Introduction
Osteoarthritis (OA) was once considered as a
non-inflammatory form of arthritis, but studies have demonstrated
that synovitis is associated with major symptoms of OA, such as
pain and the degree of joint dysfunction, and may promote more
rapid cartilage degeneration (1–3).
Synovial inflammation is an important factor involved in the
acceleration of cartilage degeneration (4,5). It is
likely that multiple joint tissues contribute to joint inflammation
(6). Fibroblast-like cells (FLS) are
the main functional synovial cells that produce cytokines, such as
tumor necrosis factor (TNF)-α, interleukin (IL)-1β, IL-1β and IL-6,
which are involved in the degradation of cartilage (7). FLS can also affect the natural course
of arthritis by releasing prostaglandin E2 (PGE2)
(8).
It is now known that several cell types are able to
generate active glucocorticoids within their cytoplasm through
expression of the 11β-hydroxysteroid dehydrogenase type 1
(11β-HSD1) enzyme. The generation of active glucocorticoids in the
synovium is strongly linked to the level of inflammation. Hardy
et al (9) reported that
11β-HSD1 mRNA was highly expressed in synovial tissues affected by
OA, and its activity was increased, as indicated by IL-1β and
TNF-α. Synovial fibroblasts have been demonstrated to maintain the
balance between intracellular glucocorticoid activation and
inactivation, and execute biological effects by producing 11β-HSD1
and binding with its receptor (9,10). It
has been reported that, in fetal membranes and adipose tissues,
11β-HSD1 mRNA expression and protein levels are upregulated by
pro-inflammatory cytokines (11,12). Sun
and Myatt reported a coordinated induction effect existed for the
regulation of 11β-HSD1 by glucocorticoids and pro-inflammatory
cytokines (11). Glucocorticoids
usually play an opposing role to proinflammatory cytokines at sites
of inflammation (13,14). However, how the glucocorticoid and
pro-inflammatory mediators induce their effects on 11β-HSD1, or
whether 11β-HSD1 correlates with PGE2 expression in the
synovial fibroblasts, remains unclear.
Therefore, we hypothesized that glucocorticoid
activity correlated with PGE2 synthesis, and that the
glucocorticoid pre-receptor regulator, 11β-HSD1, may have an effect
on relieving synovial inflammation by inhibiting microsomal
prostaglandin E synthase-1 (mPGES-1) and PGE2
expression. In the present study, a model cell, fibroblast-like
synovial cell, derived from rats, was stimulated with IL-1β and the
effect of treatment with corticosterone and
4′-cyano-biphenyl-4-sulfonic acid (6-amino-pyridin-2-yl)-amide was
evaluated. PGE2 levels in culture supernatants were
assayed, the mRNA expression of 11β-HSD1, mPGES-1, IL-1β and TNF-α
by the cells was analyzed and reverse transcription-qualitative
polymerase chain reaction and western blot analysis were used to
detect protein expression of 11β-HSD1 and mPGES-1. The
anti-inflammatory mechanism of glucocorticoid in suppressing IL-β
induced mPGES-1 expression through regulation of 11β-HSD1
bioactivity in synovial fibroblasts in vitro was
explored.
Materials and methods
Isolation and culture of
Sprague-Dawley (SD) rat synoviocytes
Synovial fibroblasts were isolated from the knee
synovial tissue of 10 female healthy 3-month SD rats (200±30 g;
Laboratory Animal Centre, Guangdong Medical College, Zhanjiang,
China). Rats were kept under regular conditions with a temperature
of 25°C, humidity of 50% and a natural day and night cycle. All
rats were able to access food and water freely. Rats were
euthanatized with an intraperitoneal overdose (130 mg/kg) of
phenobarbital sodium (cat. no. P5178; Sigma-Aldrich; Merck
Millipore, Darmstadt, Germany) in accordance with National Animal
Care guidelines. The protocol was approved by the Ethics Committee
of Guangdong Medical College. Synovial tissues were cut into pieces
(2–3 mm2) and subsequently immersed in Dulbecco's
modified Eagle's medium (DMEM) supplemented with 15% fetal bovine
serum, 100 U/ml penicillin and 100 µg/ml streptomycin (all Gibco™;
Thermo Fisher Scientific, Inc., Waltham, MA, USA). Tissue pieces
were transferred into a culture flask, with 15–20 pieces in each
bottle. Culture bottles were inverted and 2 ml medium was added.
Tissue pieces were cultured at 37°C in a humidified atmosphere,
containing 5% CO2 for 2–3 h. Culture bottles were
reversed when tissue blocks adhered to the bottom. Medium was
replenished every 2 days. Cells were separated from the synovial
tissue and passaged when confluent. Synovial fibroblasts after
three passages were analyzed using flow cytometry [for cluster of
differentiation (CD) 90] and vimentin immunocytochemical
staining.
Flow cytometric analysis
The passage 3 synovial cells were digested with 1 ml
0.25% trypsin (cat. no. 25-200-114; Gibco; Thermo Fisher
Scientific, Inc). FBS-DMEM medium (10%, 3 ml) was added to
terminate the digestion when the morphology of cell became rounded
and the cells were removed from the bottle wall. The digested cell
suspension was loaded into a 15-ml EP tube and centrifuged at 1,344
× g for 5 min. The supernatant was removed and the cells
were suspended and divided into two EP tubes (1.5 ml). One tube of
cells was labeled with 5 µl phycoerythrin (PE)-labeled anti-rat
CD90 (cat. no. 205903; Biolegend Inc., San Diego, CA, USA). The
other was labeled with the same host-derived IgG (cat. no. 400111;
Biolegend Inc.) as a negative control. The cells were incubated at
4°C in the dark for 30 min and then centrifuged at 1,344 × g
for 5 min. The supernatant was removed and resuspended with 500 µl
PBS. The cell populations were analyzed using a FACSCalibur Flow
Cytometer (BD FACSCanto; BD Biosciences, San Jose, CA, USA).
Vimentin immunocytochemical
staining
The cells were fixed with 4% formaldehyde for 30
min. Then, the cells were washed in PBS for 5 min, incubated in 3%
H2O2 and in 0.3% Triton X-100 solution for 30
min, respectively, and then blocked with BSA at room temperature
for 10 min. The cells were incubated with primary antibody vimentin
mouse anti-rat antibody (l:300 dilution; cat. no. BM0135; Wuhan
Boster Biological Technology, Ltd., Wuhan, China). Instead of
primary antibody, PBS was added as a negative control. The cells
were incubated at 4°C overnight. The next day, the slices were
washed in PBS for 5 min, 3 times, and then incubated with secondary
antibody (polymeric HRP-Conjugated Anti-Goat IgG Super Vision Assay
kit; cat. no. SV0003-1; Wuhan Boster Biological Technology, Ltd.)
at room temperature for 30 min. DAB chromogen was used according to
the manufacturer's guidance (Wuhan Boster Biological Technology,
Ltd.).
Study design
Synovial fibroblasts, preserved in DMEM, were
removed of glucocorticoid using 10% active carbon and subsequently
stimulated with 10 ng/ml IL-1β (R&D Systems, Inc., Minneapolis,
MN, USA) for 24 h. Synovial fibroblast cells were washed with PBS
at 37°C for 5 min, three times. After 24 h, cells were treated with
different components depending on the allocated group: Group A,
treated with DMEM without glucocorticoid; group B, treated with 10
ng/ml IL-1β; group C, treated with 10 ng/ml IL-1β and
10−6 mmol/l corticosterone (Sigma-Aldrich); and group D,
treated with 10 ng/ml IL-1β, 10−6 mmol/l corticosterone
and 100 nmol/l PF915275 (Tocris Bioscience, Bristol, UK). Following
a further 24 h, PGE2 levels were assayed in culture
supernatants by ELISA. Cells were harvested for mRNA evaluation of
11β-HSD1, mPGES-1, IL-1β and TNF-α levels; and protein expression
level detection of 11β-HSD1 and mPGES-1.
RNA extraction and reverse
transcription-quantitative real-time polymerase chain reaction
(RT-qPCR) analysis
Total RNA was isolated from synovial fibroblast
layers using TRIzol reagent (Takara Biotechnology Co., Ltd.,
Dalian, China) following the manufacturer's protocol. RNA quality
was detected by agarose gel electrophoresis. Reverse transcription
was carried out using the PrimeScript RT Reagent kit (Takara
Biotechnology Co., Ltd.). The mRNA expression of IL-1β, TNF-α,
11β-HSD1 and mPGES-1 was determined using the SYBR Premix Ex Taq™
kit (Takara Biotechnology Co., Ltd.). The volume of the reaction
system was 15 µl, and the reaction system contained 7.5 µl SYBR
Premix Ex Taq II, 0.6 µl each upstream and downstream primer, 1.5
µl DNA template and 4.8 µl sterilized distilled water. Target mRNA
levels were normalized to β-actin expression levels. Primers used
in the experiment are listed below. The following cycling
conditions were used: 95°C for 2 min, followed by 40 cycles of 95°C
for 15 sec and 60°C for 30 sec. Three replications of this
experiment were performed for the same reaction. A Real-time
Quantitative PCR instrument (Bio-Rad Laboratories, Inc., Hercules,
CA, USA) and the 2−∆∆Cq method (15) with LERTPA-V1.0 software (http://www.biostatistic.net/portal.php)
was used to analyze the relative gene expression data: Experimental
group (Cq target gene-Cq housekeeping gene)-blank group (Cq target
gene- Cq housekeeping gene). The Cq value in this formula
represented the number of amplification cycles required for a
fluorescent signal in each well to reach a specific threshold. The
Cq value was automatically calculated by the computer based on the
amplification curve. Gene-specific primer pairs used were as
follows: IL-1β, forward 5′-CTTCAAATCTCACAGCAGCATC-3′ and reverse
5′-GCTGTCTAATGGGAACATCACA-3′; TNF-α, forward
5′-GTGCCTCAGCCTCTTCTCATT-3′ and reverse
5′-CTCTGCTTGGTGGTTTGCTAC-3′; 11β-HSD1, forward
5′-AAAATGACCCAGCCTATGATTG-3′ and reverse
5′-GGACACAGAGAGTGATGGACAC-3′; mPGES-1, forward
5′-GTGATGGAGAACAGCCAGGT-3′ and reverse
5′-CAAGGAAGAGGAAGGGGTAGAT-3′; and β-actin, forward
5′-CCATCTATGAGGGTTACGC-3′ and reverse
5′-TTTAATGTCACGCACGATTTC-3′.
Assays for PGE2 and
cortisol
Levels of PGE2 in culture supernatants
were determined using an ELISA kit (cat. no. KGE004B; R&D
Systems, Inc.) in accordance with the manufacturer's instructions.
Assays were based on the combined use of a monoclonal antibody
against PGE2 and an alkaline phosphatase-conjugated
polyclonal antibody; p-nitrophenyl phosphate substrate was added,
and the absorbance at 405 nm was analyzed using a micro Multiskan
plate reader. The limits of detection were 10 and 1.4 pg/ml for
PGE2. Positive controls were used in each
experiment.
Cortisol levels were also determined in culture
supernatants using an ELISA kit (cat. no. KGE008B; R&D Systems,
Inc.), in accordance with manufacturer's instructions to indicate
the conversion rate of cortisone. The assay required microtiter
plates coated with purified antibody and the standards or samples,
anti-cortisol antibody and horseradish peroxidase-labeled avidin
were subsequently added. Absorbance (optical density) was read
using a Multiskan microplate reader at 450 nm, following a
substrate 3,3′,5,5′-tetramethylbenzidine color reaction. Sample
concentrations were calculated using a standard curve.
Western blot analysis
Synovial fibroblasts, seeded in 6-well plates and
grown to 80% confluency, were washed twice with ice-cold PBS and
scraped off the wells in TRIzol lysates, containing 1% protease
inhibitors. Cells were collected in 1.5 ml Eppendorf tubes and
centrifuged 19,419 at × g for 10 min at 4°C, prior to determination
of protein concentration, using a Bradford-based assay (Beyotime
institute of Biotechnology, Shanghai, China). Protein samples (100
µg) were separated by 30% SDS-PAGE, and electroblotted on a
polyvinylchloride membrane. Following 1-h incubation in blocking
buffer [Tris-buffered saline-Tween-20 (TBS-T) with 5% non-fat dried
milk], membranes (Millipore Saint-Quentin-en-Yvelines, France) were
incubated overnight at 4°C with β-actin (1:2,000; cat. no. 4967;
Cell Signaling Technology, Inc., Danvers, MA, USA), 11β-HSD1 (cat.
no. sc-20175; Santa Cruz Biotechnology, Dallas, TX, USA) and
mPGES-1 (both 1:500; cat. no. 13035; Cell Signaling Technology,
Inc) primary antibodies diluted in TBS-T with 5% bovine serum
albumin. Following three washes with TBS-T, the membrane was
incubated for 2 h at room temperature with anti-rabbit IgG
conjugated with horseradish peroxidase (cat. no. sc-2374; Santa
Cruz Biotechnology, Inc.) at 1:2,000 dilution in TBS-T containing
5% non-fat dried milk. Cells were washed three times for 10 min
with TBS-T and protein bands were detected by chemiluminescence,
using a Phototope Detection system in accordance with the
manufacturer's instructions (Beyotime Institute of Biotechnology,
Shanghai, China). The gray values of protein bands were analyzed
using Image J software (ver. 1.46; National Institutes of Health,
Bethesda, MD, USA). The relative expression of the target protein
was calculated according the following formula: Relative expression
of target protein = target protein gray value/corresponding
internal reference value.
Statistical analysis
Results are expressed as the mean ± standard
deviation. A minimum of three assays were performed. Comparisons
were made using analysis of variance analysis, followed by a least
significant difference test and subsequent homogeneity of variance
test. Correlation analysis between the groups was performed using
Pearson correlation analysis. SPSS 21.0 software (IBM SPSS, Armonk,
NY, USA) was used for statistical analyses. P<0.05 was
considered to indicate a statistically significant difference.
Results
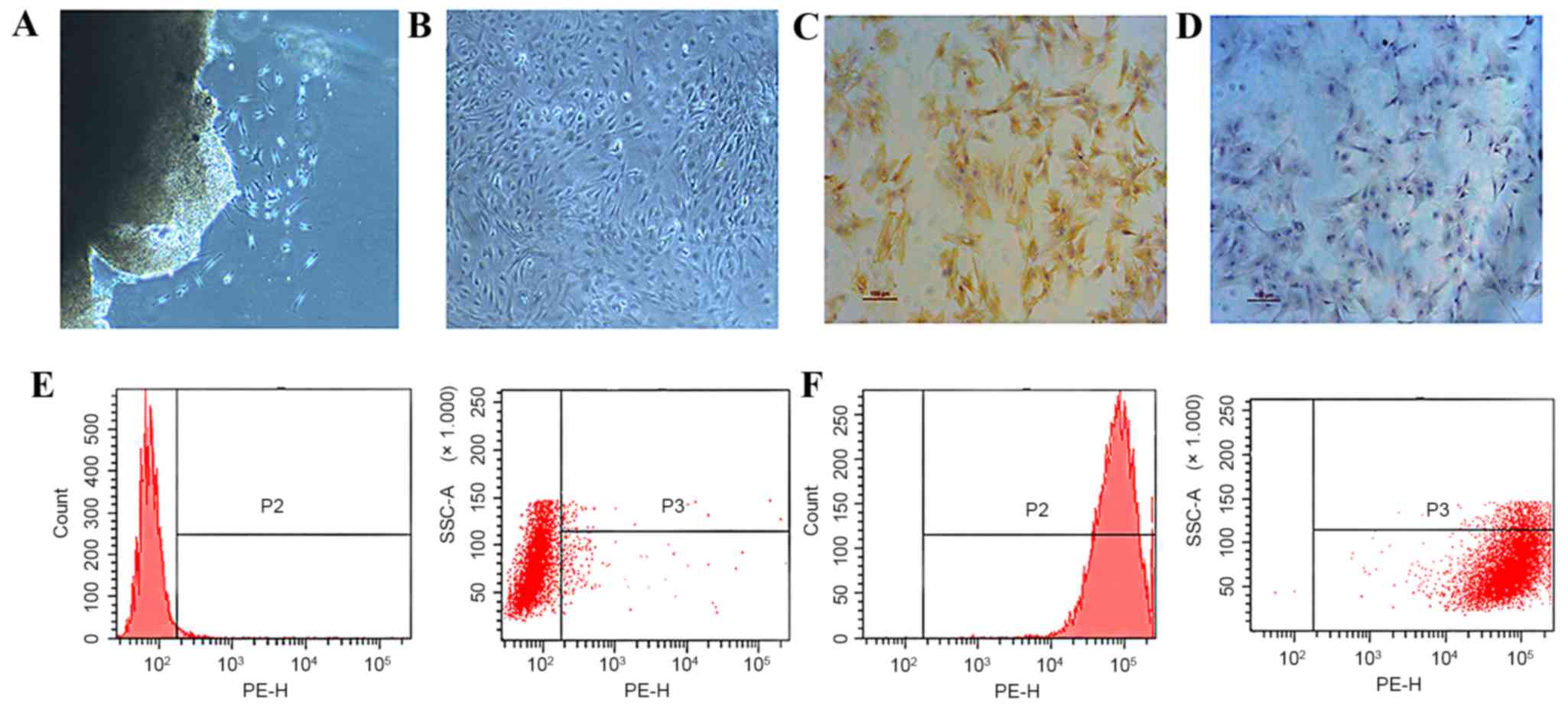
Evaluation of the isolated primary
synovial fibroblasts
Primary synovial fibroblasts, derived from the
fibrous membrane of SD rat knees, were used as the model cells for
the present study. Cells were isolated using the tissue culture
(Fig. 1A) method and were passaged
to the third generation (Fig. 1B)
and subsequently evaluated by immuohistochemical staining and flow
cytometry. Type B synoviocytes, or FLS, are mesenchymal cells that
display various characteristics of fibroblasts, including
expression of type IV and V collagens, vimentin, and CD90. Cells
that were stained brown indicated the presence of vimentin
(Fig. 1C), compared with the cells
in negative control group (Fig. 1D).
Using flow cytometry, while cells marked with homotype-negative
phycoerythrin-labeled IgG antibodies showed negative result
(Fig. 1E), CD90-positive cells were
detected and confirmed the phenotype of type B synovial fibroblasts
(Fig. 1F).
IL-1β induces the expression of
fibroblast inflammatory cytokines
Primary synovial fibroblasts derived from the
fibrous membrane of SD rat knees were used as model cells to
examine the expression levels of inflammatory factors induced by
IL-1β. Expression levels of the inflammatory cytokines, IL-1β and
TNF-α were significantly increased following induction with 10
ng/ml IL-1β for 24 h, when compared with the control, group A
(P<0.05; Fig. 2), which indicated
that IL-1β leads to an inflammatory state in a cell. However, the
expression levels of inflammatory cytokines significantly decreased
compared with those in group B following the administration of
corticosterone (P<0.05; Fig. 2),
suggesting an inhibitory effect of corticosterone on IL-1β-induced
inflammation. However, when cells were treated with PF915275 (group
D), an 11β-HSD1 inhibitor, the decreased expression levels of
inflammatory factors (suppressed by corticosterone) exhibited a
reversal effect, with a significant increase (P<0.05) in the
expression levels of IL-1β and TNF-α compared with those in group C
(Fig. 2). In comparison with group
B, a relatively low expression level of the inflammatory factors
IL-1β and TNF-α was observed in group D (P<0.05).
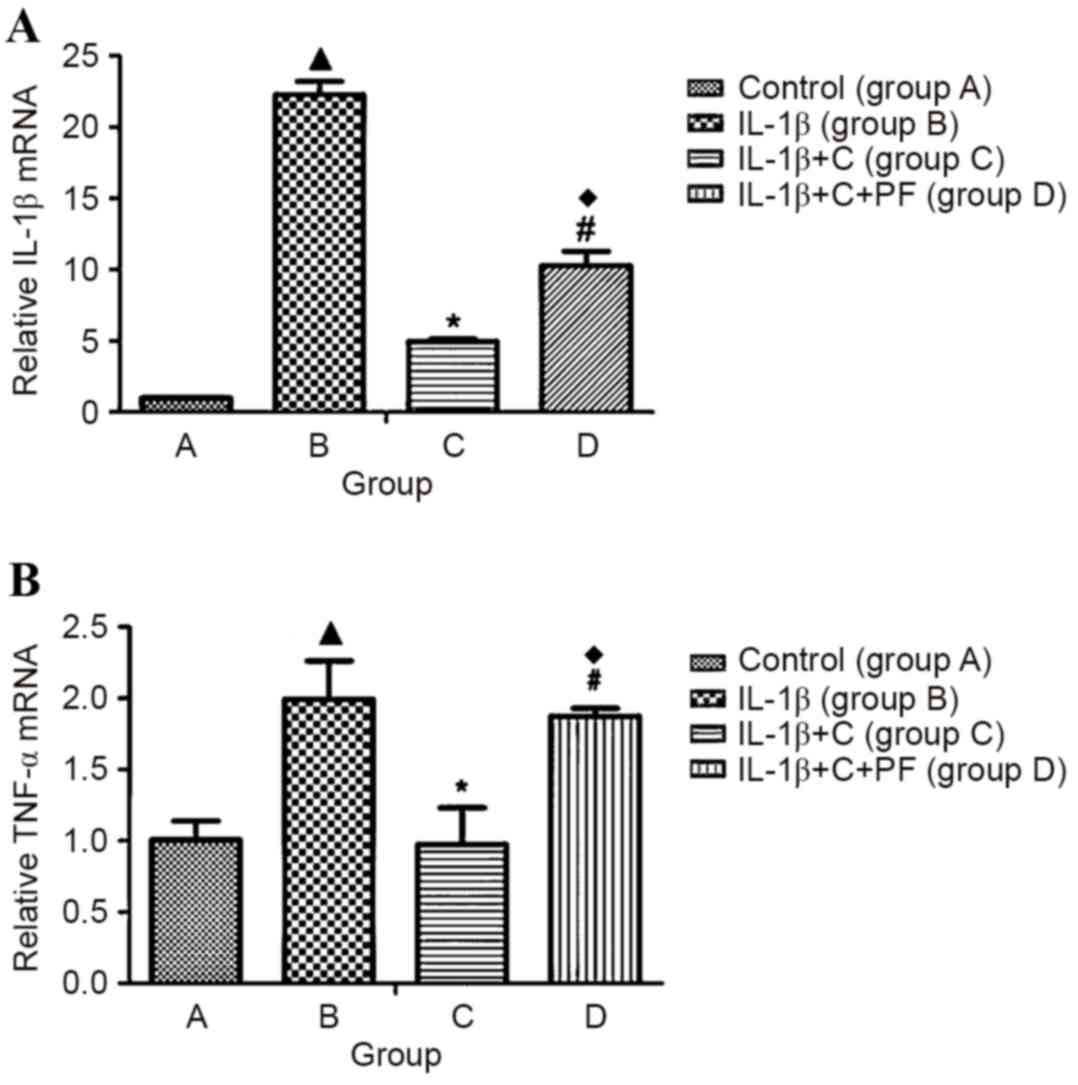 | Figure 2.IL-1β inducible inflammatory factor
expression in synovial fibroblasts. Synovial fibroblasts were
stimulated with 10 ng/ml IL-1β for 24 h. Following a further 24 h,
cells in group A were treated with Dulbecco's modified Eagle's
medium medium without glucocorticoid; group B, with 10 ng/ml IL-1β;
group C, with 10 ng/ml IL-1β and 10−6 mmol/l
corticosterone; and group D, as group C plus 100 nmol/l PF.
Expression of (A) IL-1β and (B) TNF-α mRNA increased significantly
in the IL-1β- induced synovial fibroblasts. When corticosterone was
applied, the expression of inflammatory cytokines significantly
decreased. The 11β-HSD1 inhibitor, PF, reversed the reduction in
expression of inflammatory factors caused by corticosterone.
Results are expressed as the mean ± standard deviation and a
minimum of three assays were performed. ▲P<0.05 vs. control;
*P<0.05 vs. group B; #P<0.05 vs. group C;
♦P<0.05 vs. group B. IL, interleukin; TNF-α, tumor necrosis
factor- α; 11β-HSD1, 11β-hydroxysteroid dehydrogenase type 1; C,
corticosterone; PF, PF915275 [4′-cyano-biphenyl-4-sulfonic acid
(6-amino-pyridin-2-yl) -amide]. |
PGE2 is a key factor involved in the
development and perpetuation of inflammation in disease, such as
with rheumatoid arthritis. In this disease, local inflammation of
synovial tissue is characterized, in part, by increased local
levels of PG, predominantly PGE2 (5). Inducible mPGES-1 has an essential role
in the localized increase of PGE2 during inflammatory
arthritis. Expression notably increased when cells were induced by
pro-inflammatory cytokines (IL-1β and TNF-α) in vitro
(6). In the present study, mPGES-1
mRNA expression and PGE2 production of synovial
fibroblasts were significantly increased following treatment with
10 ng/ml IL-1β for 24 h, when compared with the control, group A
(P<0.05; Fig. 3).
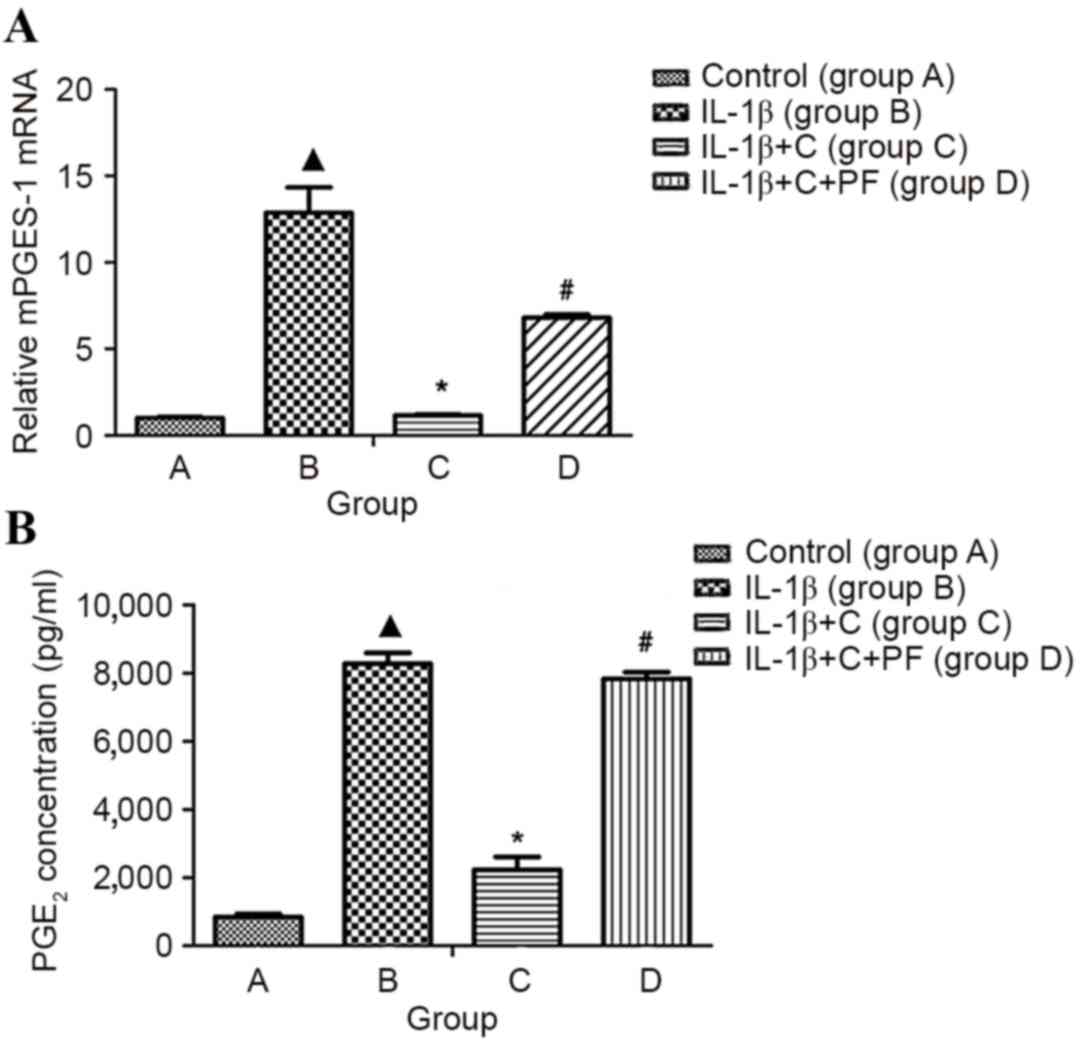 | Figure 3.mPGES-1 mRNA and PGE2
expression in the inflammatory synovial fibroblasts. Total RNA was
isolated from synovial fibroblasts layers, and mRNA was quantified
using reverse transcription-quantitative polymerase chain reaction.
PGE2 was detected by ELISA. (A) mPGES-1 mRNA and (B)
PGE2 expression levels were significantly increased in
the synoviocytes induced by IL-1β when compared with the control,
group A. However, IL-1β-induced expression was inhibited
significantly by corticosterone, and the inhibitory effect of
corticosterone was significantly reversed by the 11β-HSD1 inhibitor
PF. Group A, synovial fibroblasts treated with medium without
glucocorticoid (control); group B, synovial fibroblasts treated
with 10 ng/ml IL-1β; group C, synovial fibroblasts treated with 10
ng/ml IL-1β and 10−6 mmol/l corticosterone; group D,
synovial fibroblasts treated as group C plus 100 nmol/l PF.
▲P<0.05 vs. control; *P<0.05 vs. group B;
#P<0.05 vs. group C. C, corticosterone; PF, PF915275
[4′-cyano-biphenyl-4-sulfonic acid (6-amino-pyridin-2-yl)-amide];
mPGES-1, microsomal prostaglandin E synthase-1; PGE2,
prostaglandin E2. |
IL-1β induces 11β-HSD1 expression in
fibroblasts by suppressing corticosterone activation
11β-HSD1 is capable of converting inactive
glucocorticoids (cortisone and prednisone) into the active
counterparts, cortisol and prednisolone. Synovial fibroblasts and
osteoblasts generate active glucocorticoids through the expression
of 11β-HSD1 (7). Such activity
increases, in vitro, in response to pro-inflammatory
cytokines or glucocorticoids. The present study indicated that
IL-1β significantly increased 11β-HSD1 expression levels in
synoviocytes (P<0.05), whereas these expression levels markedly
decreased when corticosterone was added to the media (Fig. 4), which may be due to an increase in
the production of active cortisol (Fig.
5) and the inhibition of the synoviocyte inflammation. When the
activity of 11β-HSD1 was inhibited by PF915275, the 11β-HSD1 mRNA
and protein expression levels that had previously been suppressed
by corticosterone were reversed, and the expression levels were
higher than those of cells stimulated with IL-1β (P<0.05;
Fig. 4).
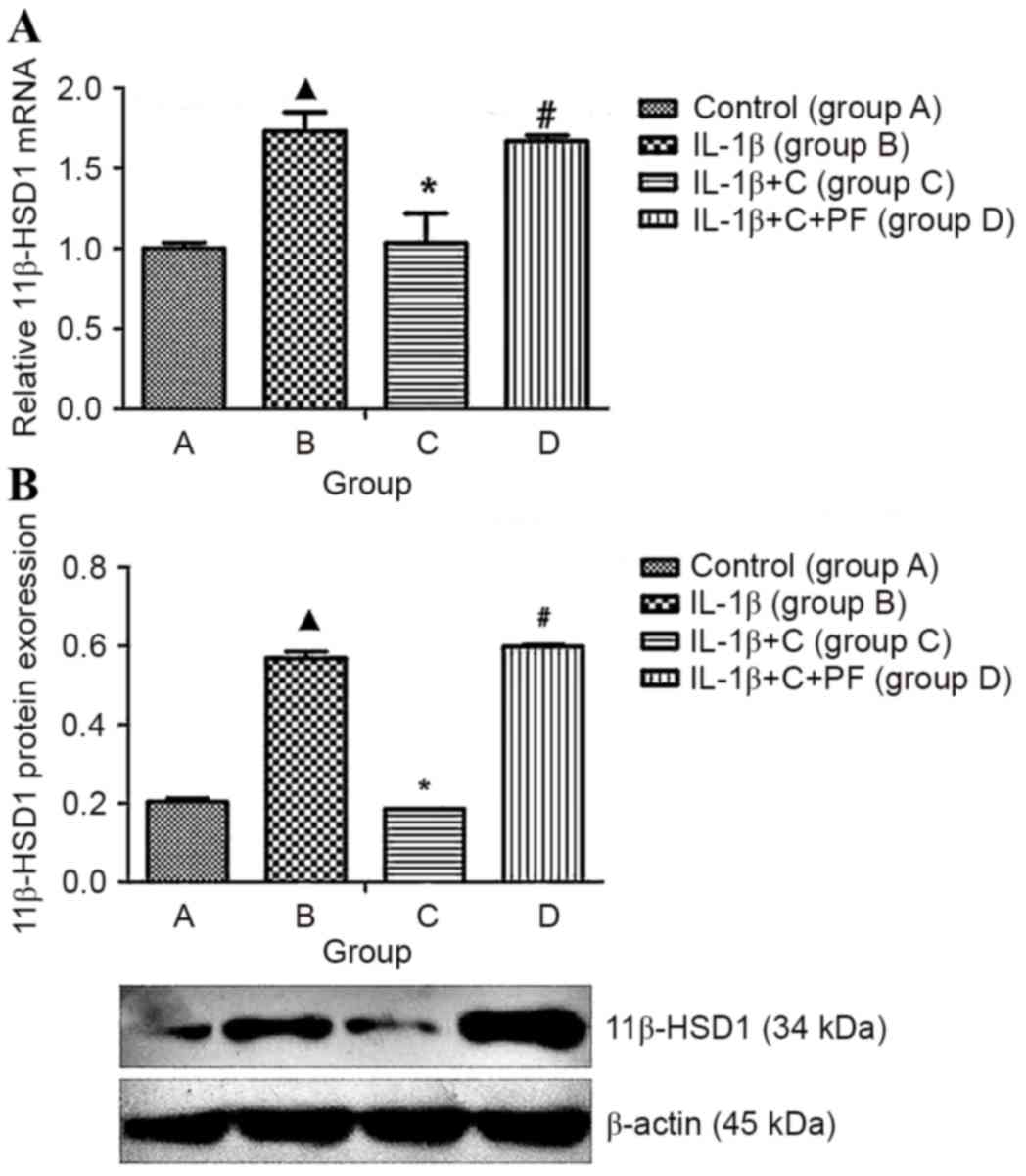 | Figure 4.IL-1β induced 11β-HSD1 expression and
biosynthesis in synovial fibroblasts. 11β-HSD1 RNA isolated from
synovial fibroblasts layers was quantified using reverse
transcription-quantitative polymerase chain reaction, and protein
levels were determined by western blotting. 11β-HSD1 expression was
increased at the (A) mRNA and (B) protein levels when compared with
the control, group A. When treated with corticosterone, the
expression levels were inhibited significantly. Inhibitory effects
were reversed by treatment with the 11β-HSD1 inhibitor PF. Results
are expressed as the mean ± standard deviation and a minimum of
three assays were performed. Group A, synovial fibroblasts treated
with medium without glucocorticoid (control); group B, synovial
fibroblasts treated with 10 ng/ml IL-1β; group C, synovial
fibroblasts treated with 10 ng/ml IL-1β and 10−6 mmol/l
corticosterone; group D, synovial fibroblasts treated as group C
plus 100 nmol/l PF. ▲P<0.05 vs. control; *P<0.05 vs. group B;
#P<0.05 vs. group C. 11β-HSD1, 11β-hydroxysteroid
dehydrogenase type 1; C, corticosterone; PF, PF915275
[4′-cyano-biphenyl-4-sulfonic acid (6-amino-pyridin-2-yl)
-amide]. |
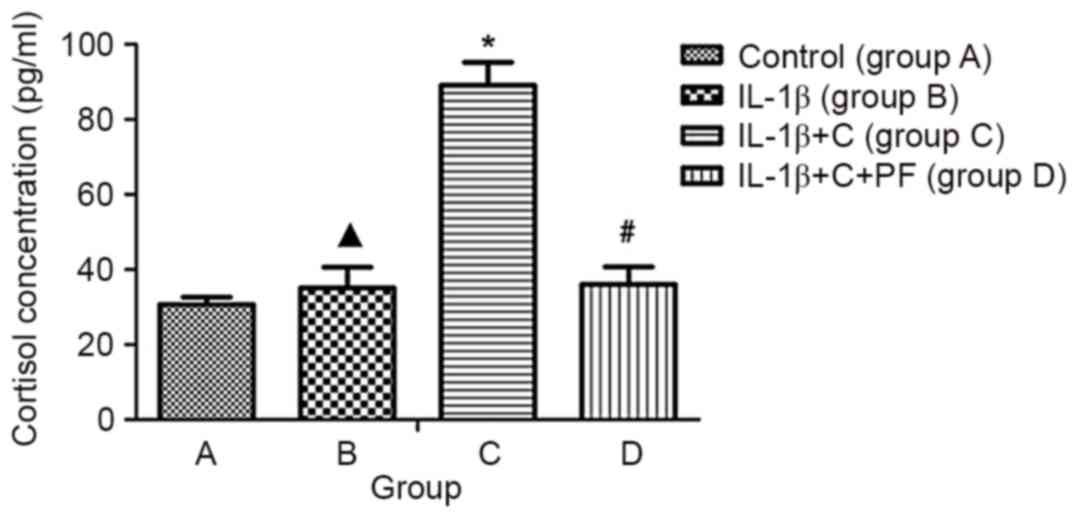 | Figure 5.ELISA of cortisol concentration in
the medium. Cortisol concentration was assayed in culture
supernatants using an ELISA kit to indicate the conversion rate of
cortisone. The absorbance (OD) was read using a micro Multiskan
plate reader at 450 nm after the substrate TMB color reaction.
IL-1β did not affect the cortisol concentration in the medium.
Cortisol concentration increased significantly following the
addition of corticosterone; however, this was reversed when PF was
included. Results are expressed as the mean ± standard deviation
and a minimum of three assays were performed. ▲P>0.05 vs.
control; *P<0.05, vs. control; #P<0.05 vs. group
C. Group A, synovial fibroblasts treated with medium without
glucocorticoid (control); group B, synovial fibroblasts treated
with 10 ng/ml IL-1β; group C, synovial fibroblasts treated with 10
ng/ml IL-1β and 10−6 mmol/l corticosterone; group D,
synovial fibroblasts treated as group C plus 100 nmol/l PF. OD,
optical density; TMB, 3,3′,5,5′-tetramethylbenzidine; 11β-HSD1,
11β-hydroxysteroid dehydrogenase type 1; C, corticosterone; PF,
PF915275 [4′-cyano-biphenyl-4-sulfonic acid (6-amino-pyridin-2-yl)
-amide]. |
Corticosterone suppresses synoviocyte
PGE2 production by promoting the activity of
11β-HSD1
Synovial fibroblasts expressed high levels of
mPGES-1 mRNA when treated with IL-1β, and PGE2
concentration was higher in the media when compared with the
control, group A, as detected by ELISA (P<0.05; Fig. 3). Corticosterone appeared to
counteract the effects of IL-1β, as indicated by the reduction in
expression of mPGES-1 mRNA and PGE2 (P<0.05; Fig. 3). This inhibitory effect may result
from a low level of active corticosterone that inhibited 11β-HSD1
expression; thus corticosterone may be effectively converted by
synoviocytes (Fig. 5). In the
present study, PF915275 was used to suppress the conversion
activity of 11β-HSD1. Suppression by PF915275 caused a significant
increase in the levels of mPGES-1 mRNA (Fig. 3A) expression in synoviocytes and the
concentration of PGE2 (Fig.
3B) in the media, when compared with the control and
corticosterone groups (groups A and C, respectively; P<0.05).
mPGES-1 mRNA and PGE2 were not inhibited when the
conversion activity of enzyme 11β-HSD1 was blocked (P>0.05,
compared with the control group; Fig.
3). These results suggest that PGE2 production by
the synoviocytes may be suppressed by activating the 11β-HSD1
pathway.
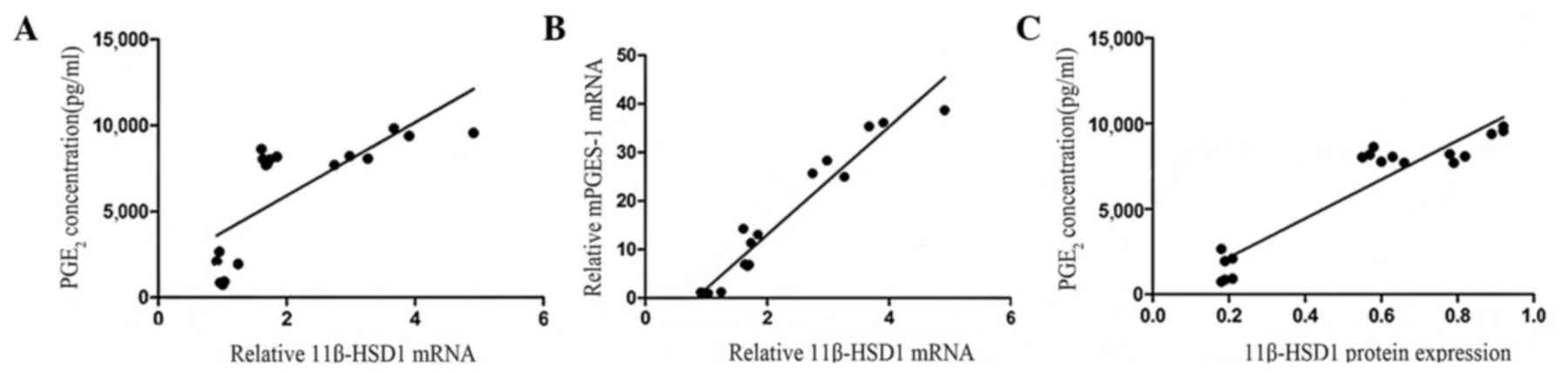
11β-HSD1 expression positively
correlates with PGE2 concentration in inflammatory
synoviocytes
Synovial fibroblasts treated with IL-1β exhibited
significantly increased mRNA levels of 11β-HSD1 (Fig. 4A) and mPGES-1 (Fig. 3A) when compared with the normal
synoviocytes (P<0.05). 11β-HSD1 and PGE2 proteins
were increasingly expressed in the inflammatory synoviocytes;
however, the association between 11β-HSD1 and PGE2 was
unclear. 11β-HSD1 mRNA and protein expression levels in the
inflammatory synoviocytes were found to be positively correlated
with PGE2 concentration (r=0.74, P<0.01 and r=0.94,
P<0.01; Fig. 6). The positive
correlation between 11β-HSD1 and mPGES-1 mRNA expression levels was
significant (r=0.97, P<0.01; Fig.
6).
Discussion
Synovial fibroblasts are important functional
compartments that contribute to the production of lubricious
synovial fluid and diffusion of the nutrients (16). Synovial fibroblasts are mesenchymal
cells that display multiple characteristics of fibroblasts,
including expression of type IV and V collagens, vimentin, and CD90
(Thy-1) (16). CD90, which is
evolutionarily conserved and developmentally regulated, often has
marked effects on cell phenotype and is a specific surface marker
expressed by synovial fibroblasts (17,18). In
isolated situations, the expression or lack of expression of CD90
has been used to separate fibroblast subsets (19). Vimentin is the specific protein
marker expressed by cells derived from the mesoderm and is used for
cell isolation (20). In the present
study, purified cells derived from synovial membranes in SD rat
knees were stained vimentin-positive, due to the expression of the
surface marker CD90. These results indicated that the cells were
confirmed to be synovial fibroblasts, which was consistent with the
literature (21). However, the
proliferative activity of synovial fibroblasts declined
significantly in the seven generations of culturing. Therefore, FLS
at passage three, with high purity and suitable activity were used
in the present study.
Synovial inflammation is an important contributor to
OA pathogenesis (2,22,23).
Synovitis has been demonstrated to correlate with OA symptom
severity, and hormonal factors, such as cytokines and chemokines,
are important for crosstalk in joint tissues (23,24).
These mediators have a critical role in the development of
inflammation and induce catabolic changes in joint tissues
(2,23,25,26).
Studies have demonstrated that FLS produce inflammatory cytokines
(such as TNF-α and IL-1β) and chondrolytic mediators, including
matrix metalloproteinases (27).
IL-1β and TNF-α, which are the two most extensively studied
factors, have been implicated in the pathogenesis of OA (22,23). In
the present study, when stimulated by IL-1β, FLS expressed
increased levels of IL-1β, TNF-α and mPGEs-1 and produced high
concentrations of PGE2 in the medium. The present data
indicated that the inflammatory state in the FLS induced by IL-1β
was suitable for this study.
The tissue availability of active glucocorticoids is
dependent on their rate of synthesis from cholesterol, downstream
metabolism, excretion and interconversion (28). The latter is mediated by 11β-HSDs.
11β-HSD is a glucocorticoid pre-receptor regulating enzyme that
regulates the local concentration of glucocorticoid through its
oxidation effect (9). Non-oxidized
corticosteroids, such as cortisone and 11-dehydrogenation of
corticosterone, cannot combine with the glucocorticoid receptor and
thus have an effective role in biological function. The only way to
promote activation is by 11β-HSD1 transformation (29). A prior study considered that the
sustained inflammatory state of arthritis is due to the partial
abnormal hormone metabolism in tissues (30). Synovitis is an important cause of
chronic, persistent OA. In animal models of arthritis, 11β-HSD1
gene knockout increases the risk of original joint inflammation
(31), suggesting a positive effect
of 11β-HSD1 on controlling the severity of arthritis. However, the
underlying mechanism remains unknown. 11β-HSD1-related genes and
proteins of IL-1β and TNF-α are upregulated in various stromal
cells (32). In this study, the
cortisol concentration in the medium was detected by ELISA to
determine the conversion activity of 11β-HSD1; 11β-HSD1 was highly
expressed when stimulated by IL-1β, whereas cortisol concentration
remained at a low level. As the substrate corticosterone was added,
the concentration increased significantly. By contrast, 11β-HSD1
levels decreased, indicating 11β-HSD1 was blocked by PF915275.
These results suggested that FLS had an active transformation
ability in cortisol activation; and the cortisol/cortisone ratio in
the medium and 11β-HSD1 expression were regulated under a feedback
mechanism.
The present study also demonstrated that normal
synovial fibroblasts, induced by IL-1β, highly expressed 11β-HSD1
gene and protein. Li et al (33) reported that IL-1β and other
inflammatory mediators are able to jointly upregulate 11β-HSD1 mRNA
in amnion; however, it has not yet been reported in the literature
whether a similar regulating mechanism in synovial fibroblasts
exists. In the present study, interactions between IL-1β and
glucocorticoid were identified. When synovial fibroblasts were
interfered with IL-1β and corticosterone, the expression of
11β-HSD1 decreased, which suggested that functional normal synovial
cells can be activated by the enzymatic conversion of
corticosterone and produce corticosteroids (cortisol) locally to
exert anti-inflammatory effects. Therefore, high expression of
11β-HSD1 may attenuate the inflammatory state. By contrast, when
enzyme activity and/or glucocorticoid receptor was inhibited, the
concentration of local activated cortical hormone decreased and the
probability of binding with its receptor also decreased, thus
11β-HSD1 failed to suppress the local inflammatory state
effectively. However, when the involved inflammatory factors
increased to high levels, expression of 11β-HSD1 was initiated and
increased in response to inflammation. These data suggest that
hormone levels regulated by 11β-HSD1 pre-receptor have a critical
role in controlling synovitis, and such regulation may have a
feedback mechanism.
In the pathological process of OA, the secretion of
inflammatory factors, such as PGE2 have an important
role in the development of synovitis, cartilage matrix
disintegration and bone destruction (8). PGE2 dilates blood vessels
and induces alterations in second messenger level via
autocrine/paracrine signaling (34).
Jia et al (35) identified
that PGE2 receptors present in arthritic tissue may
combine with PGE2, and subsequently induce cartilage
degradation, glycosaminoglycan loss and collagen type II
degradation in OA animal models. However, these pathological
changes can be alleviated in PGE2 receptor knockout
mice. Sun and Myatt (11) reported
that IL-1β was able to significantly improve 11β-HSD1 mRNA
expression and activity. With prior induction of 11β-HSD1
expression by dexamethasone, cortisone induced more PGE2
production in the amnion fibroblast. This study suggests that
glucocorticoids are able to positively induce 11β-HSD1 expression
in amnion fibroblasts and this effect was further strengthened by
pro-inflammatory cytokines. However, in synovial B cells, whether
glucocorticoids affect 11β-HSD1 expression and PGE2
biosynthesis remains unclear. In the present study, IL-1β
significantly induced mPGEs-1 and PGE2 expression in
synoviocytes, and corticosterone effectively inhibited this effect.
However, when the transformation activity of 11β-HSD1 was blocked
by PF915275, the activated corticosterone (cortisol) production
decreased significantly and the inhibitive effect of
PGE2 was reversed. This indicated that the pre-receptor
activity is able to effectively regulate the expression and
biological effect of PGE2 in FLS.
Both 11β-HSD1 and mPGES are present in microsomes
and co-exist in the complex pathogenesis of OA. PGE2 is
an important inflammatory factor in the human body. 11β-HSD1 has an
important role in regulating anti-inflammatory substance levels,
and thus activating glucocorticoid hormone mPGEs-1, which is an
inducible key limited enzyme, to control PGE2 production
in inflammation process (25). In
the present study, a preliminary analysis was constructed to
elucidate the association between the expression and production of
mPGEs-1, PGE2, and 11β-HSD1 in synoviocytes under an
inflammatory state. The results revealed significant positive
linear correlations between them, both at the gene and protein
expression levels. To date, no reports exist on whether
PGE2 and 11β-HSD1 promote each other in synovial B cells
under osteoarthritis conditions. Subsequently, the underlying
mechanisms of interactions between 11β-HSD1, mPGEs-1 and
PGE2 require further investigation.
Acknowledgements
The present study was supported by the Science and
Technology Planning Project of Guangdong Province, People's
Republic of China (grant no. 2011B031800172) and the Foundation for
the PhD Start-up Fund of the Affiliated Hospital of Guangdong
Medical College, People's Republic of China (grant no.
BK201209).
References
|
1
|
Felson DT, Niu J, Neogi T, Goggins J,
Nevitt MC, Roemer F, Torner J, Lewis CE and Guermazi A; MOST
Investigators Groups, : Synovitis and the risk of knee
osteoarthritis: The MOST Study. Osteoarthritis Cartilage.
24:458–464. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Scanzello CR and Goldring SR: The role of
synovitis in osteoarthritis pathogenesis. Bone. 51:249–257. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Pessler F, Dai L, Diaz-Torne C,
Gomez-Vaquero C, Paessler ME, Zheng DH, Einhorn E, Range U,
Scanzello C and Schumacher HR: The synovitis of ‘non-inflammatory’
orthopaedic arthropathies: A quantitative histological and
immunohistochemical analysis. Ann Rheum Dis. 67:1184–1187. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wei Y and Bai L: Recent advances in the
understanding of molecular mechanisms of cartilage degeneration,
synovitis and subchondral bone changes in osteoarthritis. Connect
Tissue Res. 57:245–261. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bhattaram P and Chandrasekharan U: The
joint synovium: A critical determinant of articular cartilage fate
in inflammatory joint diseases. Semin Cell Dev Biol. May
19–2016.(Epub ahead of print). PubMed/NCBI
|
|
6
|
Bondeson J, Blom AB, Wainwright S,
Wainwright S, Hughes C, Caterson B and van den Berg WB: The role of
synovial macrophages and macrophage-produced mediators in driving
inflammatory and destructive responses in osteoarthritis. Arthritis
Rheum. 62:647–657. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Blasioli DJ, Matthews GL and Kaplan DL:
The degradation of chondrogenic pellets using cocultures of
synovial fibroblasts and U937 cells. Biomaterials. 35:1185–1191.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Roshak A, Mochan E and Marshall LA:
Suppression of human synovial fibroblast 85 KDa phospholipase A2 by
antisense reduces interleukin-1 beta induced prostaglandin E2. J
Rheumatol. 23:420–427. 1996.PubMed/NCBI
|
|
9
|
Hardy R, Rabbitt EH, Filer A, Emery P,
Hewison M, Stewart PM, Gittoes NJ, Buckley CD, Raza K and Cooper
MS: Local and systemic glucocorticoid metabolism in inflammatory
arthritis. Ann Rheum Dis. 67:1204–1210. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hardy RS, Filer A, Cooper MS, Parsonage G,
Raza K, Hardie DL, Rabbitt EH, Stewart PM, Buckley CD and Hewison
M: Differential expression, function and response to inflammatory
stimuli of 11beta-hydroxysteroid dehydrogenase type 1 in human
fibroblasts: A mechanism for tissue-specific regulation of
inflammation. Arthritis Res Ther. 8:R1082006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sun K and Myatt L: Enhancement of
glucocorticoid-induced 11beta-hydroxysteroid dehydrogenase type 1
expression by proinflammatory cytokines in cultured human amnion
fibroblasts. Endocrinology. 144:5568–5577. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tomlinson JW, Moore J, Cooper MS, Bujalska
I, Shahmanesh M, Burt C, Strain A, Hewison M and Stewart PM:
Regulation of expression of 11beta-Hydroxysteroid dehydrogenase
type 1 in adipose tissue: Tissue-specific induction by cytokines.
Endocrinology. 142:1982–1989. 2001. View Article : Google Scholar
|
|
13
|
Newton R, Kuitert LM, Slater DM, Adcock IM
and Barnes PJ: Cytokine induction of cytosolic phospholipase A2 and
cyclooxygenase-2 mRNA is suppressed by glucocorticoids in human
epithelial cells. Life Sci. 60:67–78. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hoeck WG, Ramesha CS, Chang DJ, Fan N and
Heller RA: Cytoplasmic phospholipase A2 activity and gene
expression are stimulated by tumor necrosis factor: Dexamethasone
blocks the induced synthesis. Proc Natl Acad Sci USA. 90:4475–4479.
1993. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bartok B and Firestein GS: Fibroblast-like
synoviocytes: Key effector cells in rheumatoid arthritis. Immunol
Rev. 233:233–255. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zimmermann T, Kunisch E, Pfeiffer R, Hirth
A, Stahl HD, Sack U, Laube A, Liesaus E, Roth A, Palombo-Kinne E,
et al: Isolation and characterization of rheumatoid arthritis
synovial fibroblasts from primary culture-primary culture cells
markedly differ from fourth-passage cells. Arthritis Res. 3:72–76.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bradley JE, Ramirez G and Hagood JS: Roles
and regulation of Thy-1, a context-dependent modulator of cell
phenotype. Biofactors. 35:258–265. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sorrell JM and Caplan AI: Fibroblasts-a
diverse population at the center of it all. Int Rev Cell Mol Biol.
276:161–214. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ota F, Maeshima A, Yamashita S, Ikeuchi H,
Kaneko Y, Kuroiwa T, Hiromura K, Ueki K, Kojima I and Nojima Y:
Activin A induces cell proliferation of fibroblast-like
synoviocytes in rheumatoid arthritis. Arthritis Rheum.
48:2442–2449. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lefèvre S, Knedla A, Tennie C, Kampmann A,
Wunrau C, Dinser R, Korb A, Schnäker EM, Tarner IH, Robbins PD, et
al: Synovial fibroblasts spread rheumatoid arthritis to unaffected
joints. Nat Med. 15:1414–1420. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sellam J and Berenbaum F: The role of
synovitis in pathophysiology and clinical symptoms of
osteoarthritis. Nat Rev Rheumatol. 6:625–635. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Goldring MB and Otero M: Inflammation in
osteoarthritis. Curr Opin Rheumatol. 23:471–478. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Goldring MB and Marcu KB: Cartilage
homeostasis in health and rheumatic diseases. Arthritis Res Ther.
11:2242009. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Goldring MB and Goldring SR:
Osteoarthritis. J Cell Physiol. 213:626–634. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lotz M and Loeser RF: Effects of aging on
articular cartilage homeostasis. Bone. 51:241–248. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sokolove J and Lepus CM: Role of
inflammation in the pathogenesis of osteoarthritis: latest findings
and interpretations. Ther Adv Musculoskelet Dis. 5:77–94. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Schmidt M and Straub RH:
11β-hydroxysteroid dehydrogenase enzymes modulate effects of
glucocorticoids in rheumatoid arthritis synovial cells.
Neuroimmunomodulation. 22:40–45. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Cooper MS and Stewart PM:
11Beta-hydroxysteroid dehydrogenase type 1 and its role in the
hypothalamus-pituitary-adrenal axis, metabolic syndrome and
inflammation. J Clin Endocrinol Metab. 94:4645–4654. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Bailey E, Greaves MS, Murphy D and West
HF: Corticosteroid metabolism and rheumatoid arthritis. Ann Rheum
Dis. 25:516–524. 1966. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hardy RS, Seibel MJ and Cooper MS:
Targeting 11β-hydroxysteroid dehydrogenases: A novel approach to
manipulating local glucocorticoid levels with implications for
rheumatic disease. Curr Opin Pharmacol. 13:440–444. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Tomlinson JW, Walker EA, Bujalska IJ,
Draper N, Lavery GG, Cooper MS, Hewison M and Stewart PM:
11β-hydroxysteroid dehydrogenase type 1: A tissue-specific
regulator of glucocorticoid response. Endocr Rev. 25:831–866. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Li W, Gao L, Wang Y, Duan T, Myatt L and
Sun K: Enhancement of cortisol-induced 11beta-hydroxysteroid
dehydrogenase type 1 expression by interleukin 1beta in cultured
human chorionic trophoblast cells. Endocrinology. 147:2490–2495.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lemieux LI, Rahal SS and Kennedy CR: PGE2
reduces arachidonic acid release in murine podocytes: Evidence for
an autocrine feedback loop. Am J Physiol Cell Physiol.
284:C302–C309. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Jia XY, Chang Y, Sun XJ, Dai X and Wei W:
The role of prostaglandin E2 receptor signaling of dendritic cells
in rheumatoid arthritis. Int Immunopharmacol. 23:163–169. 2014.
View Article : Google Scholar : PubMed/NCBI
|