Introduction
The liver is important in metabolism, detoxification
and secretory functions in the body, and its disorder and
dysfunction may lead to severe liver damage as a result of
increasing cellular, tissue and functional disruption. Carbon
tetrachloride (CCl4) is a potent environmental
hepatotoxin (1) that has been
reported to induce acute and chronic tissue injuries (2–5). Acute
administration of CCl4 is utilized to establish a model
of severe liver damage through generation of oxidative stress,
recruitment of inflammatory cells and cell death (6–8), and
ultimately, liver architectural and functional damage (9,10). The
regeneration of damaged liver from CCl4-induced acute
liver injury involves a complex regulated cellular response
(11,12).
Oxidative stress and inflammatory responses may be
critical in the chemical hepatic injury induced by CCl4,
as CCl4 exposure induces elevations in reactive and
cytotoxic lipoperoxide and free peroxide radicals (13,14). In
addition, various factors, including bacterial and viral
infections, toxins, dietary factors and alcohol abuse may promote
liver inflammation. Pro-inflammatory chemokines and immune cells
have an important role in the regulation of the inflammatory
response. Nuclear factor-κB (NF-κB) is involved in several
inflammatory cytokine responses, including pathways mediated by
endotoxins that cause liver damage (15,16).
Blocking NF-κB signaling inhibits a positive feedback loop that is
mediated by pro-inflammatory cytokines such as interleukin (IL)-1
and tumor necrosis factor (TNF)-α that attenuate inflammation
(17).
Natural remedies from medicinal plants are
considered safe and effective alternative treatments against
hepatotoxicity. The present study investigated the hepatoprotective
effects of Pien Tze Huang Gan Bao (GB), which contains Calculus
bovis, Panax notoginseng, Artemisia capillaris,
snake gall and Radix Paeoniae alba, against CCl4-induced
hepatotoxicity in rats. GB, a classical Traditional Chinese
Medicine formula, has been used for millennia in China and
Southeast Asia. GB has demonstrated efficacy in protecting against
liver injury due to excessive alcohol consumption. However, no
scientific reports are currently available to explain the potential
protective effects of GB against chemical injury. The present study
was undertaken to evaluate the protective effect of GB on
CCl4-induced hepatotoxicity, and the antioxidant and
anti-inflammatory effects of GB in liver-injured rats were
investigated.
Materials and methods
Reagents
GB was obtained from and authenticated by the sole
manufacturer, Zhangzhou Pien Tze Huang Pharmaceutical Co. Ltd.,
(Zhangzhou, China; Chinese Food and Drug Administration approval
no., HPK-08411). Superoxide dismutase (SOD), malondialdehyde (MDA),
thiobarbituric acid reactive substances (TBARS), glutathione (GSH)
and glutathione peroxidase (GSP-PX) assay kits were purchased from
Nanjing Jiancheng Biotech Co., Ltd. (Nanjing, China). BCA Protein
Assay Kit was purchased from Tiangen Biotech Co., Ltd., (Beijing,
China). IL-1β and TNF-α ELISA kits were purchased from Shanghai
Xitang Biotech Co., Ltd. (Shanghai, China). TRIzol reagent was
obtained from Life Technologies (Thermo Fisher Scientific, Inc.,
Waltham, MA, USA). A PrimeScript™ RT reagent kit with gDNA Eraser
was purchased from Takara Bio Inc., (Tokyo, Japan). CCl4
was purchased from Shanghai Lingfeng Chemical Co., Ltd. (Shanghai,
China).
Animals
A total of 60 male 6-week-old Sprage-Dawley rats
(Slike Co. Ltd., Shanghai, China), weighing 180–200 g, were housed
at five per cage in an environmentally controlled room at 22±1°C
and relative humidity of 40–60% with a 12-h light/dark cycle.
Animals were allowed access to food and water ad libitum for
one week prior to the start of the study. All experiments involving
animals were approved by the Fujian Institute of Traditional
Chinese Medicine Animal Ethics Committee (Fuzhou, China). All
experimental procedures were performed in accordance with the
Guidelines for Animal Experimentation of Fujian University of
Traditional Chinese Medicine (Fuzhou, China).
CCl4-induced hepatotoxicity
and experimental design
The rats were randomly divided into six groups (n=10
in each): Group 1, Control group; group 2, CCl4 injury
model group; group 3, silymarin (Jiangsu ZTE Pharmaceutical Co.,
Ltd., Jiangsu, China) treatment group [pre-treated with silymarin,
50 mg/kg by oral gavage (per os; p.o.), for 7 days]; group 4,
low-dose treatment group (pre-treated with GB, 150 mg/kg p.o., for
7 days); group 5, medium-dose treatment group (pre-treated with GB,
300 mg/kg p.o., for 7 days), and group 6, high-dose treatment group
(pre-treated with GB, 600 mg/kg p.o., for 7 days). Rats in groups 1
and 2 were pre-treated with phosphate-buffered saline (0.5 ml/100
g) p.o. for 7 days. On the final day, rats from groups 2–6 received
an intraperitoneal (i.p.) injection of CCl4 at a dose of
2.0 ml/kg in a 50% corn oil solution, while group 1 received 2.0
ml/kg of corn oil only. At 24 h after CCl4
administration, the animals were anesthetized (40 mg/kg
pentobarbital; Sigma-Aldrich; Merck Millipore, Darmstadt, Germany;
i.p.), sacrificed by cervical dislocation and subjected to
laparotomy. Blood was collected from the aorta abdominalis into
non-heparinized tubes and centrifuged (980 × g at 4°C for 10 min)
to obtain serum for biochemical tests. The livers were quickly
excised, washed, and a portion of the liver was dissected and fixed
in 4% formaldehyde saline solution for histological analysis; the
remaining tissue was snap frozen in liquid nitrogen and stored at
−70°C until use for determination of oxidative stress markers and
for molecular analysis.
Biochemical assays
Aspartate aminotransferase (AST), alanine
aminotransferase (ALT), alkaline phosphatase (ALP), gamma glutamyl
transpeptidase (γ-GT) and total bilirubin (TB) levels were
evaluated to assess hepatic function using an automatic biochemical
analyzer (Bayer ADVIA 2400; Bayer, Siemens, Germany), according to
the manufacturer's instructions.
Histopathological analysis
Extracted livers were fixed in 10% buffered formalin
for a minimum of 48 h, processed and embedded in paraffin. Paraffin
sections were then prepared by an automatic tissue processor (RM2;
Leica Microsystems GmbH, Wetzlar, Germany) and cut into 5 µm-thick
sections by a rotary microtome. Sections then were stained with
haematoxylin-eosin dye and analyzed for histopathological
alterations. Photomicrographs of stained tissue sections were
obtained using a DMRB/E light microscope (Leica Microsystems
GmbH).
Assessment of oxidative stress
Liver tissue was homogenized in cold 50 mM potassium
phosphate buffer (pH 7.0) using a Potter-Elvehjem homogenizer
(SRH4000-30) to give a 10% (w/v) liver homogenate, which was
centrifuged at 980 × g for 10 min. The supernatant was used for
analysis of TBARS, MDA, SOD, GSH and GSH-PX. Hepatic TBARS were
assayed according to previously described methods (18). In brief, TBARS were determined using
the thiobarbituric acid reaction with 1.0 mM EDTA added to the
reaction medium as a minor modification. The concentration of
hepatic TBARS was expressed as MDA equivalents. SOD activity was
determined by measurement of the inhibition of cytochrome C
reduction via assessment of the absorbance at 550 nm and expressed
in U/mg protein (19), whereas
GSH-PX activity was determined via assessment of nicotinamide
adenine dinucleotide phosphate oxidation of t-butyl peroxide by
measuring the absorbance at 412 nm and expressed in U/mg protein.
The GSH concentration in homogenates was tested using a previously
described method (20). Absorbance
was measured at 420 nm and the results are expressed as mg/g
protein. Endogenous lipid peroxidation was determined by measuring
MDA at 532 nm and the results are expressed as nmol/mg protein.
Immunohistochemistry
NF-κB expression was examined immunohistochemically
using rabbit anti-rat polyclonal NF-κB p65 immunoglobulin G
antibody (cat. no. sc-372; Santa Cruz Biotechnologies, Inc.,
Dallas, TX, USA). In brief, sections were subjected to antigen
retrieval, blocking of endogenous peroxidase activity and
incubation at 4°C overnight with rabbit polyclonal anti-NF-κB
primary antibody (diluted 1:200). The sections were then incubated
at 37°C for 20 min with appropriate biotinylated secondary antibody
(diluted 1:1,000) followed by horseradish peroxidase-conjugated
streptavidin and reaction with 3,3-diaminobendizine (Sigma-Aldrich;
Merck Millipore) working solution at room temperature for 6 min.
Immunolabeled sections were counterstained with Harris hematoxylin
(Sigma-Aldrich; Merck Millipore). To rule out any nonspecific
labeling, negative controls were used, in which primary antibodies
were replaced with PBS. The average proportion of positive cells in
each field was counted using a true color multi-functional cell
image analysis management system (Image-Pro Plus, Media
Cybernetics, Bethesda, MD, USA).
Quantification of mRNA expression by
reverse-transcription quantitative polymerase chain reaction
(RT-qPCR)
TRIzol reagent was used to isolate total RNA from
liver tissues according to the manufacturer's instructions.
Following quantification by measuring the absorbance at 260 nm, the
RNA was reverse-transcribed into complementary (c)DNA using the
PrimeScript™ RT reagent kit with the gDNA Eraser Kit (Takara,
Kyoto, Japan) according to the manufacturer's instructions. A total
of 1.0 µg total RNA from each sample was added to a mixture of 1.0
µl MultiScribe reverse transcriptase, 2.0 µl 10X reverse
transcriptase random primers, 0.8 µl 25X deoxynucleotide
triphosphate mix (100 mM), 2.0 µl 10X reverse transcriptase buffer
and 3.2 µl nuclease-free water. The reaction conditions were as
follows: 25°C for 10 min, 37°C for 15 min and 85°C for 5 sec,
followed by cooling to 4°C. The cDNA was then subjected to PCR
amplification using SYBR Premix Ex Taq II (Takara) in an ABI 7500
Fast instrument (Applied Biosystems, Thermo Fisher Scientific,
Inc.). PCR thermal cycling was performed as follows: 95°C for 30
sec, followed by 40 cycles of 95°C for 3 sec and 60°C for 30 sec,
and one cycle of 95°C for 15 sec, 60°C for 1 min and 95°C for 15
sec. The PCR mixture contained: 2.0 µl cDNA, 10 µl SYBR Premix Ex
Taq II (2X), 0.8 µl PCR forword primer (10 µm), 0.8 µl PCR reverse
primer (10 µM), 0.4 µl ROX reference dye II and 6 µl
dH2O. mRNA expression values were determined as
∆Cq=Cqsample-CqGAPDH and relative quantities
between different samples were determined as
∆∆Cq=∆Cqsample1-∆Ctsample2. The values were
expressed as 2−∆∆Ct. All qPCR reactions were performed
in triplicate.
The sequences of the primers used for amplification
of NF-κB, IL-1β, TNF-α and β-actin transcripts were as follows:
NF-κB forward, 5′-ACGCAAAAGGACCTACGAGACC-3′ and reverse,
5′-ATGTTGAAAAGGCATAGGGCTG-3′; IL-1β forward,
5′-TGTTCTTTGAGGCTGAC-3′ and reverse, 5′-CTTTGGGATTTGTTTGG-3′; TNF-α
forward, 5′-CAGCAGATGGGCTGTACCTT-3′ and reverse,
5′-AAGTAGACCTGCCCGGACTC-3′; and β-actin forward,
5′-CGGTCAGGTCATCACTATCGGC-3′ and reverse,
5′-GTGTTGGCATAGAGGTCTTTACGG-3′.
ELISA
A commercial ELISA kit was employed to determine
TNF-α or IL-1β expression. The method used was a solid-phase
sandwich ELISA utilizing a monoclonal antibody specific for rat
TNF-α or IL-1β coated on a 96-well plate. Standards and samples
were added to the wells and incubated to allow for any present
TNF-α or IL-1β to bind to immobilized antibody. The plates were
washed and biotinylated polyclonal anti-rat TNF-α or IL-1β antibody
was added. Following an additional wash, avidin-horseradish
peroxidase was added to form an antibody-antigen-antibody sandwich.
Upon repeating the wash, a substrate solution was added to generate
a blue color proportional to the amount of rat TNF-α or IL-1β
present in the sample. Stop buffer was added to terminate the
reaction, leading to a change in color from blue to yellow. The
absorbance of the plates at 450 nm was read and the amount of rat
TNF-α or IL −1β was expressed as pg/mg protein.
Statistical analysis
Values are expressed as the mean ± standard
deviation of three measurements or assessments. Data were analyzed
using the SPSS software package for Windows (version 11.5; SPSS,
Inc., Chicago, IL, USA). Analysis of differences between groups was
performed by one-way analysis of variance. P<0.05 was considered
to indicate a statistically significant difference.
Results
GB prevents CCl4-induced
increases in liver parameters
The serum biochemical parameters providing
information regarding the hepatoprotective influence of GB in
CCl4-intoxicted rats are shown in Table I. CCl4-treated rats
developed extensive hepatic damage, evidenced by significant
increases in the levels of serum AST, ALT, ALP, γ-GT and TB levels
compared with those in the control group, which was significantly
inhibited by GB at doses of 150, 300 and 600 mg/kg (P≤0.05),
particularly in the case of AST, ALT and ALP. The standard control
drug silymarin also prevented the elevation of serum enzymes and
TB. Treatment with 150, 300 and 600 mg/kg GB and 50 mg/kg silymarin
had protective effects as shown by decreases in ALT levels by
32.84, 30.78, 58.38 and 45.68%, AST levels by 56.06, 82.96, 89.68
and 45.35% and ALP levels by 26.41, 34.28, 33.17 and 35.26%,
respectively.
 | Table I.Effect of GB on ALT, AST, ALP, γ-GT,
TB and LDH in serum of rats. |
Table I.
Effect of GB on ALT, AST, ALP, γ-GT,
TB and LDH in serum of rats.
| Group | ALT (U/l) | AST (U/l) | ALP (U/l) | γ-GT (U/l) | TB (µmol/l) |
|---|
| Control |
48.70±9.38 | 128.40±20.30 | 295.22±42.86 | 1.00±0.67 | 0.75±0.19 |
|
CCl4 |
518.67±84.53a |
1252.83±95.49a |
359.43±59.18a |
1.80±0.79a | 1.71±0.62 |
|
Silymarin+CCl4 |
281.67±60.47b |
684.71±85.82b |
232.71±49.55b | 1.67±0.99 | 1.52±0.39 |
| GB (150 mg/kg) +
CCl4 |
348.33±42.46b |
550.50±96.11b |
264.50±45.79b | 1.11±0.60 | 1.66±0.46 |
| GB (300 mg/kg) +
CCl4 |
359.00±47.60b |
213.38±73.39b |
236.20±36.17b | 1.71±0.73 | 0.61±0.44 |
| GB (600 mg/kg) +
CCl4 |
215.86±46.57b |
129.29±36.18b |
240.20±42.55b | 1.22±0.83 | 0.10±0.47 |
GB prevents CCl4-induced
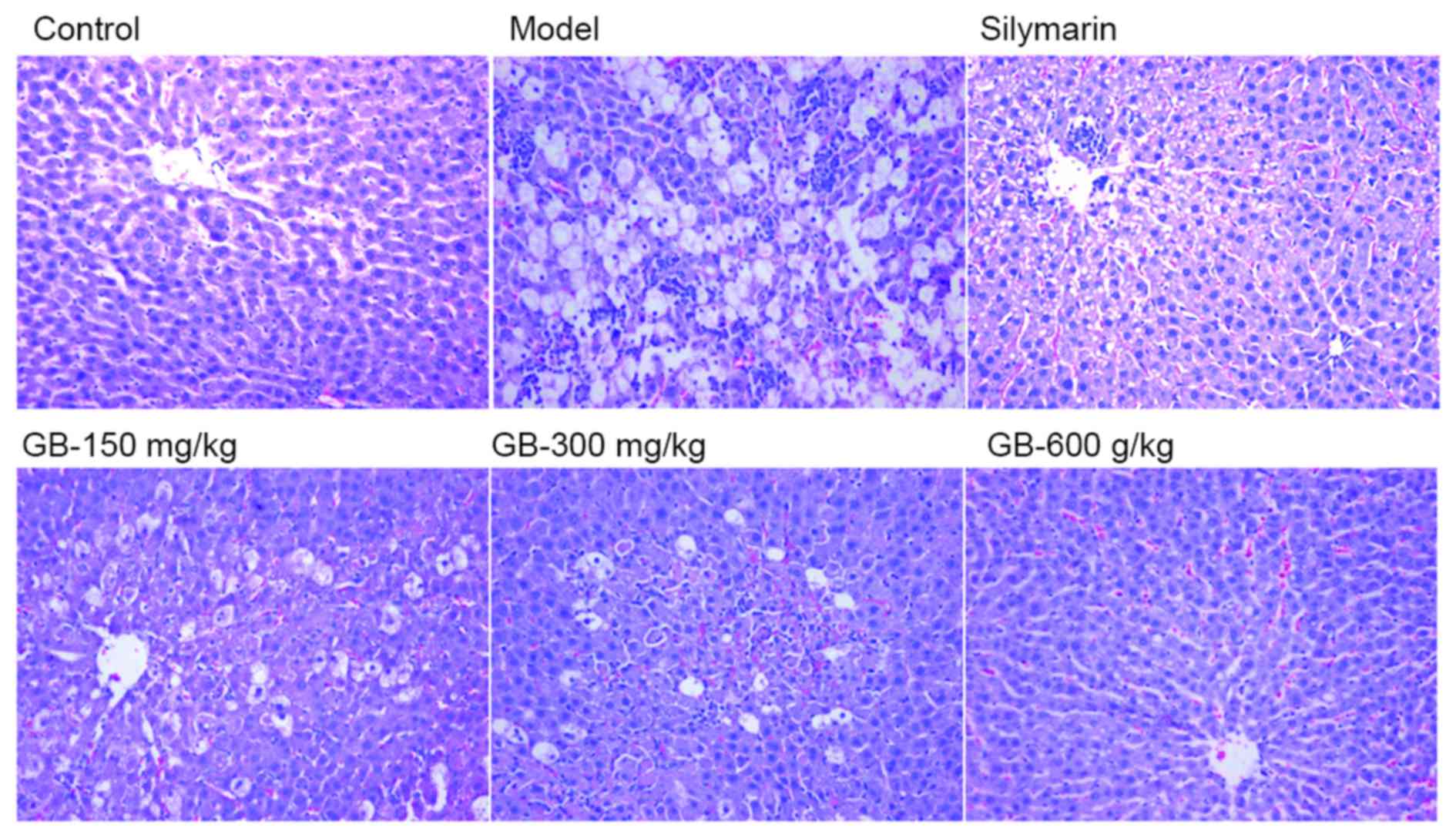
morphological changes in liver tissues
To evaluate the influence of GB therapy on
hepatocellular necrosis and inflammation, a histopathologic
analysis of liver tissues was performed to support the evidence
provided by the biochemical parameters. Liver histology of the
control group revealed a lobular architecture and hepatic cells
with well-preserved cytoplasm, prominent nucleus and nucleolus,
visible central veins and thin sinusoids (Fig. 1). By contrast, the injury model group
showed the most extensive damage among all groups, exhibiting
centrilobular necrosis, broad inflammatory cell infiltration,
ballooning degeneration and the loss of cellular boundaries. Liver
sections of rats pretreated with GB (150, 300 and 600 mg/kg) or
silymarin revealed that these treatments reduced or prevented the
development of histopathological damage, particularly 600 mg/kg GB
(Fig. 1).
GB inhibits CCl4-induced
oxidative stress in the liver
CCl4 administration induced acute
hepatotoxicity and caused severe oxidative damage in vivo
(Table II). CCl4
significantly decreased the activity of SOD, GSH, GSH-Px and
increased MDA and TBARS in liver samples (P<0.05). This
increased lipid peroxidation and the resulting reduced activities
of antioxidant enzymes were markedly attenuated by silymarin and GB
(150, 300 and 600 mg/kg) (P<0.05).
 | Table II.Effect of GB on MDA and TBARS levels
as well as SOD, GSH-Px and GSH activities. |
Table II.
Effect of GB on MDA and TBARS levels
as well as SOD, GSH-Px and GSH activities.
| Group | SOD (U/mg
prot) | MDA (nmol/mg
prot) | GSH (mg/g
prot) | GSH-PX (U/mg
prot) | TBARS (nmol/mg
prot) |
|---|
| Control | 502.92±46.61 | 2.70±0.40 | 14.36±2.63 | 122.04±32.90 | 8.66±0.98 |
|
CCl4 |
267.73±51.00a |
5.08±0.48a |
6.32±0.50a |
57.73±12.55a |
28.13±0.98a |
|
Silymarin+CCl4 |
420.32±43.14b |
1.48±0.16b | 6.03±0.36 |
116.10±24.32b |
8.36±0.82b |
| GB (150 mg/kg) +
CCl4 |
379.45±58.08b |
1.46±0.07b | 9.08±1.67 |
114.86±21.18b |
11.48±1.43b |
| GB (300 mg/kg) +
CCl4 |
372.98±14.54b |
1.22±0.08b |
10.79±1.21b |
113.02±12.88b |
7.80±1.28b |
| GB (600 mg/kg) +
CCl4 |
435.22±44.17b |
1.37±0.21b |
12.46±1.64b |
117.62±24.16b |
9.38±2.01b |
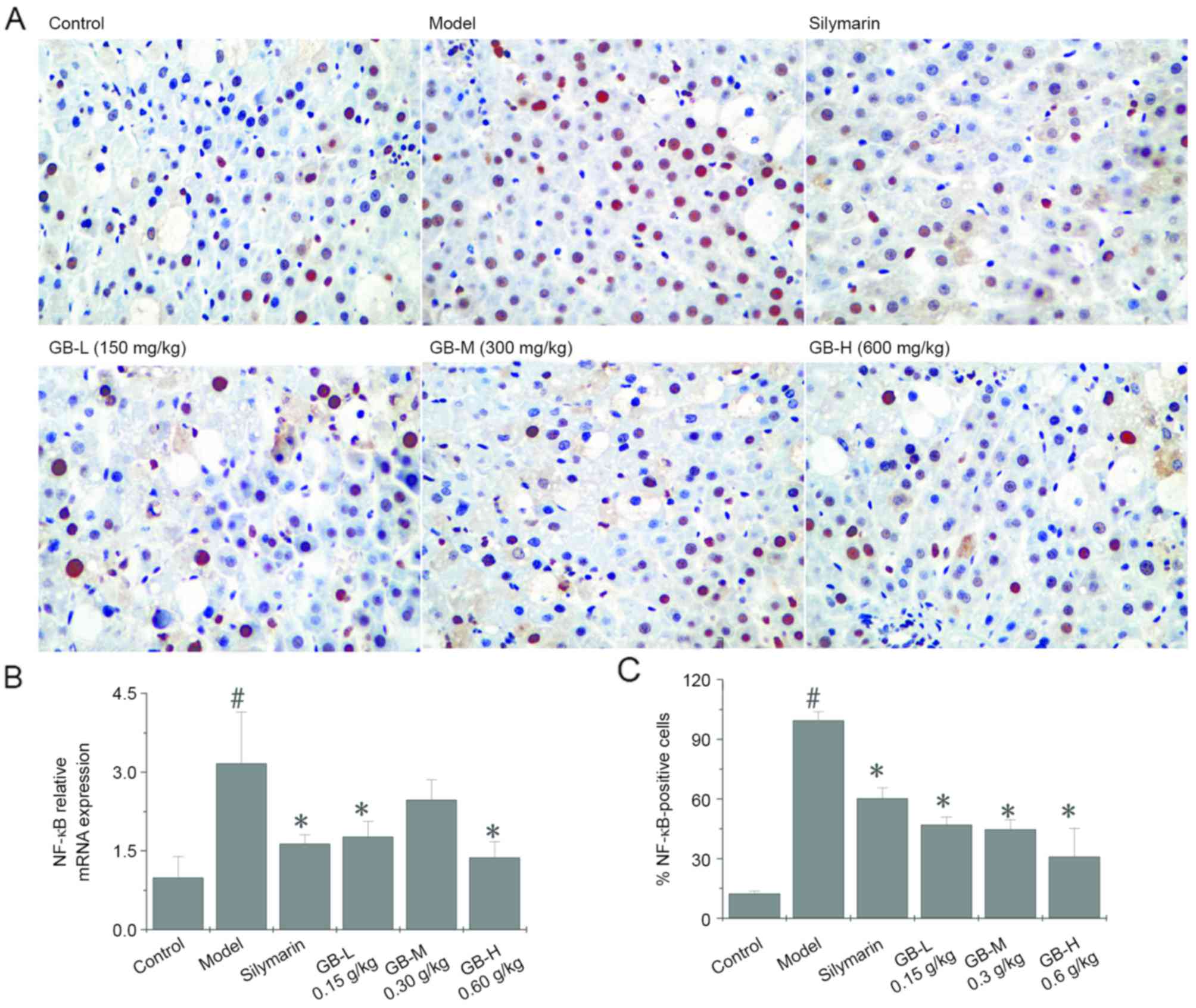
GB prevents liver inflammation induced by
CCl4. To identify the possible molecular mechanisms
responsible for the protective effects of GB against
CCl4-induced hepatotoxicity, NF-κB expression Acute
CCl4 toxicity induced NF-κB expression was assessed by
RT-qPCR analysis and immunohistochemistry. As shown in Fig. 2A and B, NF-κB-positive cells in the
injury model group were significantly increased compared to those
in the control group (P<0.05). Pre-treatment with silymarin or
GB (150, 300 or 600 mg/kg) prevented these CCl4-induced
increases in NF-κB expression. The results on mRNA expression of
NF-κB were consistent with those on the protein expression
(Fig. 2C), as
CCl4-induced increases were prevented by pre-treatment
with silymarin or GB (150, 300 and 600 mg/kg). However, no
significant difference was observed between the injury model and
the 300 mg/kg GB pre-treatment groups.
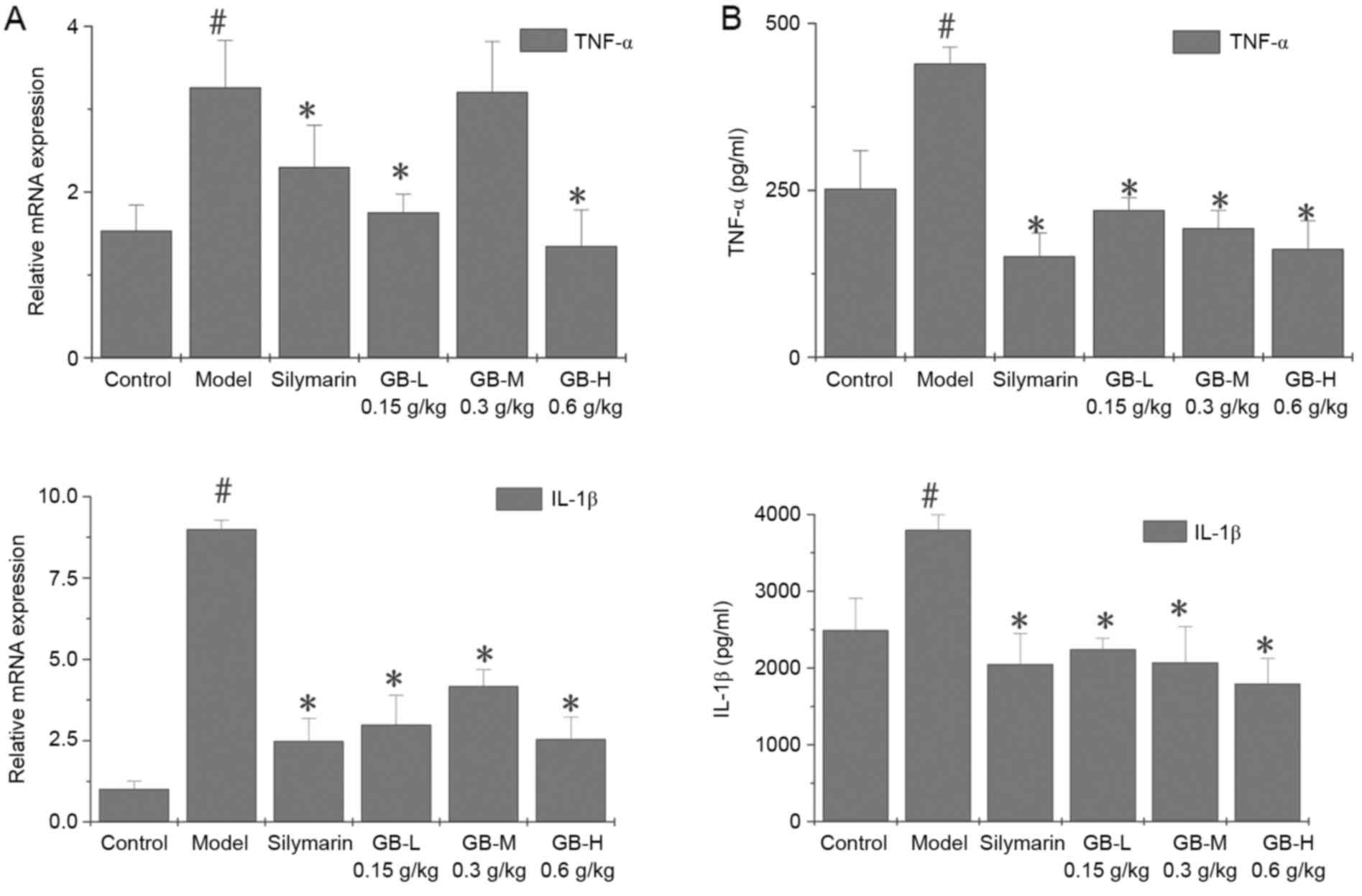
Certain key genes regulating hepatocellular necrosis
and inflammation are known to mediate liver injury. The present
study examined the effects of GB on proinflammatory gene expression
of acutely liver-injured rats by measuring TNF-α and IL-1β mRNA by
RT-qPCR in liver tissues (Fig. 3A).
The results showed significant increases in TNF-α and IL-1β mRNA in
livers from CCl4-treated rats. However, pre-treated with
GB or silymarin prevented these increases in the proinflammatory
cytokines. Inhibition of TNF-α and IL-1β gene expression by GB was
confirmed by measuring TNF-α and IL-1β protein using ELISA
(Fig. 3B). TNF-α and IL-1β protein
was elevated by CCl4 intoxication but inhibited by
pre-treatment with GB or silymarin.
Discussion
For decades, CCl4-induced liver injury
has been utilized as an experimental model, and biochemical and
histological changes associated with CCl4 are similar to
those resulting in acute viral hepatitis (21). The present study verified that
CCl4 induced acute liver damage, as demonstrated by
increased serum markers of hepatic injury and histology.
Specifically, the damage was associated with increased lipid
peroxidation products and reduced antioxidative enzymes (22), indicating oxidative stress. Moreover,
NF-κB was elevated, and TNF-α and IL-1β, which are pro-inflammatory
cytokines regulated by this factor, were also increased. Of note,
GB treatment prevented all the alterations induced by
CCl4. It is indicated that the antioxidant properties of
GB and its ability to inactivate NF-κB, and thus proinflammatory
cytokine production (23), are the
most likely mechanisms of action of GB.
Under normal conditions, serum hepatobiliary
enzymes, including AST, ALT and ALP, are present in high
concentrations in the liver. The serum enzyme levels of these
enzymes become increased upon onset of hepatocyte membrane damage
and necrosis, as they are released into the circulation (24). In CCl4-induced animals,
hepatic cell damage is indicated by increased serum AST, ALT and
ALP levels (25), as was observed in
the present study. CCl4-induced increases in serum AST,
ALT, ALP and γ-GT were reduced or prevented by pre-treatment with
GB or silymarin, indicating hepatoprotective and curative
abilities. Bilirubin is a useful clinical indicator of necrotic
severity, as it is produced during breakdown of heme in red blood
cells, with hyperbilirubinemia indicating liver pathophysiology and
bilirubin accumulation being a measure of liver cell conjugation,
binding and excretory ability. The results of the present study
showed that CCl4-induced rats produced more total
bilirubin, which was inhibited by pre-treatment with GB or
silymarin. However, no statistical significance was observed, which
may in part be due to liver function restoration.
Lipid peroxidation is an important indicator of
oxidative stress. It has been reported that CCl4-induced
hepatic tissue damage causes lipid peroxidation and ultimately
triggers MDA production (26).
Furthermore, TBARs, the final metabolites of peroxidized
polyunsaturated fatty acids, are considered a late biomarker of
oxidative stress (27). Increased
liver MDA and TBARS levels induced by CCl4 suggest
enhanced lipid peroxidation, leading to hepatic tissue damage and
failure of antioxidant defense. In agreement with previous studies
of CCl4-induced oxidative stress, the present study
showed that CCl4 treatment caused an increase of hepatic
MDA and TBARS. Of note, pre-treatment with silymarin or GB
significantly decreased these markers. GSH is a first line of
defense that scavenges free reactive oxygen species (ROS) and
exists in its reduced and its oxidized, disulfide state (GSSG). In
healthy cells and tissues, >90% of total glutathione is present
in the reduced form, while <10% exists in the GSSG form. An
elevated GSSG-to-GSH ratio is considered indicative of oxidative
stress (28). GSH-dependent enzymes
offer a second line of protection by primarily detoxifying noxious
byproducts of ROS and preventing free radical dissemination.
Peroxides are detoxified by GSH-PX through its reaction with GSH,
converting it into GSSG, which is then reduced to GSH by its
specific reductase (29). Endogenous
antioxidant enzymes such as SOD are also affected by free radicals.
The present study revealed that treatment with CCl4 in
rats markedly altered the activity of antioxidant enzymes, while
silymarin and GB reversed these effects.
Various studies have shown that hepatocellular
injury is not primarily due to the damaging agent itself, but
rather a result of inflammatory cells that attack the stressed
hepatocytes (30). This inflammatory
response is mediated by cytokines, of which TNF-α and IL-1β can
induce an acute-phase response (31). NF-κB is an integral inflammatory
response regulator, controlling cytokine gene expression. It has
been shown that inflammatory molecules are activated or increased
following activation of NF-κB by CCl4-mediated release
of TNF-α and IL-1β (13). Thus, a
self-amplifying, detrimental feedback loop is set forth in
hepatocytes: TNF-α and IL-1β promote NF-κB activation, and NF-κB
enhances production of additional TNF-α and IL-1β. This cycle
eventually modifies hepatocyte structures and morphology, and
impairs liver cell function (32).
As a result, continued NF-κB activation promotes prolonged
inflammatory responses, which makes it a key target for various
anti-inflammatory drugs used for treating various diseases
(33). The present study showed that
CCl4 treatment led to increased NF-κB activation and
release of the inflammatory factors TNF-α and IL-1β in the livers
of rats, which was prevented by silymarin and GB. The effect of 300
mg/kg of GB was poorer than that of 150 or 600 mg/kg of GB,
particularly regarding the gene expression of NF-κB, TNF-α and
IL-1β. A possible explanation may be the complexity of mechanisms
of action of GB leading to the different effect on gene
transcription from that on protein expression.
In conclusion, the findings of the present study
indicated that GB was highly effective in preventing
CCl4-induced acute liver damage, oxidative stress, the
expression of pro-inflammatory cytokines and the activation of
NF-κB. Based on the known effects of GB, the results of the present
study suggested that GB prevented transcription factor NF-κB
expression and activation to suppress the expression of a series of
pro-inflammatory agents, thereby preventing liver injury. Further
study is necessary to more broadly assess the therapeutic dose
range and mechanism of action of BR in preventing acute
hepatotoxicity. However, the present study shed light on the
potential clinical efficacy and mode of action of GB in treating
liver disorders.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (grant no. 81303125) as well as by the
Developmental Fund of Chen Keji Integrative Medicine (grant no.
CKJ2013015).
References
|
1
|
Güven A, Güven A and Gülmez M: The effect
of kefir on the activities of GSH-Px, GST, CAT, GSH and LPO levels
in carbon tetrachloride-induced mice tissues. J Vet Med B Infect
Dis Vet Public Health. 50:412–416. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ogeturk M, Kus I, Colakoglu N, Zararsiz I,
Ilhan N and Sarsilmaz M: Caffeic acid phenethyl ester protects
kidneys against carbon tetrachloride toxicity in rats. J
Ethnopharmacol. 97:273–280. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jaramillo-Juárez F, Rodríguez-Vázquez ML,
Rincón-Sánchez AR, Consolación Martínez M, Ortiz GG, Llamas J,
Anibal Posadas F and Reyes JL: Acute renal failure induced by
carbon tetrachloride in rats with hepatic cirrhosis. Ann Hepatol.
7:331–338. 2008.PubMed/NCBI
|
|
4
|
Galligani L, Lonati-Galligani M and Fuller
GC: Collagen synthesis in explant cultures of normal and
CCl4-treated mouse liver. Toxicol Appl Pharmacol. 48:131–137. 1979.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Recknagel RO, Glende EA Jr, Dolak JA and
Waller RL: Mechanisms of carbon tetrachloride toxicity. Pharmacol
Ther. 43:139–154. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Slater TF: Free-radical mechanisms in
tissue injury. Biochem J. 222:1–15. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Poli G: Liver damage due to free radicals.
Br Med Bull. 49:604–620. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Johnson SJ, Hines JE and Burt AD:
Macrophage and perisinusoidal cell kinetics in acute liver injury.
J Pathol. 166:351–358. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sasaki S, Yoneyama H, Suzuki K, Suriki H,
Aiba T, Watanabe S, Kawauchi Y, Kawachi H, Shimizu F, Matsushima K,
et al: Blockade of CXCL10 protects mice from acute colitis and
enhances crypt cell survival. Eur J Immunol. 32:3197–3205. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kalinichenko VV, Bhattacharyya D, Zhou Y,
Gusarova GA, Kim W, Shin B and Costa RH: Foxf1 +/− mice
exhibit defective stellate cell activation and abnormal liver
regeneration following CCl4 injury. Hepatology.
37:107–117. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Steinman L, Martin R, Bernard C, Conlon P
and Oksenberg JR: Multiple sclerosis: Deeper understanding of its
pathogenesis reveals new targets for therapy. Annu Rev Neurosci.
25:491–505. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Morio LA, Chiu H, Sprowles KA, Zhou P,
Heck DE, Gordon MK and Laskin DL: Distinct roles of tumor necrosis
factor-alpha and nitric oxide in acute liver injury induced by
carbon tetrachloride in mice. Toxicol Appl Pharmacol. 172:44–51.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Weber LW, Boll M and Stampfl A:
Hepatotoxicity and mechanism of action of haloalkanes: Carbon
tetrachloride as a toxicological model. Crit Rev Toxicol.
33:105–136. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Miyazaki T, Bouscarel B, Ikegami T, Honda
A and Matsuzaki Y: The protective effect of taurine against hepatic
damage in a model of liver disease and hepatic stellate cells. Adv
Exp Med Biol. 643:293–303. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kolios G, Valatas V and Kouroumalis E:
Role of Kupffer cells in the pathogenesis of liver disease. World J
Gastroenterol. 12:7413–7420. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Luckey SW and Petersen DR: Activation of
Kupffer cells during the course of carbon tetrachloride-induced
liver injury and fibrosis in rats. Exp Mol Pathol. 71:226–240.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yang L, Magness ST, Bataller R, Rippe RA
and Brenner DA: NF-kappaB activation in Kupffer cells after partial
hepatectomy. Am J Physiol Gastrointest Liver Physiol.
289:G530–G538. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ohkawa H, Ohishi N and Yagi K: Assay for
lipid peroxides in animal tissues by thiobarbituric acid reaction.
Anal Biochem. 95:351–358. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
McCord JM and Fridovich I: Superoxide
dismutase. An enzymic function for erythrocuprein (hemocuprein). J
Biol Chem. 244:6049–6055. 1969.PubMed/NCBI
|
|
20
|
Beutler E, Duron O and Kelly BM: Improved
method for the determination of blood glutathione. J Lab Clin Med.
61:882–888. 1963.PubMed/NCBI
|
|
21
|
Suja SR, Latha PG, Pushpangadan P and
Rajasekharan S: Evaluation of hepatoprotective effects of
Helminthostachys zeylanica (L.) Hook against carbon
tetrachloride-induced liver damage in Wistar rats. J
Ethnopharmacol. 92:61–66. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kwak JH, Kim HJ, Lee KH, Kang SC and Zee
OP: Antioxidative iridoid glycosides and phenolic compounds from
Veronica peregrina. Arch Pharm Res. 32:207–213. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Abe Y, Hashimoto S and Horie T: Curcumin
inhibition of inflammatory cytokine production by human peripheral
blood monocytes and alveolar macrophages. Pharmacol Res. 39:41–47.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Drotman RB and Lawhorn GT: Serum enzymes
as indicators of chemically induced liver damage. Drug Chem
Toxicol. 1:163–171. 1978. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wolf PL: Biochemical diagnosis of liver
disease. Indian J Clin Biochem. 14:59–90. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Cemek M, Aymelek F, Büyükokuroğlu ME,
Karaca T, Büyükben A and Yilmaz F: Protective potential of Royal
Jelly against carbon tetrachloride induced-toxicity and changes in
the serum sialic acid levels. Food Chem Toxicol. 48:2827–2832.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Cheeseman KH: Mechanisms and effects of
lipid peroxidation. Mol Aspects Med. 14:191–197. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Pompella A, Visvikis A, Paolicchi A, De
Tata V and Casini AF: The changing faces of glutathione, a cellular
protagonist. Biochem Pharmacol. 66:1499–1503. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Maritim AC, Sanders RA and Watkins JB III:
Effects of alpha-lipoic acid on biomarkers of oxidative stress in
streptozotocin-induced diabetic rats. J Nutr Biochem. 14:288–294.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ramadori G and Armbrust T: Cytokines in
the liver. Eur J Gastroenterol Hepatol. 13:777–784. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Simpson KJ, Lukacs NW, Colletti L,
Strieter RM and Kunkel SL: Cytokines and the liver. J Hepatol.
27:1120–1132. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Neuman MG: Cytokines-central factors in
alcoholic liver disease. Alcohol Res Health. 27:307–316.
2003.PubMed/NCBI
|
|
33
|
Oakley F, Mann J, Nailard S, Smart DE,
Mungalsingh N, Constandinou C, Ali S, Wilson SJ, Millward-Sadler H,
Iredale JP and Mann DA: Nuclear factor-kappaB1 (p50) limits the
inflammatory and fibrogenic responses to chronic injury. Am J
Pathol. 166:695–708. 2005. View Article : Google Scholar : PubMed/NCBI
|