressing TRP-2
provide potent therapeutic effect on melanoma through activation of
humoral and cellular immunity. PLoS One. 5:e126702010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hong S, Qian J, Li H, Yang J, Lu Y, Zheng
Y and Yi Q: CpG or IFN-α are more potent adjuvants than GM-CSF to
promote anti-tumor immunity following idiotype vaccine in multiple
myeloma. Cancer Immunol Immunother. 6:561–571. 2012. View Article : Google Scholar
|
|
21
|
Cubillos C, de la Torre BG, Bárcena J,
Andreu D, Sobrino F and Blanco E: Inclusion of a specific T cell
epitope increases the protection conferred against foot-and-mouth
disease virus in pigs by a linear peptide containing an
immunodominant B cell site. Virol J. 9:662012. View Article : Google Scholar : PubMed/NCBI
|