Introduction
Acinetobacter baumannii (A. baumannii) are a
type of gram-negative non-fermenting bacteria that widely exist in
nature and on the surface of human skin. In the presence of a
compromised immune system, A. baumannii may cause serious
infections, including ventilator-associated pneumonia, sepsis,
urinary tract infections and meningitis (1). Due to the use of invasive operation,
broad-spectrum antibiotics and immunosuppressive agents in the last
decade, A. baumannii has transformed from a
monodrug-resistant to a multidrug-resistant or pandrug-resistant
organism, and is now becoming the major pathogen of severe fatal
nosocomial infections (2). Previous
results have suggested that numerous mechanisms may lead to this
resistance, and the active efflux mechanism is an important factor
for mutidrug-resistance in A. baumannii (3). Since it is the unique efflux pump to
A. baumannii, the AdeABC system is important in mediating
drug-resistance, which belongs to the
resistance-nodulation-division multidrug efflux system (4).
Therefore, the present study was designed to analyze
the association between the differences in the expression level of
the efflux pump adeB gene using an in vitro induction of a
drug-resistance test in A. baumannii, and to verify the
significance of the efflux mechanism in the induced drug-resistance
among the three drugs, respectively. Therefore, research on the
mechanisms underlying induced multidrug resistance is aimed at
finding a solution to antimicrobial resistance.
Materials and methods
Bacterial strains
A. baumannii strains that were sensitive to
amikacin (n=19), netilmicin (n=17) and imipenem (n=25) were
isolated from clinical blood or drainage samples in the First
Affiliated Hospital of China Medical University (Shenyang, China)
between January 2009 and December 2010. Strains were identified and
initial antimicrobial susceptibilities were determined by the Vitek
system (BioMérieux, Marcy-l'Étoile, France). All of the strains
were non-repetitive, meaning that only one strain was collected
from each patient.
Instruments and reagents
The instruments that were used for the experiments
included a VITEK-2 automated microbial analyzer and Sakuma MIT-P
bacterial multipoint inoculator (Sakuma Co., Ltd., Matsudo, Japan).
A quantitative polymerase chain reaction (qPCR) kit was purchased
from Takara Biotechnology Co., Ltd., Dalian, China. Reference
standards of amikacin, netilmicin and imipenem were purchased from
The European Pharmacopoeia (EP; Strasbourg, France). Luria-Bertani
and Mueller-Hinton (MH) broths and MH agar were purchased from
Oxoid Ltd. (Nepean, ON, Canada). AdeB and 16S rRNA primers were
synthesized by Sangon Biotech Co., Ltd. (Shanghai, China). RNA
extraction kits were purchased from Thermo Fischer Scientific, Inc.
(Waltham, MA, USA). Quantitative and reverse transcription PCR kits
were purchased from GeneCopoeia, Inc. (Rockville, MD, USA), diethyl
pyrophosphate from Tiangen Biotech Co., Ltd. (Beijing, China), and
carbonyl cyanide 3-chlorophenylhydrazone (CCCP) and acetone from
Sigma-Aldrich (Merck KGaA, Darmstadt, Germany).
Susceptibility testing
The minimum inhibitory concentration (MIC) values of
all the strains to each drug were tested by the broth microdilution
assay method, which was recommended by the Clinical and Laboratory
Standards Institute (CLSI; 2012) (5). A 0.5 McFarland standard bacterial
suspension was prepared, which was then diluted using MH broth
(10-fold and twice) and were placed in the 96-well plates at
different concentrations. The A. baumannii strains that were
sensitive to amikacin (n=19), netilmicin (n=17) and imipenem (n=25)
were selected for this experiment according to the CLSI standards
(2012) (5). Furthermore,
Pseudomonas aeruginosa (P. aeruginosa) ATCC27853 and
Escherichia coli ATCC29522 were both used as control
strains.
Multi-step stable in vitro induction
of drug-resistance
Selective medium containing an appropriate
concentration of one antibiotic and MH broth was prepared in a
96-well plate. The initial concentration of the antibiotic was half
of the MIC of the testing strains. The final concentration of
bacterial suspension was 4–8×105 colony forming
units/ml. In total a volume of 200 µl was prepared in each well of
the 96-well plate, and each well contained 100 µl antimicrobial
solution and 100 µl bacterial suspension. The suspension was
inoculated into the MH broth medium with ascending concentrations
of antimicrobial drugs, until the drug concentration reached 128
µg/ml. The 128 µg/ml was then selected as the final concentration
for induction. The resistant strains were cultured another five
times at this final concentration. Next, the surviving strains were
transferred to the drug-free broth medium and inoculated twice to
maintain resistant stability. Then the resistant strains were
re-inoculated in the media with a drug concentration of 128 mg/l.
The surviving strains were considered to have stable
drug-resistance. Finally, the stably resistant strains were
inoculated on a blood plate to preserve the strains. When the
strains grew poorly, the induction was repeated with the same or
reduced concentration. If there was no growth for two generations
(the same concentration) the strains were discarded. In addition,
the drug-free and strain-free control broth wells and the culture
in the blood plate were also established. Finally, the duration of
induction for each drug was ~4 weeks.
Detection of an efflux phenotype
The 2-fold agar dilution method, recommended by the
2012 CLSI, was used for this experiment. The MIC values of
amikacin, netilmicin and imipenem were detected. Meanwhile, the MH
agar containing CCCP was prepared. CCCP was fully dissolved in 10
µg/ml acetone and the solution was passed through a sterile filter
(0.22-µm; Bioer, Hangzhou, China). The final concentration of CCCP
was 10 µg/ml in which the strains could survive. The criteria for a
positive efflux phenotype were defined as a MIC value with CCCP
that decreased 4-fold or more compared to the MIC values without
CCCP (6). Both the sensitive and
resistant groups were used for the test, and P. aeruginosa
ATCC27853 was selected for the quality control strain.
Detection of the expression level of
efflux pump gene adeB by qPCR
Following bacterial RNA extraction, the total RNA
was reverse transcribed into cDNA and RT-qPCR was performed. AdeB
was the target gene and 16S rRNA served as an internal reference
gene (4). The reaction was 20 µl in
total: 10 µl 2X All-in-one qPCR mix, 2 µl PCR forward and 2 µl PCR
reverse primers, 2 µl cDNA templates, 0.4 µl 50X Rox Reference Dye
and 3.6 µl purified water. The reaction conditions consisted of
three phases: Predenaturation at 95°C for 10 min; 40 cycles of
denaturation at 95°C for 10 sec, annealing at 55°C for 20 sec,
extension at 72°C for 15 sec; and one cycle of dissociation at 95°C
for 10 sec. For both the target and the internal reference genes,
RT-qPCR reactions were conducted in two tubes simultaneously, and
each reaction was repeated six times. The results were analyzed by
the ∆∆Cq method (7). One positive
strain (for adeB) was selected for the control sample.
Standardization was made by the subtraction of the mean Cq value of
each adeB gene and the mean Cq value of the corresponding 16S rRNA
(repeated three times for each sample), yielding a corrected ∆Cq
value. Following correction, subtraction of the ∆Cq value of the
resistant strain from the mean ∆Cq value of the positive strain
yielded ∆∆Cq. Finally, the relative mRNA expression level of the
adeB gene in the resistant strain was calculated using the
2−∆∆Cq method.
Results
In vitro induction of
drug-resistance
There were 19, 17 and 25 strains (sensitive to
amikacin, netilmicin and imipenem, respectively) selected for in
vitro induction under a single drug-condition. All the MICs of
the 19 amikacin-sensitive strains were no more than 16 µg/ml, 17
netilmicin-sensitive strains were no more than 8 µg/ml and for the
25 imipenem-sensitive strains were ≤4 µg/ml. Following the in
vitro induction test, 11, 15 and 8 resistant strains were
generated with stable mutations, respectively. Among them, the
imipenem-resistant group had the longest induction time.
Result of the efflux phenotype
The MIC values of all the sensitive strains
decreased less than 2-fold under CCCP-containing conditions for the
three drugs, demonstrating the negative efflux phenotype. On the
other hand, there were 10 strains resistant to amikacin showing
decreased MIC values for 4-fold or more after addition of CCCP, 14
strains for netilmicin but none for imipenem. All the strains grew
well on the MH plates containing only CCCP. Furthermore, decreased
MIC values for 4-fold or more with CCCP showed a significant efflux
pump phenotype. The details of the efflux pump phenotype are listed
in Table I.
 | Table I.Detection of efflux phenotype of in
vitro-induced isolates by three drugs. |
Table I.
Detection of efflux phenotype of in
vitro-induced isolates by three drugs.
|
| MIC (µg/ml) | MIC (µg/ml) | MIC (µg/ml) |
|---|
|
|
|
|
|
|---|
| Strain number | Imipenem | +CCCP | Netilmicin | +CCCP | Amikacin | +CCCP |
|---|
| 1 | 256 | 256 | 256 | 64 | 512 | 128 |
| 2 | 256 | 128 | 256 | 64 | 512 | 128 |
| 3 | 256 | 256 | 256 | 64 | 512 | 128 |
| 4 | 256 | 256 | 256 | 256 | 512 | 128 |
| 5 | 256 | 256 | 256 | 16 | 512 | 128 |
| 6 | 256 | 256 | 256 | 32 | 512 | 128 |
| 7 | 256 | 256 | 256 | 32 | 512 | 128 |
| 8 | 256 | 256 | 256 | 32 | 512 | <0.015 |
| 9 |
|
| 256 | 32 | 512 | 128 |
| 10 |
|
| 256 | 64 | 512 | 128 |
| 11 |
|
| 256 |
0.5 | 128 | 128 |
| 12 |
|
| 256 | 64 |
|
|
| 13 |
|
| 256 | 64 |
|
|
| 14 |
|
| 256 | 64 |
|
|
| 15 |
|
| 256 | 64 |
|
|
Results of the adeB gene expression
levels by RT-qPCR
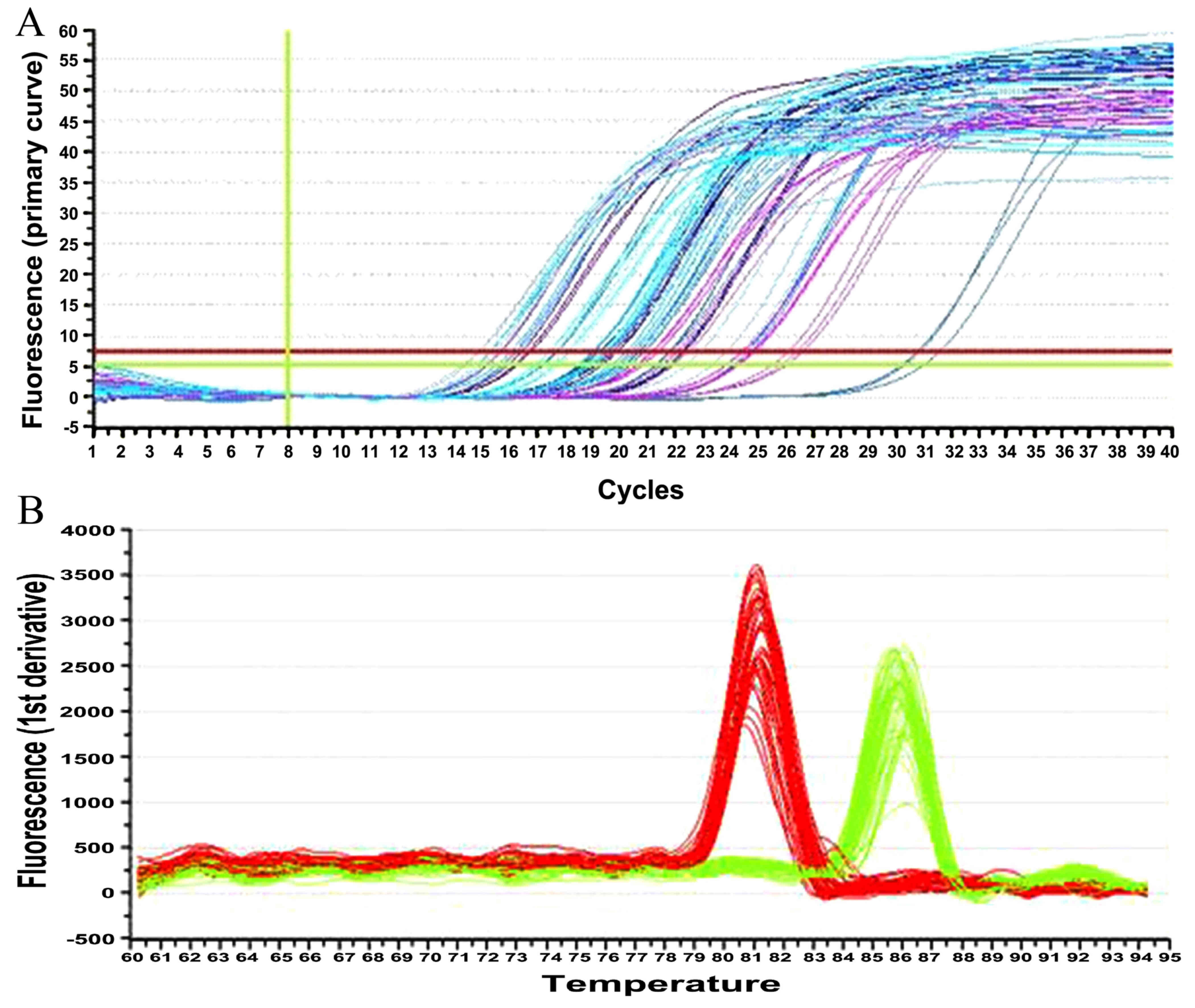
The amplification curves of the target and the
internal reference genes were all presented as typical S-shape
kinetic curves, with evident exponential and plateau phases. The
corresponding melting curves only had a single peak, indicating
good specificity. Furthermore, no adeB gene expression was observed
in any of the initially susceptible strains or the
imipenem-resistant group. The expression levels of all the internal
reference 16S rRNA standards were good. Furthermore, there were 10
and 5 strains, respectively, for the amikacin- and
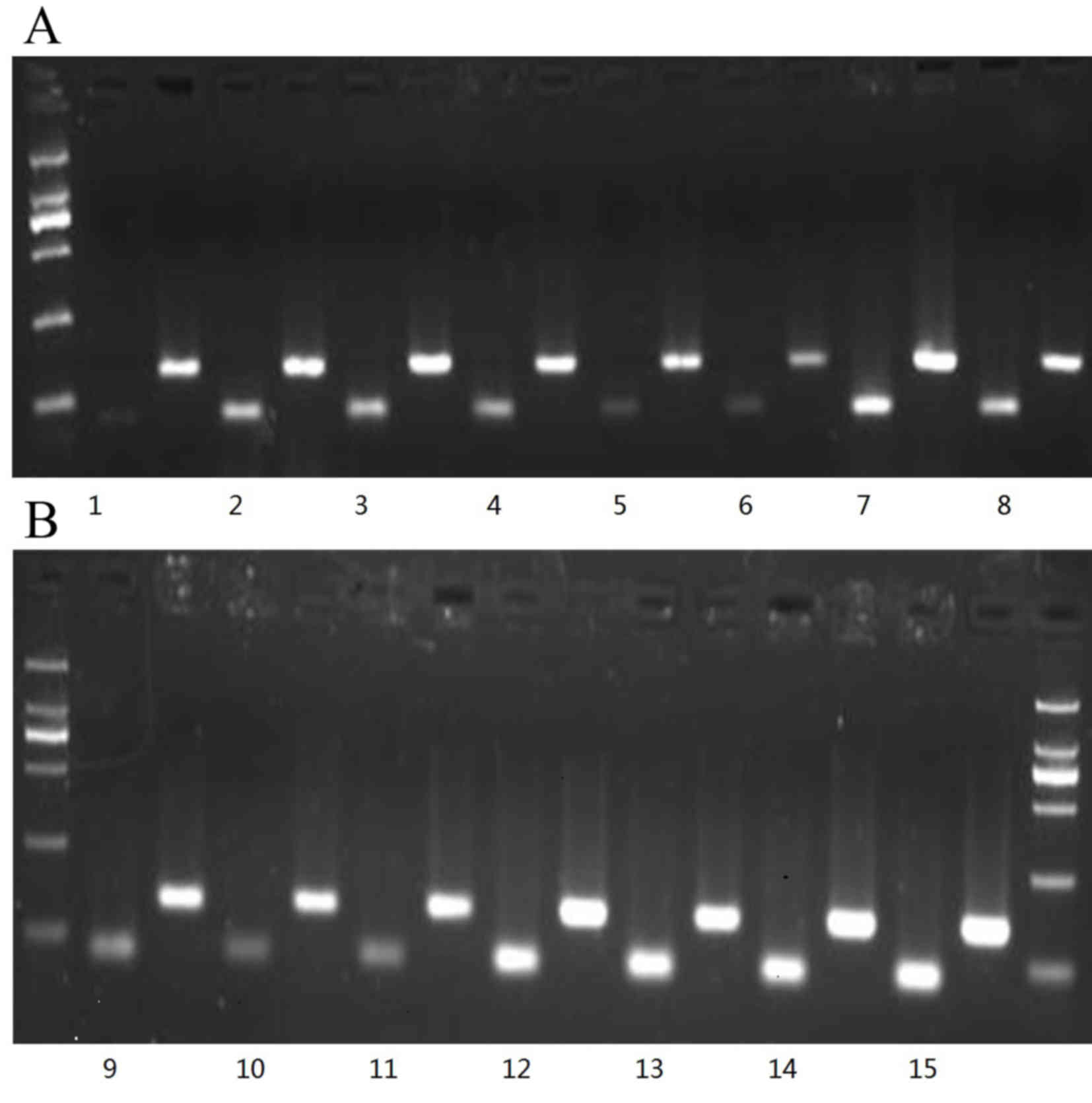
netilmicin-resistant groups, as shown in Table II and Fig. 1. In addition, the electrophoretograms
of the strains with positive expression of the efflux pump adeB
gene are shown in Fig. 2, in which
lanes 1–10 showed the results from the strains in the
amikacin-resistant group and the last five lanes show the results
from strains 3, 5, 11, 13 and 15 of the netilmicin-resistant group.
These data indicated different copy number between the groups;
specifically, the netilmicin-resistant groups had a higher copy
number.
 | Table II.Detection of the expression levels of
the active efflux pump gene adeB in the amikacin and netilmicin
mutation groups by RT-qPCR. |
Table II.
Detection of the expression levels of
the active efflux pump gene adeB in the amikacin and netilmicin
mutation groups by RT-qPCR.
|
| Cq | Cq | ΔCq | ΔΔCq |
|
|---|
|
|
|
|
|
|
|
|---|
| Strains | adeB | 16S rRNA | Trial group | Trial group-control
group | Copy number |
|---|
| Amikacin 1 | 27.26 | 21.32 | 5.94 | −3.41 | 10.6294 |
| Amikacin 2 | 19.09 | 14.74 | 4.35 | −5.00 | 32.0000 |
| Amikacin 3 | 19.35 | 14.87 | 4.48 | −4.87 | 29.2426 |
| Amikacin 4 | 24.46 | 20.66 | 3.80 | −5.55 | 46.8507 |
| Amikacin 5 | 24.32 | 20.13 | 4.29 | −4.56 | 23.5883 |
| Amikacin 6 | 22.36 | 17.02 | 5.34 | −4.61 | 24.4201 |
| Amikacin 7 | 19.54 | 15.48 | 4.06 | −5.29 | 39.1244 |
| Amikacin 8 | 25.42 | 21.87 | 3.55 | −5.80 | 55.7152 |
| Amikacin 9 | 19.87 | 15.20 | 4.67 | −4.68 | 25.6342 |
| Amikacin 10 | 23.87 | 19.29 | 4.58 | −4.77 | 27.2843 |
| Netilmicin 3 | 19.61 | 15.75 | 4.86 | −4.49 | 22.4711 |
| Netilmicin 5 | 19.50 | 17.16 | 2.34 | −7.01 | 128.2903 |
| Netilmicin 11 | 21.82 | 17.82 | 4.00 | −5.35 | 40.7859 |
| Netilmicin 13 | 20.16 | 15.86 | 4.30 | −5.05 | 33.1284 |
| Netilmicin 15 | 20.81 | 17.46 | 3.35 | −6.00 | 64.0000 |
Discussion
A. baumannii has evolved from being mono- to
multi-drug resistant or even into a pandrug-resistant organism.
They have become a major pathogen involved in serious and fatal
nosocomial infections, which is an important global issue (8). Previous studies show that the resistant
mechanisms of A. baumannii include formation of inactivated
enzymes, gene mutations in chromosomes, changes in outer membrane
porins and the active drug efflux mechanism (6,9,10). Among these, the active efflux
mechanism is the main factor causing multi-drug resistance. Since
the AdeABC-type active efflux system in A. baumannii BM4454
(3) was discovered by French
researchers in 2001, a greater number of studies on the mechanisms
of multi-drug resistance in A. baumannii have focused on
active efflux. Although AdelJK, AdeDE, AdeFGH and other systems
have been studied in recent years, the AdeABC-type active efflux
system still remains unique and most closely associated to A.
baumannii (11,12).
As an inhibitor of the efflux systems, CCCP can
freely diffuse across both sides of the lipid membrane. CCCP is a
strong uncoupler that transmits protons to destroy the
transmembrane electrochemical gradient, resulting in a loss of
energy supply for transport proteins. CCCP can damage the active
effects of the efflux system, increase drug accumulation in
bacteria and recover the susceptibility of bacteria to drugs
(13). A previous study on active
efflux pump inhibitors by Chinese researchers has demonstrated that
their reported inhibitory effects are different to the presently
reported effects (6), but it is not
clear how suitable CCCP is as an inhibitor for screening the AdeABC
efflux pump in A. baumannii and the difference between
different antibiotics.
The results of the present in vitro induction
of drug-resistance tests demonstrate that amikacin and netilmicin
are more prone to producing drug-resistance mutations than
imipenem. Following induction by amikacin, netilmicin and imipenem
in vitro, there were 10, 14 and 0 resistant strains
inhibited by CCCP, respectively. MIC values decreased 4-fold or
more after addition of the efflux pump inhibitor, CCCP. The most
notable changes were in the amikacin- and netilmicin-resistant
groups with 11 and 15 breakpoints, respectively, indicating a
marked efflux effect. These results demonstrate that amikacin and
netilmicin are more likely to produce resistance caused by the
AdeABC-type efflux system than imipenem in vitro. There were
15 strains that showed a positive efflux phenotype in
netilmicin-resistant groups, but the expression of the adeB gene
was detected only in 5 of all the 15 strains. In addition, the MIC
value of strain no. 8 decreased 15 breakpoints when accompanied
with CCCP, but its copy number was only 55.7152, as shown by
RT-qPCR. Those results indicate the difference between different
drugs in inducing resistance, and the application of CCCP for
inhibition of efflux systems in different drugs.
The results demonstrated that among the
drug-resistant strains that were induced in vitro, 10 and 5
strains expressed the adeB gene in the amikacin- and
netilmicin-resistant groups, respectively. RT-qPCR fluorescence
quantitative detection of resistant strains demonstrated that
netilmicin-resistant strain no. 5 had a higher mRNA level of the
adeB efflux pump and its copy number reached 128.2903. Furthermore,
amikacin-resistant strain nos. 1, 5, 6, 9 and 10 and
netilmicin-resistant strain no. 3 had lower mRNA levels of the adeB
efflux pump. The copy number of amikacin-resistant strain no. 1 was
10.6294, which was significantly lower than that of other strains.
These results indicate that there are differences in the efficacy
of common clinically used antimicrobial drugs due to the AdeABC
efflux system in different strains. The induction time of
netilmicin strain no. 5 was 13 days and the amikacin strain no. 1
was 19 days. Therefore, we can conclude that the induction time has
no direct association with the expression of adeB. Furthermore, no
expression of the adeB was detected by qPCR in initially
susceptible strains, indicating that the active efflux mechanism is
important in mediating the induction of drug resistance in A.
baumannii (14–18). There were a number of differences
between the adeB expression results and the number of in
vitro induction of resistant strains. These findings indicate
that drug-resistant strains possess efflux pump mechanisms or
important resistance mechanisms other than the AdeABC efflux
system, including extended-spectrum β-lactamases enzymes and
changes in and loss of membrane permeability, all of which need to
be studied further. By contrast to the reports on the mechanisms of
carbapenem-resistance in A. baumannii, the resistance
mechanisms have regional differences that may be caused by a
different phenotype or genotype of the clinically collected strains
(19–21).
The present study used an in vitro
drug-induction test to confirm that susceptible A. baumannii
could develop a drug-resistance following long-term exposure at low
doses of antimicrobial drugs. In addition, the presence of an
active efflux mechanism and the involvement of other mechanisms in
drug resistance induction also increase the resistance against
commonly used clinical antimicrobial drugs. Therefore, the rational
use of antimicrobial drugs and strengthened management of treatment
regimens can reduce or avoid formation of drug-resistant strains
and evolution of resistance. Therefore, rational antibiotic use is
an important risk management strategy used to prevent and reduce
opportunistic pathogen infections in hospitals, and the development
of drug-resistance.
Acknowledgements
The present study was funded by the Liaoning
Province Natural Science Foundation (grant no. 2013021091).
References
|
1
|
Bergogne-Bérézin E and Towner KJ:
Acinetobacter spp. as nosocomial pathogens: Microbiological,
clinical, and epidemiological features. Clin Microbiol Rev.
9:148–165. 1996.PubMed/NCBI
|
|
2
|
Guardado Rodríguez A, Blanco A, Asensi V,
Pérez F, Rial JC, Pintado V, Bustillo E, Lantero M, Tenza E,
Alvarez M, et al: Multidrug-resistant Acinetobacter
meningitis in neurosurgical patients with intraventricular
catheters: Assessment of different treatments. J Antimicrob
Chemother. 61:908–913. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Magnet S, Courvalin P and Lambert T:
Resistance-nodulation-cell division-type efflux pump involved in
aminoglycoside resistance in Acinetobacter baumannii strain
BM4454. Antimicrob Agents Chemother. 45:3375–3380. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Peleg AY, Adams J and Paterson DL:
Tigecycline efflux as a mechanism for nonsusceptibility in
Acinetobacter baumannii. Antimicrob Agents Chemother.
51:2065–2069. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Clinical and Laboratory Standards
Institute (CLSI): Performance Standards for Antimicrobial
Susceptibility Testing: Twenty-First Informational SupplementCLSI
documen M100-S22. CLSI; Wayne, PA: 2012
|
|
6
|
Shi WF, Jiang JP, Xu N, Huang ZM and Wang
YY: Inhibitory effects of reserpine and carbonyl cyanide
m-chloro-phenylhydrazone on fluoroquinolone resistance of
Acinetobacter baumannii. Chin Med J (Engl). 118:340–343.
2005.PubMed/NCBI
|
|
7
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Giannouli M, Cuccurullo S, Crivaro V, Di
Popolo A, Bernardo M, Tomasone F, Amato G, Brisse S, Triassi M,
Utili R and Zarrilli R: Molecular epidemiology of
multidrug-resistant Acinetobacter baumannii in a tertiary
care hospital in Naples, Italy, shows the emergence of a novel
epidemic clone. J Clin Microbiol. 48:1223–1230. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fernandez-Cuenca F, Martínez-Martínez L,
Conejo MC, Ayala JA, Perea EJ and Pascual A: Relationship between
beta-lactamase production, outer membrane protein and
penicillin-binding protein profiles on the activity of carbapenems
against clinical isolates of Acinetobacter baumannii. J
Antimicrob Chemother. 51:565–574. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lee JK, Martínez-Martínez L, Conejo MC,
Ayala JA, Perea EJ and Pascual A: Mutations in the gyrA and gyrC
genes in ciprofloxacin-resistant clinical isolates of
Acinetobacter baumannii in Korea. Microbiol Immunol.
49:647–653. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yoon EJ, Courvalin P and Grillot-Courvalin
C: RND-type efflux pumps in multidrug-resistant clinical isolates
of Acinetobacter baumannii: Major role for AdeABC
overexpression and AdeRS mutations. Antimicrob Agents Chemother.
57:2989–2995. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Rajamohan G, Srinivasan VB and Gebreyes
WA: Molecular and functional characterization of a novel efflux
pump, AmvA, mediating antimicrobial and disinfectant resistance in
Acinetobacter baumannii. J Antimicrob Chemother.
65:1919–1925. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ni W, Li Y, Guan J, Zhao J, Cui J, Wang R
and Liu Y: Effects of efflux pump inhibitors on colistin resistance
in multidrug-resistant gram-negative bacteria. Antimicrob Agents
Chemother. 60:3215–3218. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Principe L, D'Arezzo S, Capone A,
Petrosillo N and Visca P: In vitro activity of tigecycline in
combination with various antimicrobials against multidrug resistant
Acinetobacter baumannii. Ann Clin Microbiol Antimicrob.
8:182009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ruzin A, Keeney D and Bradford PA: AdeABC
multidrug efflux pump is associated with decreased susceptibility
to tigecycline in Acinetobacter calcoaceticus-Acinetobacter
baumannii complex. J Antimicrob Chemother. 59:1001–1004. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lin L, Ling BD and Li XZ: Distribution of
the multidrug efflux pump genes, adeABC, adeDE and adeIJK, and
class 1 integron genes in multiple-antimicrobial-resistant clinical
isolates of Acinetobacter baumannii-Acinetobacter
calcoaceticus complex. Int J Antimicrob Agents. 33:27–32. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Poole K: Outer membranes and efflux: The
path to multidrug resistance in Gram-negative bacteria. Curr Pharm
Biotechnol. 3:77–98. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang JP, Zhu W, Tian SF, Chu YZ and Chen
BY: Molecular characteristics and resistant mechanisms of
imipenem-resistant Acinetobacter baumannii isolates in
Shenyang, China. J Microbiol. 48:689–694. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Heritier C, Poirel L, Lambert T and
Nordmann P: Contribution of acquired carbapenem-hydrolyzing
oxacillinases to carbapenem resistance in Acinetobacter
baumannii. Antimicrob Agents Chemother. 49:3198–3202. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hu WS, Yao SM, Fung CP, Hsieh YP, Liu CP
and Lin JF: An OXA-66/OXA-51-like carbapenemase and possibly an
efflux pump are associated with resistance to imipenem in
Acinetobacter baumannii. Antimicrob Agents Chemother.
51:3844–3852. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Rhomberg PR and Jones RN: MYSTIC Program
(USA) Study Group: Antimicrobial spectrum of activity for meropenem
and nine broad spectrum antimicrobials: Report from the MYSTIC
program (2002) in North America. Diagn Microbiol Infect Dis.
47:365–372. 2003. View Article : Google Scholar : PubMed/NCBI
|