Introduction
Community-acquired pneumonia (CAP) is a leading
disease associated with a high mortality and requiring
hospitalization (1,2). In the United States, ~4 million people
are diagnosed with CAP annually, with ~600,000 of these patients
requiring hospitalization (3). In
Turkey, according to the 2014 health statistics by the Turkish
Ministry of Health, pneumonia accounts for 2.6% of mortality cases
(4).
It is not always possible to establish the disease
etiology for patients with pneumonia (5). In addition, although the equipment
required to identify the etiologic agent is available, isolation of
the agent may take up to seven days (6). A review of the CAP literature in Turkey
shows an identification rate of 21–62.8% (5). Holter et al (7) and Qu et al (8) reported that viral agent was present in
~1/3 of all pneumonias in Norway and China, respectively. In
patients with CAP, a delay >4-8 h in treatment results in an
increased mortality rate (9).
Pneumonia is initially treated with empiric therapy, since
isolation of the agent is challenging and time-consuming. Two newly
published studies have shown that viruses in CAP patients were
detected as causative agents in 34% and 27.5%, respectively.
(7,8). In these studies, influenza and human
rhinovirus were the most common causative agents. Other agents
included parainfluenza viruses, respiratory syncytial virus,
metapneumovirus, enterovirus and adenovirus (7,8).
A new strain of Influenza A virus caused an outbreak
of human infection in April 2009 in the USA and Mexico, and spread
rapidly to other regions. This strain was classified as Influenza A
H1N1 (H1N1) (10). Since 2009, this
novel virus has been the most prevalent viral pneumonia agent among
the respiratory viruses (7). The
influenza A H1N1 infection exhibits a greater impact on populations
<65 years old than other seasonal influenza viruses (10). Pandemic H1N1 is primarily a mild,
self-limiting upper respiratory tract illness, but unlike seasonal
influenza, H1N1 leads to a more severe infection and high mortality
rate in young adults (11). It was
reported that 2–5% of confirmed cases in the USA and Canada, and 6%
of cases in Mexico required hospitalization (12). The majority of hospitalized patients
had underlying conditions such as cardiovascular disease,
respiratory diseases including asthma and chronic obstructive
pulmonary disease, auto-immune disorders, obesity, diabetes or
cancer (12). In addition, secondary
bacterial infections may be accompained by influenza pneumonia
(7). Treatment with oseltamivir in
H1N1 infections should be initiated within the first 48 hours
(13). Therefore, it is necessary to
ensure early diagnosis of viral pneumonias.
The present multicenter study retrospectively
examined the clinical, demographic and prognostic characteristics
of patients diagnosed with epidemic viral pneumonia in Turkey.
Patients and methods
Patients and viral pneumonia
diagnosis
A total of 92 patients were included in the study.
The male/female ratio was 42/50, and the mean age was 48.74±16.65
years. Patients were from 11 individual secondary and tertiary care
hospitals located in various regions of Turkey and the study was
performed retrospectively from January 2015 to April 2015. The
patients had all been previously diagnosed with viral pneumonia.
The patient files were retrospectively reviewed and included in the
study. Viral pneumonia was diagnosed according to clinical and
radiological examination results, and the exclusion of alternative
causes. To confirm the diagnosis of viral pneumonia,
nasopharyngeal, oropharyngeal and nasal swab specimens were placed
into Virocult transport media (Medical Wire & Equipment,
Wiltshire, UK), and transferred to the Virology Laboratory of the
Ankara Refik Saydam Hygiene Institute (Ankara, Turkey) in
accordance with the cold chain principles (14), and were evaluated using the
quantitative polymerase chain reaction (qPCR) method assay as
recommended by the World Health Organization (15). Following appropriate preparations,
accepted laboratory samples were tested with PCR to detect the
presence of influenza A/influenza B via targeting the conservative
gene region of the influenza virus. A second PCR reaction was
carried out using H1, H3 and H5 primers to identify the subtype of
the influenza virus present in the samples. Based on the full
genomic sequence of the recently-identified H1N1 virus, incoming
samples were sequenced. Therefore, typing and genetic
characterization of virus was provided.
All patients were treated with 150 mg/day of the
antiviral therapeutic oseltamivir. Secondary bacterial infections
may be accompained by influenza pneumonia; therefore patients
additionally received intravenous fluoroquinolones or
clarithromycin (1,000 mg/day), in combination with intravenous
β-lactam antibiotics.
Monitoring of viral pneumonia
The symptoms, medical history, radiological
examination, hemogram levels, C-reactive protein (CRP) level
(normal range: 0–5 mg/l) and biochemical parameters (such as
sedimentation, urea, creatinine, aspartate aminotransferase,
alanine aminotransferase, lactate dehydrogenase, creatine kinase)
of the patients were recorded. Arterial blood gas values for those
who required intensive care admission were also recorded.
Furthermore, the following were obtained from the patient files:
Relevant information from the day of presentation after the onset
of symptoms, monitoring parameters [such as arterial blood
pressure, pulse rate, fever, and days spent in the intensive care
unit (ICU)] were recorded in the clinic and/or ICU, and the
treatment administered following disease diagnosis.
Statistical analysis
Data analysis was performed using the Minitab 14
statistical software (Minitab, Inc., State College, PA, USA).
Descriptive statistics were performed to analyze patient
characteristics (Table I). The
Student's t-test and Chi-square test were performed to analyze
quantitative data, assuming a normal distribution, and to analyze
qualitative data, respectively. P<0.05 was considered to be
statistically significant. Prognosis was used as an outcome
(response variable) to establish a binary logistic regression
model, with ‘1’ corresponding to alive, and ‘0’ corresponding to
mortality. Initially, the complete set of demographic variables and
laboratory values that were potentially associated with mortality
were included in the model. Following this, a backward-elimination
approach was performed within a multiple explanatory variable
logistic regression model in order to evaluate the model for
potential confounding effects. In this model, the
factors/covariates were removed individually in decreasing order of
P-value until the remaining factors had a two-sided P value
<0.05. Following this, the Hosmer-Lemeshow test was performed to
determine the goodness-of-fit.
 | Table I.Demographic and laboratory
characteristics of patients. |
Table I.
Demographic and laboratory
characteristics of patients.
| Variable | n | % |
|---|
| Age (mean ± SD),
year | 48.7±16.6 | – |
| Male/female | 42/50 | – |
| Smoking status |
|
|
|
Non-smoker | 47 | 51.1 |
|
Smoker | 18 | 19.6 |
|
Ex-smoker | 27 | 29.3 |
| Duration of symptoms
(median; days) | 5.5 |
|
| Past
contact with upper | 18 | 19.6 |
|
respiratory viral
infections |
| Influenza
vaccination |
|
|
| No | 69 | 75.0 |
| Yes | 3 | 3.3 |
| Arterial blood gas
(median) |
|
|
| pH | 7.43 |
|
|
PaO2 (mmHg) | 55.0 |
|
|
PaCO2 (mmHg) | 35.0 |
|
|
SaO2 (%) | 89.3 |
|
| Laboratory values
(median) |
|
|
|
Leukocytes
(/mm3) | 6,484 |
|
|
Lymphocytes
(/mm3) | 1,000 |
|
|
Neutrophils
(/mm3) | 4,507 |
|
|
Platelets
(/mm3) | 184,500 |
|
|
C-reactive protein (mg/l) | 20.3 |
|
|
Sedimentation (mm/h) | 35 |
|
| Urea
(mg/dl) | 23.7 |
|
|
Creatinine (mg/dl) | 0.88 |
|
|
Aspartate aminotransferase
(IU/l) | 36 |
|
| Alanine
aminotransferase (IU/l) | 33 |
|
| Lactate
dehydrogenase (IU/l) | 401.5 |
|
|
Creatine kinase (IU/l) | 94.2 |
|
| Chest X-ray |
|
|
|
Consolidation |
|
|
|
Bilateral | 64 | 69.6 |
|
Unilateral | 23 | 25.0 |
| Pleural
effusion | 3 |
3.3 |
| Chest
tomography |
|
|
|
Consolidation |
|
|
|
Bilateral | 38 | 79.2 |
|
Unilateral | 10 | 20.8 |
| Pleural
effusion | 4 |
8.3 |
| Virology |
|
|
| H1N1
positivity | 22 | 59.4 |
|
Influenza positivity | 5 | 12.5 |
|
Negative | 10 | 27.1 |
The study was approved by the Ethics Board of the
Abant İzzet Baysal University (Bolu, Turkey). The requirement for
informed consent was waived by the Ethics Board due to the study's
retrospective design.
Results
Patient characteristics and
symptoms
Of the 92 patients that were included in the present
study, 69 (75%) patients were unvaccinated against influenza. The
demographic characteristics, main laboratory results and
radiological findings of the patients are outlined in Table I. The most prevalent symptoms were a
cough (87%) and a fever (63%). The duration between the onset of
symptoms and presentation to the hospital was 5.51±3.27 days, and
18 patients (19.6%) had a history of contact with individuals
complaining of upper respiratory tract viral infections. The
prevalence of symptoms is depicted in Table II. No additional disorders were
found in 56.5% of patients. The prevalence of comorbidities are
shown in Table II.
 | Table II.Distribution of symptoms and comorbid
diseases in patients. |
Table II.
Distribution of symptoms and comorbid
diseases in patients.
| Variable | n | % |
|---|
| Symptoms |
|
|
|
Cough | 80 | 87.0 |
|
Fever | 58 | 63.0 |
|
Dyspnea | 54 | 58.7 |
|
Malaise | 30 | 32.6 |
|
Myalgia | 16 | 17.8 |
|
Headache | 15 | 16.3 |
|
Gastrointestinala | 7 | 7.6 |
| Upper
airwayb | 7 | 7.6 |
| Chest
pain | 6 | 6.5 |
|
Hemoptysis | 2 | 2.2 |
|
Hoarseness | 1 | 1.1 |
| Comorbidities |
|
|
|
None | 52 | 56.5 |
|
Hypertension | 16 | 17.4 |
| Chronic
obstructive pulmonary disease | 14 | 15.2 |
|
Diabetes mellitus | 11 | 12.0 |
|
Asthma | 6 | 6.5 |
|
Coronary artery disease | 5 | 5.4 |
|
Malignancy | 2 | 2.2 |
|
Cerebrovascular disease | 1 | 1.1 |
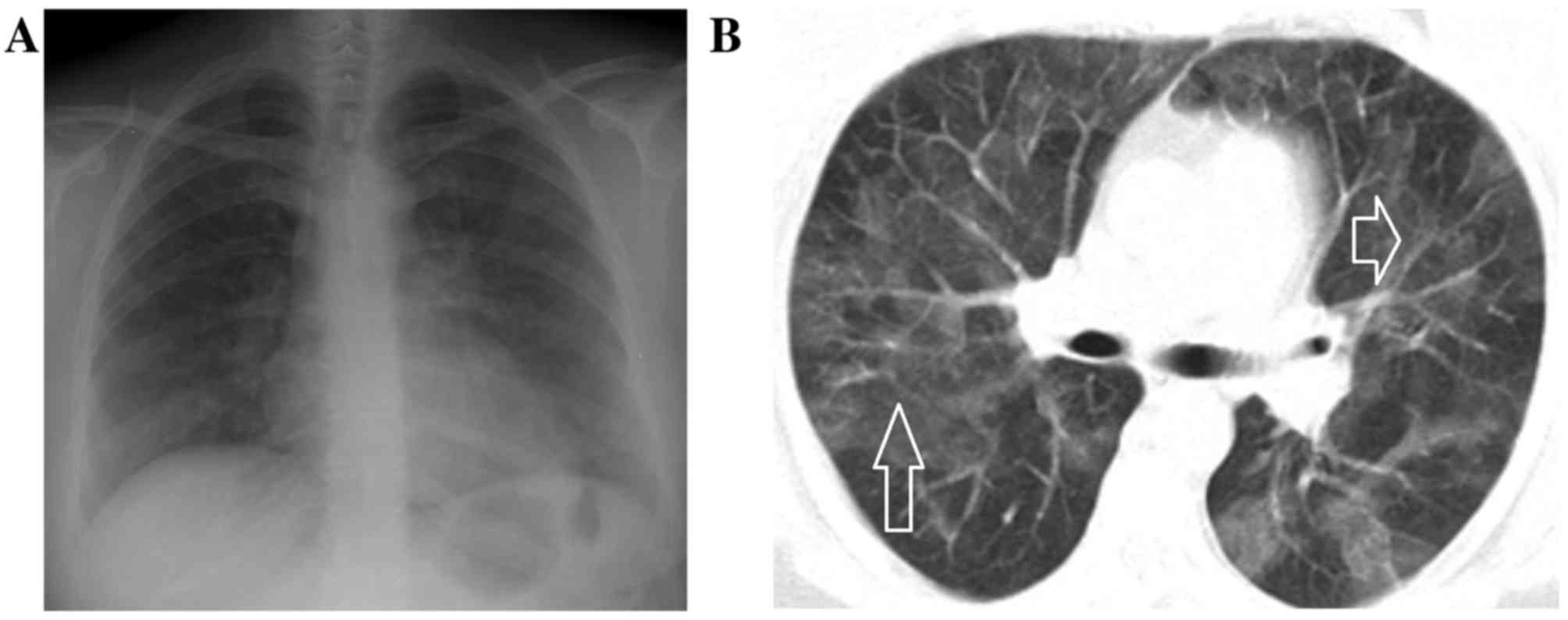
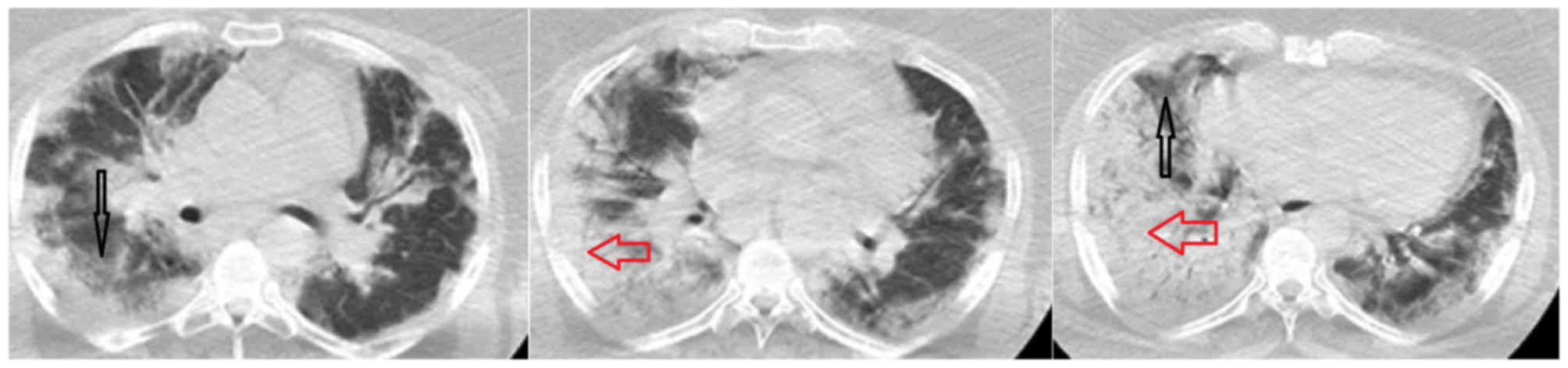
Clinical indications of pneumonia
Chest radiographies revealed bilateral patchy
pneumonic infiltrates in 69.6% of patients. Chest computed
tomography (CT) identified peripheral multiple patchy areas of
ground glass density in 38 patients (41.3%). Bilateral
alveolar-interstitial consolidations and ground-glass opacities
were observed in five patients by chest X-ray and CT imaging
(Figs. 1–5). From the laboratory findings, leukocyte
count was found to be within the normal range,
>10,000/mm3, in 54 patients (58.7%). The lymphocyte
count was found to be below the normal limit, at a median value of
1,000/mm3, in 34 patients (37%). The laboratory data are
detailed in Table I.
The throat and nasal swab specimens of 37 patients
with clinical and radiological findings suggestive of viral
pneumonia were sent to the laboratory. Treatment was initiated on
the same day. A total of 22 (59.4%) of these patients had H1N1, and
5 (12.5%) patients had influenza B. Patients with suspected viral
pneumonia were administered the antiviral drug oseltamivir, on a
dose of 150 mg/day, and monitored accordingly.
Outcomes of patients admitted to the
ICU
Of the 92 patients in total, 38 (41.3%) patients met
the criteria for admission to the ICU. Of these patients, 20
(52.63%) were monitored with a mechanical ventilator, with a
noninvasive mechanical ventilator (NIMV) being adequate for 10
(26.32%) of the patients. A smaller group of 8 (8.7%) patients were
monitored with NIMV during weaning from mechanical ventilation. The
length of stay in the ICU was 6.45±5.97 days (1–30 days), and the
duration of mechanical ventilation (MV) was 5.06±4.69 days (1–18
days). A total of 12 (13.04%) patients did not survive treatment.
Logistic regression analysis indicated that among the parameters
potentially associated with mortality, current smoking increased
the mortality risk 7.08-fold compared with non-smokers and
ex-smokers (95% confidence interval: 1.81–27.67) (P=0.005). No
significant relationship was found between mortality and other
parameters, including age, gender, comorbid diseases, platelet
count, lactate dehydrogenase (LDH) level, arterial blood oxygen
level at the time of presentation and creatine kinase (CK).
Discussion
The present study demonstrated that the majority of
patients diagnosed with viral pneumonia were middle-aged
individuals presenting with major symptoms of a cough, fever and
shortness of breath. The patients displayed a rapid progression of
patchy infiltrates in their chest radiographies and a large
proportion required admission to the ICU. A total of 38 (41.3%)
patients were monitored at the ICU, and 20 (52.63%) required MV.
The rate of mortality was 13.04%. Active smoking caused a 7.08-fold
increase in the mortality risk.
In the present study, the mean age was 48.74±16.65
years, with no significant difference in gender. A past study
comparing patients with bacterial CAP and viral pneumonias found
the mean age to be 60.0±20.2 years for bacterial CAP patients vs.
49.7±18.7 years for viral pneumonia patients (16). In a general study of patients with
CAP, the mean age was 66 years, with a range of 52–78 years
(7). Another study found that 90.3%
of patients with H1N1 were younger than 65 years (17). In the present study, the mean age was
found to be lower for viral pneumonia compared to that recorded
previously for bacterial pneumonia (5,16).
However, the present results are consistent with the previously
recorded mean ages of patients with viral pneumonia (16,18).
A study including 1,088 cases performed in 2009
during the H1N1 pandemic reported that the clinical status of
patients deteriorated rapidly; 31% required intensive care,
mortality was higher in those >50 years-old, overall mortality
was 11% and the rate of comorbidity was 68% (19). Similarly, Gürgün et al
reported that 25% of patients diagnosed with influenza pneumonia
required intensive care (16), while
Almirall et al found that the rate of ICU admissions was 7%
in patients with bacterial CAP vs. 19.3% in patients with viral CAP
(20). Furthermore, Rello et
al reported an ICU admission rate of 62.5% in H1N1 pneumonia
(21). For the patients of the
present study, the rate of ICU admission was 41.3%. This higher
rate relative to that documented for bacterial pneumonia suggests
that patients with suspected viral pneumonia may require closer
monitoring (5).
An analysis of several demographic and laboratory
phenotypes (including age, gender, CRP level, sedimentation, urea,
creatinine, aspartate aminotransferase, alanine aminotransferase,
lactate dehydrogenase and creatine kinase) associated with
mortality indicated that active smoking increased the mortality
risk 7.08-fold. Previously published studies on CAP have revealed
that 72% of pneumonia cases requiring intensive care were
predisposed by smoking (22).
Similarly, smoking has been found to be a risk factor in patients
requiring admission to the ICU for viral pneumonia (23). Cigarette smoke disrupts the pulmonary
defense mechanism by reducing mucociliary clearance (24). This deterioration in the defence
mechanism may be associated with the increased rate of mortality
with pneumonia in current smokers. Although it is established that
mortality is higher in patients admitted to the ICU, a direct
association between active smoking and mortality in viral pneumonia
remains to established. Previous analysis of the radiological
imaging methods indicated that bilateral involvement was greater in
viral pneumonias (25), while other
studies found that radiological presentation varies substantially,
and thus, has no predictive value in differential diagnosis
(26). Similar to the results by
Shiley et al (25), present
results of the chest radiography and chest CT imaging revealed
bilateral pneumonic infiltrates in 69.6 and 79.2% of patients,
respectively.
The current study found that 69 (75%) patients were
unvaccinated against influenza. This may be due to the proportion
of young adults included in the patient group, or that only 26.09%
of cases exhibited comorbidities. As viral pneumonias are thought
to progress rapidly following the onset of symptoms (21), and the present study found a
mortality rate of 13.04%, these results suggest that influenza
vaccination should not be overlooked by individuals who have no
contraindications for vaccination. In the present study, 1 out of
12 of the patients who did not survive were vaccinated against
influenza.
Similar to previous studies, the current study found
that the majority (59.4%) of viral swab specimens taken from
patients with suspected viral pneumonia were positive for influenza
A (7,8). The patients from whom specimens where
not requested consisted of those who had suspected viral pneumonia
based on clinical, radiological and laboratory findings, however,
lacked the growth of any other microorganisms. In clinical practice
in Turkey, swab specimens from patients who are suspected to have
viral pneumonia, are analysed only during the ‘influenza season’
(i.e., December-March). When cases of influenze are less prevalent,
diagnoses are made according to clinical and radiological findings.
Nevertheless, an association between early antiviral treatment and
better survival has been demonstrated by previous studies (19,27). In
the present study, patients were treated with oseltamivir.
Secondary bacterial infections may be accompained by influenza
pneumonia (7,28); therefore, patients additionally
received fluoroquinolone or clarithromycin, in combination with
intravenous β-lactam antibiotics.
The current study had a number of limitations. In
January 2015, the cases of viral pneumonias were considered to be
sporadic and not at an epidemic level. Therefore, specimens were
not obtained from each patient to identify the strain types. By
contrast, when increasing numbers of cases were reported by
multiple centers, particularly in February and March 2015,
specimens were collected from the patients to confirm the diagnosis
of viral pneumonia. The present study was performed retrospectively
and was multicentric; as such, some laboratory results could not be
obtained from all patients.
In conclusion, viral pneumonia remains a major
health problem during the winter period. In affected patients, the
H1N1 virus is a potential etiologic agent. Therefore, H1N1 should
be considered, particularly in patients presenting with symptoms of
pneumonia. These include fever, shortness of breath and muscle pain
during the influenza season, coupled with clinical characteristics,
including high levels of CRP, LDH and CK, and radiological evidence
of bilateral pneumonic infiltrates that progress rapidly. Smoking
habits should be particularly questioned, due to findings of an
association between active smoking and increased mortality. As
viral pneumonia is associated with high mortality and the need for
ICU admission, antiviral treatment should be rapidly initiated upon
clinical suspicion of the disease. However, there is still no
method to quickly isolate the pneumonia agent. Further research is
required to elucidate the role of active smoking on pneumonia
mortality. Additionally, further studies should be performed to
identify a method for rapid and definite pneumonia agent
isolation.
References
|
1
|
Welte T, Torres A and Nathwani D: Clinical
and economic burden of community-acquired pneumonia among adults in
Europe. Thorax. 67:71–79. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mandell LA, Wunderink RG, Anzueto A,
Bartlett JG, Campbell GD, Dean NC, Dowell SF, File TM Jr, Musher
DM, Niederman MS, et al: Infectious diseases society of
America/American thoracic society consensus guidelines on the
management of community-acquired pneumonia in adults. Clin Infect
Dis. 44:27–72. 2007. View
Article : Google Scholar
|
|
3
|
Fine MJ, Auble TE, Yealy DM, Hanusa BH,
Weissfeld LA, Singer DE, Coley CM, Marrie TJ and Kapoor WN: A
prediction rule to identify low-risk patients with
community-acquired pneumonia. N Engl J Med. 336:243–250. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Türkiye istatistik kurumu. http://www.tuik.gov.trMarch 16–2016.
|
|
5
|
Ozlü T, Bülbül Y and Ozsu S:
Community-acquired pneumonia based on the Turkish national data.
Tuberk Toraks. 55:191–212. 2007.(In Turkish). PubMed/NCBI
|
|
6
|
Public Health Institution of Turkey: Field
Guide for Laboratory Diagnosis of Contagious Diseases. http://mikrobiyoloji.thsk.saglik.gov.tr/ums/B/Bakteriyel%20pnomoniler.pdfDecember
21–2016.
|
|
7
|
Holter JC, Müller F, Bjørang O, Samdal HH,
Marthinsen JB, Jenum PA, Ueland T, Frøland SS, Aukrust P, Husebye
E, et al: Etiology of community-acquired pneumonia and diagnostic
yields of microbiological methods: A 3-year prospective study in
Norway. BMC Infect Dis. 15:642015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Qu JX, Gu L, Pu ZH, Yu XM, Liu YM, Li R,
Wang YM, Cao B and Wang C; Beijing Network for Adult
Community-Acquired Pneumonia (BNACAP), : Viral etiology of
community-acquired pneumonia among adolescents and adults with mild
or moderate severity and its relation to age and severity. BMC
Infect Dis. 15:892015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tang CM and Macfarlane JT: Early
management of younger adults dying of community acquired pneumonia.
Respir Med. 87:289–294. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gómez-Gómez A, Magaña-Aquino M,
Garcia-Sepúlveda C, Ochoa-Pèrez UR, Falcón-Escobedo R, Comas-García
A, Aranda-Romo S, Contreras-Treviño HI, Jimenéz-Rico PV,
Banda-Barbosa MA, et al: Severe pneumonia associated with pandemic
(H1N1) 2009 outbreak, San Luis Potosi, Mexico. Emerg Infect Dis.
16:27–34. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sertogullarindan B, Ozbay B, Gunini H,
Sunnetcioglu A, Arisoy A, Bilgin HM, Cilingir B Mermit, Duran M,
Yildiz H, Ekin S and Baran A: Clinical and prognostic features of
patients with pandemic 2009 influenza A (H1N1) virus in the
intensive care unit. Afr Health Sci. 11:163–170. 2011.PubMed/NCBI
|
|
12
|
Girard MP, Tam JS, Assossou OM and Kieny
MP: The 2009 A (H1N1) influenza virus pandemic: A review. Vaccine.
28:4895–4902. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fiore AE, Shay DK, Haber P, Iskander JK,
Uyeki TM, Mootrey G, Bresee JS and Cox NJ; Advisory Committee on
Immunization Practices (ACIP), ; Centers for Disease Control and
Prevention (CDC), : Prevention and control of influenza.
Recommendations of the advisory committee on immunization practices
(ACIP), 2007. MMWR Recomm Rep. 56(RR-6): 1–54. 2007.PubMed/NCBI
|
|
14
|
Wang X, Zoueva O, Zhao J, Ye Z and Hewlett
I: Stability and infectivity of novel pandemic influenza A (H1N1)
virus in blood-derived matrices under different storage conditions.
BMC Infect Dis. 11:3542011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jain S, Kamimoto L, Bramley AM, Schmitz
AM, Benoit SR, Louie J, Sugerman DE, Druckenmiller JK, Ritger KA,
Chugh R, et al: Hospitalized patients with 2009 H1N1 influenza in
the United States, April-June 2009. N Engl J Med. 361:1935–1944.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gürgün A, Bacakoğlu F, Başoğlu OK,
Taşbakan MS, Pullukçu H and Sayiner A: Comparison of the patients
with pandemic (H1N1) influenza A virus pneumonia and
community-acquired pneumonia. Tuberk Toraks. 58:357–365. 2010.(In
Turkish). PubMed/NCBI
|
|
17
|
Bakir M: Pandemic influenza situation
update in Turkey. J Infect Dev Ctries. 4:124–125. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Duru S, Köker Y, Uyanusta Ç, Şencan N,
Altaş B, Albayrak N and Ardıç S: Clinical analysis in influenza A
(H1N1) virus patients. Turkish Thoracic J. 13:45–49. 2012.
View Article : Google Scholar
|
|
19
|
Louie JK, Acosta M, Winter K, Jean C,
Gavali S, Schechter R, Vugia D, Harriman K, Matyas B, Glaser CA, et
al: Factors associated with death or hospitalization due to
pandemic 2009 influenza A(H1N1) infection in California. JAMA.
302:1896–1902. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Almirall J, Bolíbar I, Vidal J, Sauca G,
Coll P, Niklasson B, Bartolomé M and Balanzó X: Epidemiology of
community-acquired pneumonia in adults: A population-based study.
Eur Respir J. 15:757–763. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Rello J, Rodriguez A, Ibañez P, Socias L,
Cebrian J, Marques A, Guerrero J, Ruiz-Santana S, Marquez E, Del N,
ogal-Saez F, et al: Intensive care adult patients with severe
respiratory failure caused by Influenza A (H1N1)v in Spain. Crit
Care. 13:R1482009. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ediger D, Uzaslan EK, Yüksel EG, Coşkun F,
Ege E, Karadaş M and Gözü RO: Evaluation of pneumonia cases in
intensive care unit. Turkiye Klinikleri Archives Lung. 6:111–114.
2005.
|
|
23
|
Kumar A, Zarychanski R, Pinto R, Cook DJ,
Marshall J, Lacroix J, Stelfox T, Bagshaw S, Choong K, Lamontagne
F, et al: Critically ill patients with 2009 influenza A(H1N1)
infection in Canada. JAMA. 302:1872–1879. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Arcavi L and Benowitz NL: Cigarette
smoking and infection. Arch Intern Med. 164:2206–2216. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Shiley KT, Van Deerlin VM and WT Jr
Miller: Chest CT features of community-acquired respiratory viral
infections in adult inpatients with lower respiratory tract
infections. J Thorac Imaging. 25:68–75. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Miller WT Jr, Mickus TJ, Barbosa E Jr,
Mullin C, Van Deerlin VM and Shiley KT: CT of viral lower
respiratory tract infections in adults: Comparison among viral
organisms and between viral and bacterial infections. AJR Am J
Roentgenol. 197:1088–1095. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zarychanski R, Stuart TL, Kumar A,
Doucette S, Elliott L, Kettner J and Plummer F: Correlates of
severe disease in patients with 2009 pandemic influenza (H1N1)
virus infection. CMJA. 182:257–264. 2010.
|
|
28
|
Rodriguez A, Alvarez-Rocha L, Sirvent JM,
Zaragoza R, Nieto M, Arenzana A, Luque P, Socías L, Martín M,
Navarro D, et al: Recommendations of the infectious diseases work
group (GTEI) of the spanish society of intensive and critical care
medicine and coronary units (SEMICYUC) and the infections in
critically Ill patients study group (GEIPC) of the spanish society
of infectious diseases and clinical microbiology (SEIMC) for the
diagnosis and treatment of influenza A/H1N1 in seriously ill adults
admitted to the intensive care unit. Med Intensiva. 36:103–137.
2012.(In Spanish). PubMed/NCBI
|