Introduction
Video-assisted thoracoscopic surgery (VATS) has been
used as a diagnostic and therapeutic platform to perform a wide
variety of thoracic cavity surgical procedures (1), including surgical lung biopsy, cancer
staging, pneumothorax, pericardiotomy and pericardial window
creation (2). VATS is clinically
accepted as being less painful and causing less stress than
traditional open procedures. Eliminating conventional abdominal
incisions is considered to have a number of potential advantages
including faster recovery and thus a shorter hospital stay,
decreased postoperative pain, improved postoperative pulmonary
function and improved cosmetic outcome. Previous studies have also
confirmed the safety and benefits of VATS in thoracic cavity
surgery (3,4).
Peumonectomy is a lung resection technique used in
humans and animals to remove all lung lobes when bilobectomy or
lobectomy techniques are inadequate to remove the pathology in the
hemithorax. Pneumonectomy was performed to treat certain
pathological conditions including lung tumors, congenital lung
anomalies, chronic lung collapse, chronic progressive lung
inflammation, post-traumatic diffuse parenchymal laceration and
bronchial rupture (5,6).
A number of comparative studies on VATS procedures
in animals have evaluated stress parameters and postoperative
outcomes (7,8); there have also been studies into the
outcomes of VATS lobectomy (4,9).
Compared with VATS, hemithorax pneumonectomy is more difficult to
perform and there is a higher risk of intra- and post-operative
complications occurring (10); thus,
the degree of stress and the postoperative hemodynamics in VATS
compared with traditional open pneumonectomy remains to be
evaluated. Study.
Acute-phase proteins (APPs) are a series of proteins
that are sensitive to inflammation and body stress including
infection, surgical trauma, certain diseases and tissue damage
(11). The concentrations of certain
APPs, including C-reactive protein (CRP) and serum amyloid A (SAA),
may increase markedly during stress or under pathological
conditions. YKL-40 is another type of APP that increases in
proportion to body stress, indicating the respiratory function
level and pneumopathy prognosis.
To thoroughly assess the impact of minimally
invasive surgical techniques on acute-phase reactions, metabolic
changes and stress responses, the current study compared such
factors in dogs undergoing pneumonectomy via the VATS approach vs.
those undergoing open thoracotomy to compare the impacts of the two
approaches.
Materials and methods
Animals
A total of 14 mongrel dogs aged 2–5 years with a
mean body weight of 7.7 kg (range 5.4–10.5 kg) were used in the
present study. The dogs were obtained from the Experimental Animal
Center of Northeast Agricultural University (Harbin, China). The
dogs were housed at a controlled temperature (20°C) and humidity
(60°C) under a 12 h light/dark cycle. All animals were fed a
standard canine diet 3 times daily and had free access to water. Of
the 14 dogs enrolled in the study, 9 were ultimately adopted and 5
were euthanized. Dogs were sacrificed via 300 mg intravenous
xylocaine (Shandong Jincheng Pharmaceutical Co., Ltd., Zibo, China)
following anesthetizing with 0.1 mg/kg xylazine and 5 mg/kg
ketamine hydrochloride (Jiangsu Hengrui Medicine Co., Ltd.,
Lianyungang, China). A preoperative physical examination and
complete blood count indicated that all dogs were healthy. Animals
were randomly and equally divided into two groups (n=7): One group
underwent a VATS pneumonectomy and the other group underwent an
open pneumonectomy. The present study was approved by the Northeast
Agricultural University Institutional Animal Care and Use Committee
(Harbin, China), and was conducted in a manner consistent with the
U.S. National Institutes of Health ‘Guide for the Care and Use of
Laboratory Animals’, the Animal Welfare Acts (12) and the Guide for the Care and Use of
Agricultural Animals in Agricultural Research and Teaching,
including appropriate methods of euthanasia (13).
Surgical preparation
Baseline physiological parameters were determined
prior to surgery (t=0), including routine blood testing, heart rate
(HR), respiratory frequency (RF), blood pressure (BP), rectal
temperature and serum concentrations of CRP, SAA and YKL-40.
Following a 24 h fast and a 2 h water-fast, all animals were
administered the same general anesthesia and were monitored and
managed similarly.
Cefazolin (20 mg/kg; Harbin Pharmaceutical Group
Co., Ltd., General Pharm Factory, Harbin, China) was administered
intramuscularly (IM) 30 min prior to surgery to prevent
postoperative infection. Each dog was pre-medicated with 0.05 mg/kg
atropine (Beijing Shuanghe Pharmaceutical Co., Ltd., Beijing,
China) subcutaneously and ~15 min later anesthesia was induced with
intravenous propofol (4–5 mg/kg; Xi'an Libang Pharmaceutical Co.,
Ltd., Xian, China) and midazolam (0.05–0.06 mg/kg; Jiangsu Shenhua
Pharmaceutical Co., Ltd., Jiangsu, China). The dog was then placed
in the supine position and intubated with a tracheal cannula.
General anesthesia was maintained with 1.5–2% isoflurane (Hebei
Welcome Pharmaceutical Co., Ltd., Shijianzhuang, China) gas mixed
in oxygen, and respiration was maintained via mechanical
ventilation with the anesthetic gas machine (respiratory frequency
12 breaths per minute, tidal volume 15–20 ml/kg,
inspiratory:expiratory ratio 1:2). Tramadol (4 mg/kg; Zhejiang
Jiuxu Pharmaceutical Co., Ltd., Hangzhou, China) was administrated
intravenously prior to surgery for analgesia.
The surgical site (left chest from the clavicle to
the last rib and from the sternum to the spine) was shaved,
aseptically prepared and draped for surgery. A heating blanket was
placed between the animal and the operating table.
Sterile instruments were used for all open and
laparoscopic procedures. For the VATS procedures, the endoscopes
and other equipment underwent a high-level of disinfection.
VATS procedure
During the VATS procedure, 2 portals were created:
The endoscope portal (portal 1) and the surgical portal (portal 2).
Portal 1 (1 cm diameter) was created by a trocar inside a metal
tube, and was located between the eighth and ninth rib. Portal 2
(2–3 cm diameter) was created using a scalpel and endotherm knife,
and was located between the fifth and the sixth rib. A 10/11 mm
trocar-cannula unit (Hangzhou Optcla Medical Instrument Co., Ltd.,
Hangzhou, China) was inserted through the chest wall at portal 1.
An endoscope (0°, 10 mm diameter, 330 mm long; Olympus Corporation,
Tokyo, Japan) attached to a video endoscopic camera and light
source (Olympus Corporation) was then advanced through this tube
into the chest cavity. The margin of portal 2 was treated with an
electric coagulation knife for hemostasis.
Lung-grasping forceps were used to lift the lung
lobe and expose the hilum of the lung. The pulmonary artery,
pulmonary vein and bronchus were separated cautiously using
hemostatic forceps and peanut sponges (a homemade tool used for
dissociated soft tissue). The pulmonary artery and pulmonary veins
were clipped and cut off, and the bronchus was cut off following
double ligation. Bronchus ligation required an extra transfixion
ligation. All lung lobes in the left chest were excised in the
following order: Middle lobe, upper lobe and subjacent lobe. Warm
physiological saline (100–200 ml at ~40°C) was used to check the
bronchus ligation and syringe the thoracic cavity; this solution
was then extracted using a vacuum absorber.
Open procedure
A 7–8 cm incision was created using a scalpel and
electric coagulation knife between the seventh and eighth rib. An
endotherm knife was used for blood coagulation. The surgical room
was expanded through a wound spreader. The surgical procedure and
the order that lung lobes were excised was the same as for the VATS
group. Lung-grasping forceps were used to lift the lung lobe and
expose the hilum of the lung. The pulmonary artery, pulmonary vein
and bronchus were separated carefully using hemostatic forceps and
peanut sponges. The method and order of clipping and cutting off of
vessels was the same as for the VATS group. Warm physiological
saline was used to check the bronchus ligation and syringe the
thoracic cavity; this solution was then extracted using a vacuum
absorber.
Monitoring and postoperative care
Postoperative care consisted of cefazolin (20 mg/kg,
IM) twice daily for 7 days and topical erythromycin ointment
applied to the incision sites twice daily for 7 days. Tramadol (2
mg/kg) was administrated intravenously following surgery and once a
day for 3 days.
Water was offered when the animal was ambulatory and
moistened dog food was offered 6 h following surgery.
The following parameters were recorded: HR, RF,
rectal temperature, BP (preoperative, every 15 min
intraoperatively, postoperatively and on postoperative days 1, 2,
3, 5 and 7), operating time, incision size, postoperative
complications and time of standing up. Hematology examination
including white blood cells (WBC), red blood cells (RBC),
lymphocytes (LY) and granulocytes (Gran) was conducted
preoperatively, postoperatively and on days 1, 2, 3, 5, 7 and 14
following surgery.
Blood samples were collected (preoperatively,
postoperatively, 4 h, and 1, 3, 5, 7 and 14 days following surgery)
through the precaval vein and were centrifuged for 15 min at 1,000
× g at room temperature (20°C). Serum for APP analysis was
stored at −80°C and the concentration of APPs was assayed using
commercially available ELISA kits (BD Pharmingen, San Diego, CA,
USA) with specific monoclonal antibodies according to the
manufacturer's protocol. Antibodies against the following were
used: CRP (51–934DCRP1; 1:100; BD Pharmingen), SAA (F02520; 1:100;
Shanghai Westang Bio-Tech Co., Ltd., Shanghai, China) and YKL-40
(F03132; 1:100; Shanghai Westang Bio-Tech Co., Ltd.). Levels of
CRP, SAA and YKL-40 were measured preoperatively and
postoperatively on days 1, 2, 3, 5, 7 and 14.
Statistical analysis
Standard statistical methods were used to analyze
data. Data are presented as mean ± standard deviation. Statistical
differences within each group were determined by two-way analysis
of variance. The paired-sample t-test was used to compare the two
groups. P<0.05 was determined to represent statistically
significant differences. Statistical analyses were performed using
SPSS software (version 22; IBM SPSS, Armonk, NY, USA).
Results
Animals
All animals survived the follow-up period of 14
days. Total pneumonectomy via VATS and open surgery was
successfully performed, with complete removal of all left lobes in
14 animals. No difference was observed in the body weight of dogs
in either group (VATS 21.7±10.5 kg vs. open 20.4±3.8 kg;
P>0.05). The operating time for the VATS procedure was
significantly longer than for the open procedure (VATS 176.7±22.7
min vs. open 132.4±15.7 min, P<0.05).
None of the animals in either group had intra- or
peri-operative complications. There was no evidence of hemorrhage
and no areas of iatrogenic trauma from introduction of surgical
instruments. The total length of all skin incisions was 2.5–4 cm in
the VATS group and 5.5–8 cm in the open group. No other
abnormalities were identified in either group. All dogs were
considered to have returned to their normal activity levels 36 h
following surgery.
Physiological parameters
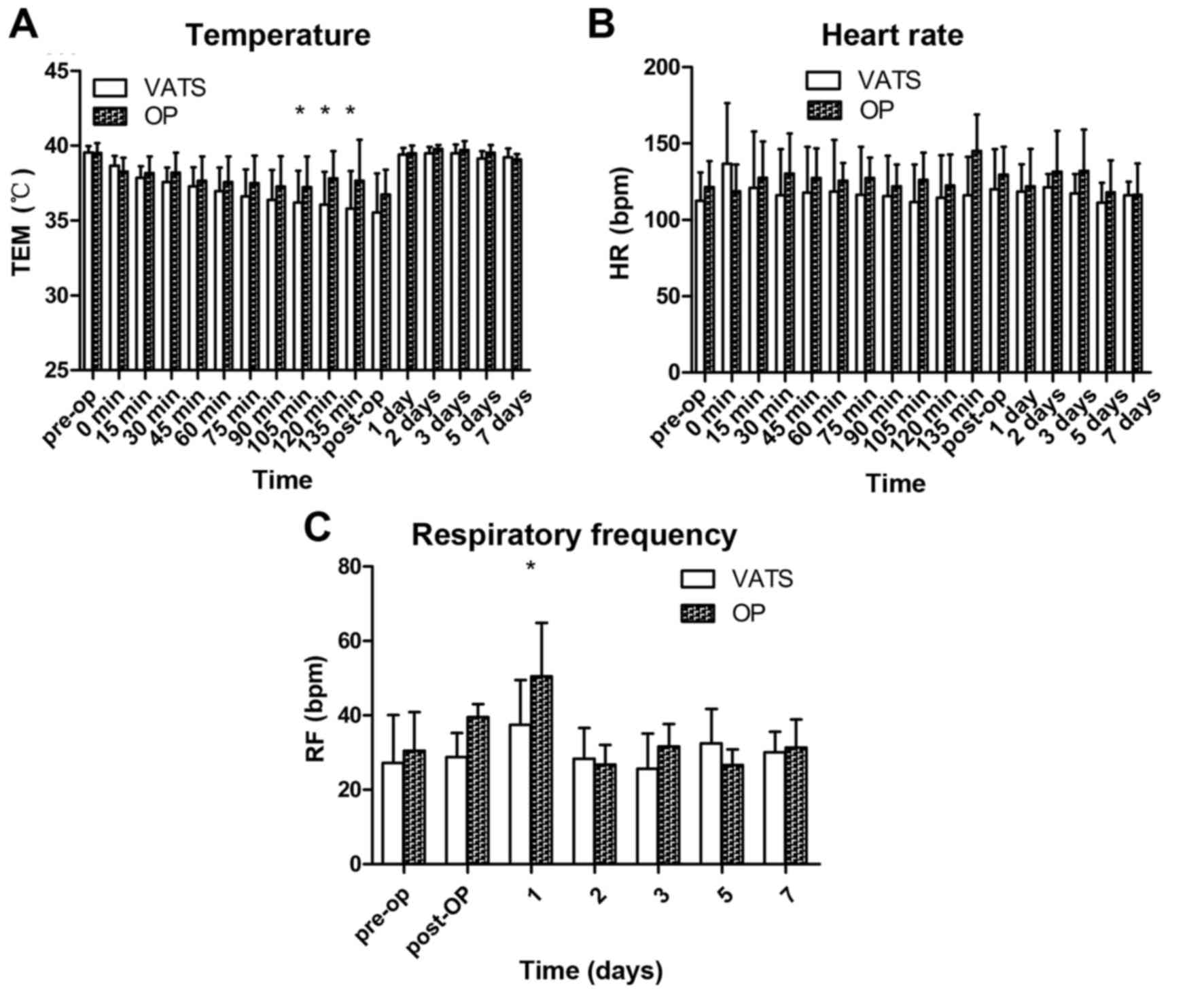
Rectal temperature was measured prior to surgery
(baseline) and at the designated intra- and post-operative time
points (Fig. 1A). Rectal temperature
in the VATS group 105, 120 and 135 min postoperation was
significantly lower than baseline (P<0.05). There was no
significant change in rectal temperature at any time point in the
open group. Three dogs in the VATS and four dogs in the open group
experienced hyperpyrexia day 1 and 2 postoperation, but returned to
baseline 5 days following surgery.
Comprehensive hemodynamic changes were recorded in
all 14 animals. There were no significant changes in HR at any time
point in either group (Fig. 1B).
Respiratory frequency at 1 day was significantly higher in the open
group than the baseline (P<0.05), and no significant changes
were observed among groups (Fig.
1C).
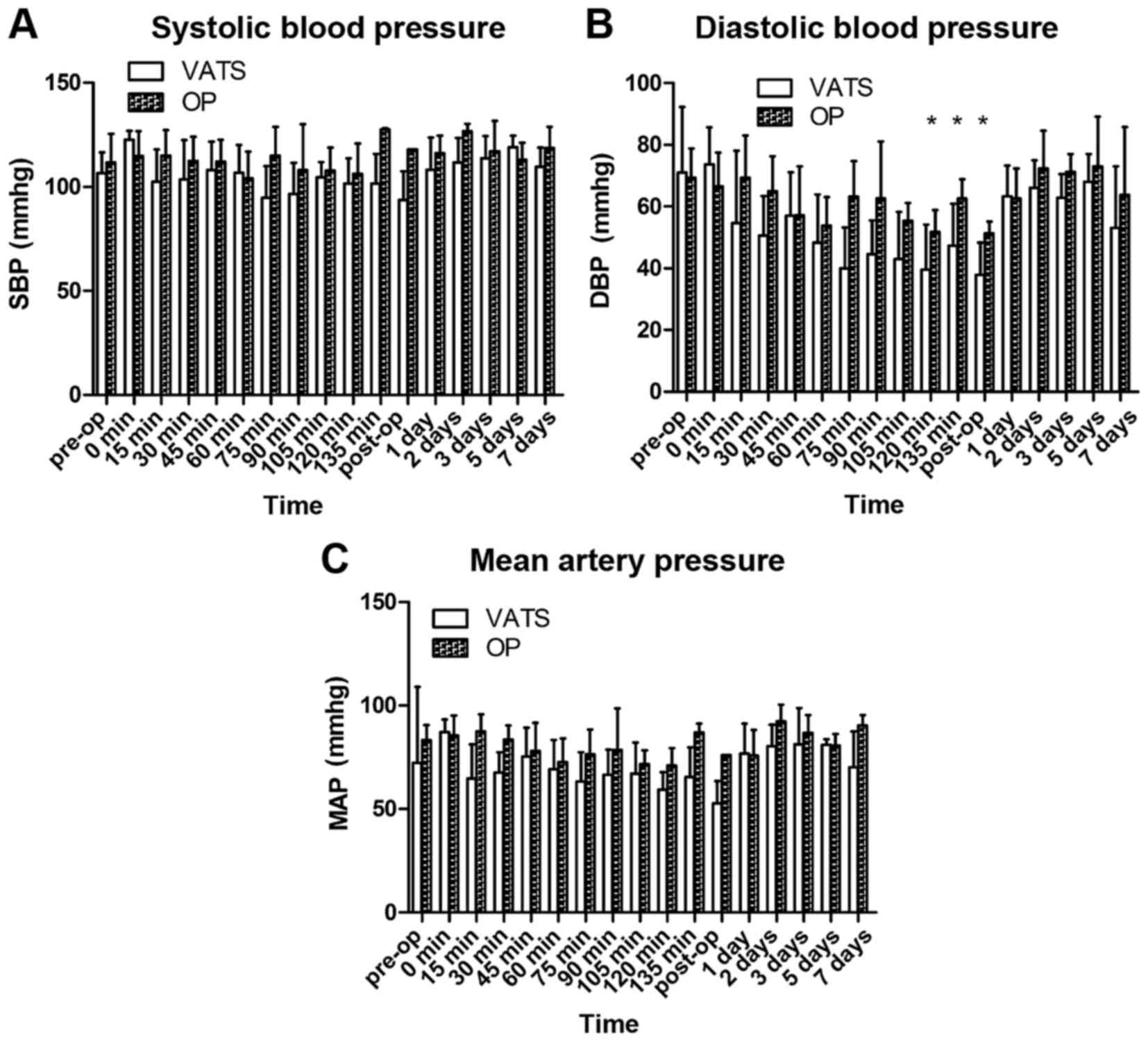
No significant change was observed in systolic BP at
any time point in either group (Fig.
2A). During the operative period, the diastolic BP and mean
arterial pressure demonstrated a declining trend in both groups,
and increased gradually back to baseline levels postoperatively
(Fig. 2B and C, respectively).
Diastolic BP at 120 min postoperation was significantly decreased
in the VATS group compared with baseline (Fig. 2B; P<0.05).
Hematology
The WBC count in both groups was significantly
increased 1, 2 and 3 days following surgery (P<0.05), and
returned to a normal level 2 weeks later (Fig. 3A). Furthermore, it was significantly
higher 1 day after surgery in the open group, compared with the
VATS group (P<0.05). There was no significant change in the
level of RBCs prior to or following the procedure in both groups
(Fig. 3B). The LY count demonstrated
an increased trend postoperatively (Fig.
3C; P>0.05) and the Gran count was significantly increased 1
day following surgery (Fig. 3D;
P<0.05).
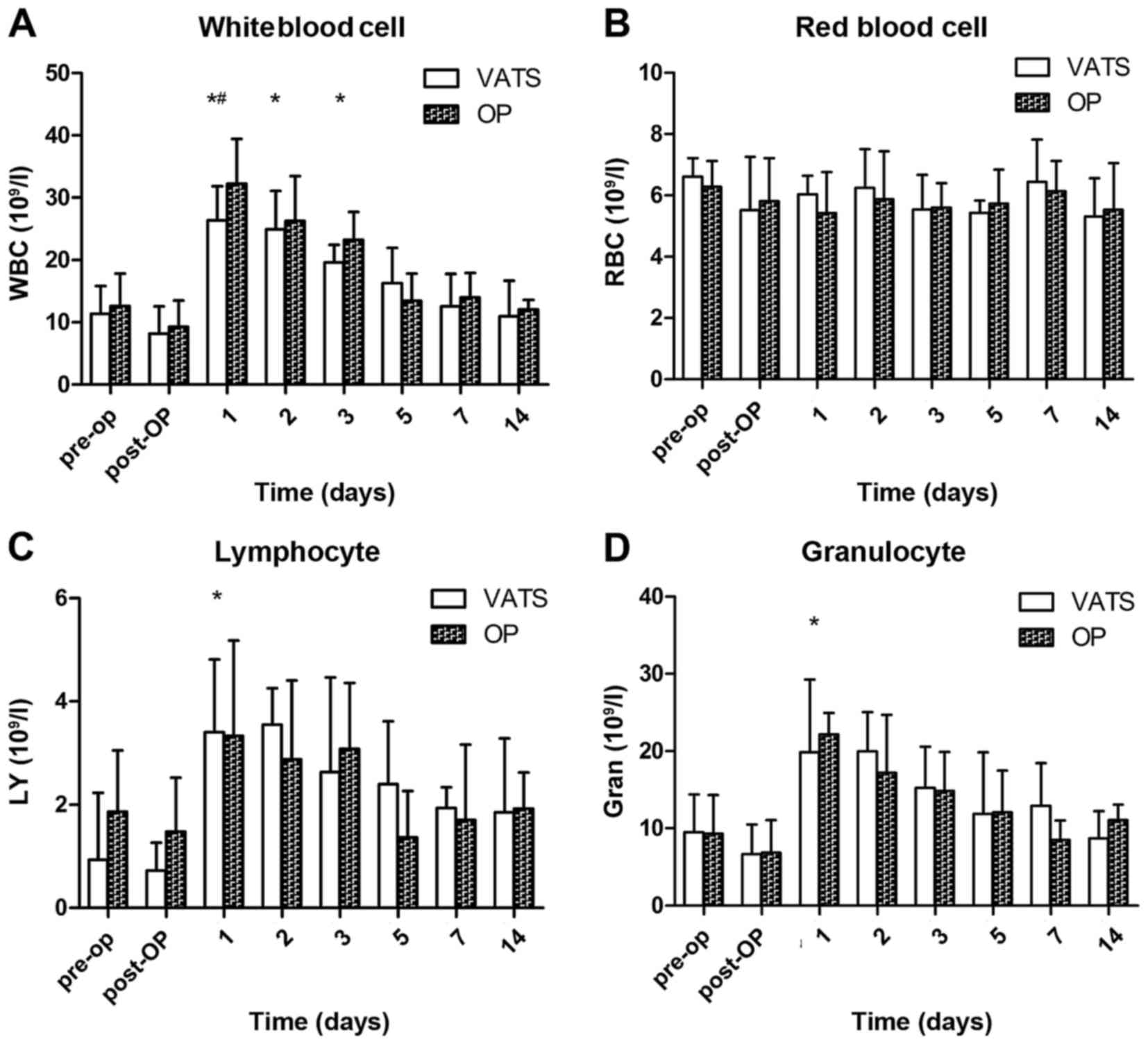 | Figure 3.Hematology assessments in all
animals. Changes in (A) WBCs, (B) RBCs, (C) LYs and (D) Grans in 14
dogs that underwent pneumonectomy (n=7 for VATS and n=7 for open
thoracotomy). *P<0.05 vs. preoperative value (baseline) and
#P<0.05 for VATS vs. transthoracic approach at the
same time point. WBC, white blood cells; RBD, red blood cells; LY,
lymphocytes; Gran, granulocyes; OP, open surgery; VATS,
video-assisted thoracoscopic surgery; pre-op, preoperation;
post-op, postoperation. |
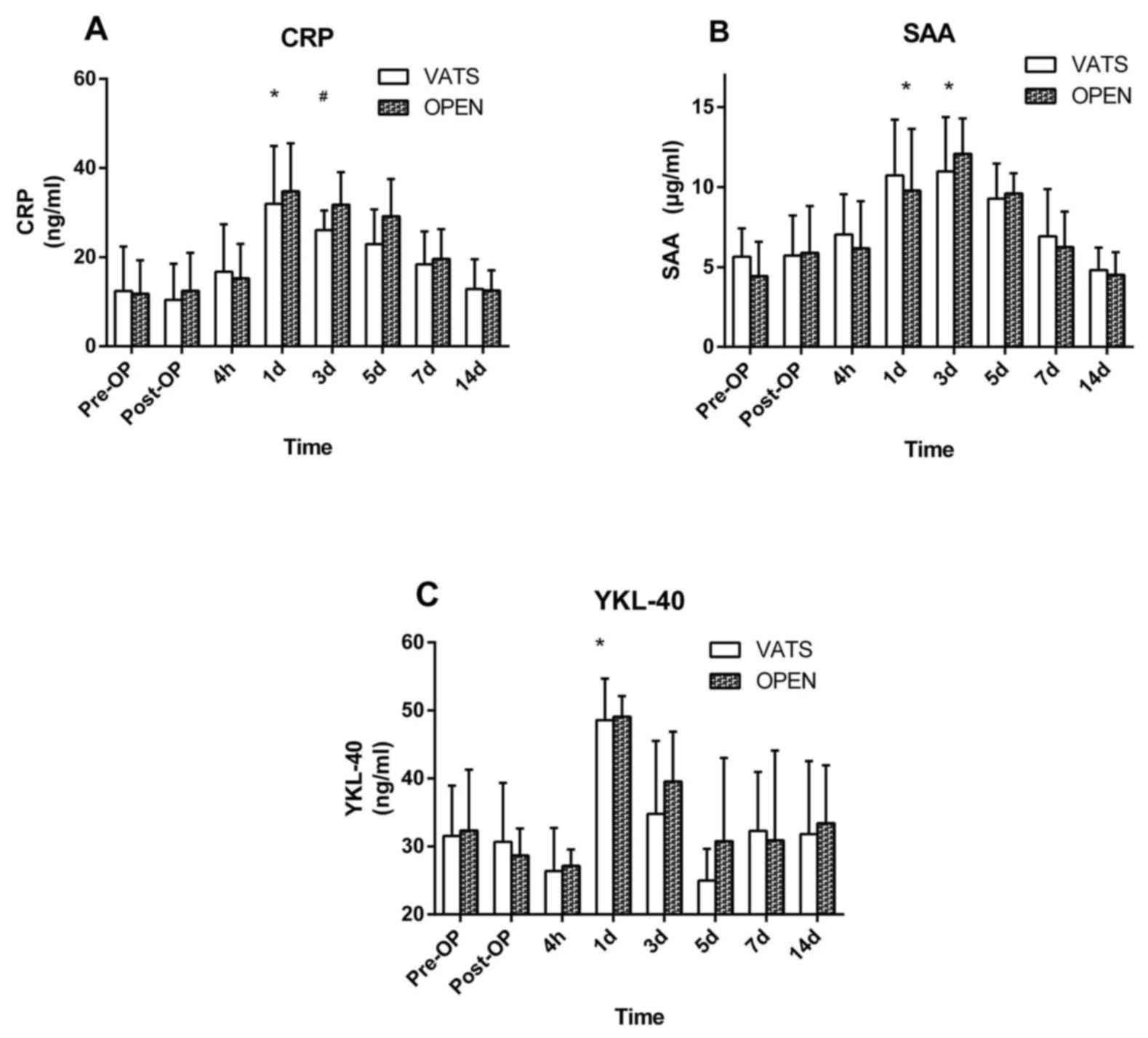
Acute-phase proteins
Serum concentrations of CRP and SAA in both groups
were significantly increased 1 day after surgery compared with
baseline (P<0.05; Fig. 4A and B).
These decreased gradually back to baseline by day 14 post-surgery
(Fig. 4A and B). The serum
concentration of CRP was significantly higher on day 3 following
surgery in the open group, compared with the VATS group (P<0.05;
Fig. 4A). In both groups, the serum
concentration of YKL-40 was not significantly decreased at 4 h
following surgery but significantly increased day 1 postoperation
compared with baseline (P<0.05; Fig.
4C) and returned to normal level day 5 postoperation. No
significant difference in SAA and YKL-40 was observed between the
two groups at any time point (Fig. 4B
and C).
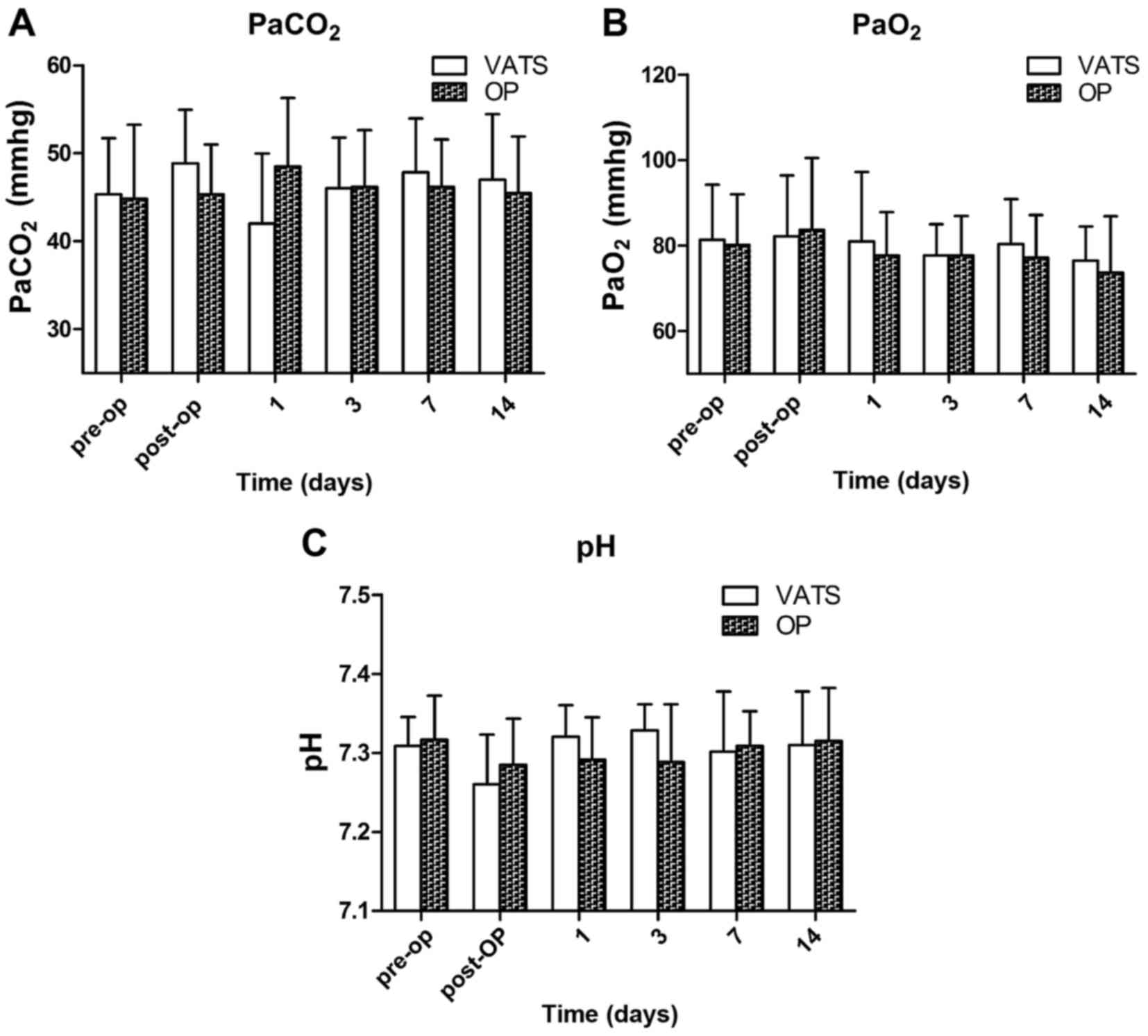
Arterial blood gas
Arterial blood gas analysis was conducted in the
animals (Fig. 5). There was no
significant change in the partial pressure of oxygen
(PaO2) before and after the procedure in either group
(Fig. 5B). The pH in both groups
decreased immediately following the procedure, and returned to
preoperative levels day 1 postoperation (P>0.05; Fig. 5C). The partial pressure of carbon
dioxide (PaCO2) was elevated immediately following the
procedure in both groups; PaCO2 returned to preoperative
level day 1 postoperation in the VATS group and day 3 postoperation
in the open group (P>0.05; Fig.
5A). There was no significant difference between the two groups
in the values of pH, PaO2, and PaCO2 at any
time point (Fig. 5).
Discussion
Lung lobes may be excised through either open
thoracotomy or VATS. In the present study, pneumonectomy in the
hemithorax was performed successfully using both of these
approaches without major intra- or post-operative complications.
Thus, both VATS and the transthoracic approach are viable
alternative techniques to total pneumonectomy. The current study
also performed thoracic exploration, surgical lung biopsy and
pericardial window creation successfully with the VATS approach in
dogs.
Pneumonectomy is a widely performed surgical
technology that is used to cure pathological conditions including
lung tumors, congenital lung anomalies, chronic lung collapse,
chronic progressive lung inflammation, post-traumatic diffuse
parenchymal laceration and bronchial rupture. VATS is considered to
be the most significant progress made in thoracic surgery at the
end of the 20th century. Since the first pioneering lobectomy
completed using VATS (14,15), the techniques and reliability of such
surgeries have considerably improved (16–19).
VATS is now well established as an alternative treatment to open
thoracotomy for major resections of lung cancer and benign disease.
VATS has the advantage of providing an improved visual field and
cosmesis. Previous studies have verified that VATS has other
advantages, such as a lower concentration of inflammatory cytokines
(20), a lower risk of developing
chest infection, reduced pain and improved lung function (21,22). An
increasing body of evidence has indicated that patients undergoing
VATS experience a shorter hospital stay, have their chest tubes
removed earlier (23,24) and have an improved prognosis
(25). Aujesky et al
(26) observed good outcomes in
patients who underwent video-assisted laparoscopic resection of the
esophagus for carcinoma following neoadjuvant therapy.
Body temperature decreased during all surgeries in
the current study. A decrease in rectal temperature was observed
postoperatively in both groups; although heating blankets may help,
intraoperative hypothermia is inevitable. A lower rectal
temperature was observed postoperatively in the VATS group compared
with the open group, this is potentially due to the operating time
in the VATS group being much longer.
WBC, Gran and LY are factors that influence
inflammation and stress. The serum level of these factors was
raised on day 1 following surgery; this may be due to the severe
trauma caused by surgery and anesthesia. However, the inflammatory
reaction reflected through WBC difference on day 1 suggested that
the thoracotomy caused more severe body stress, as the longer
incision increases the likelihood of infection. Considering this
with the change in APPs, no influence on the dogs owing to a longer
operating time or a bigger incision was observed; however, suturing
the incision took longer in the open surgery.
APPs may cause rapid response to infections, burns,
invasive surgery, inflammation and tissue injury stress. Levels of
the APPs CRP, SAA, and YKL-40 increase as a result of the
inflammatory response to infection or tissue damage, and have been
used to evaluate surgery technique, infection and pathology
progress. CRP and SAA levels increase in a number of pathological
states, including surgical trauma (27–30),
esophageal neoplasia, infection with H3N2 swine influenza virus,
alcoholic liver and systemic inflammation (11,31–34). In
the present study, levels of all APPs increased significantly on
day 1 postoperatively compared with baseline (preoperative levels)
and decreased over the subsequent time point measurements. This
change suggested that the surgery resulted in tissue trauma and
inflammation. Although animals in the thoracotomy group exhibited
more injury and pain postoperatively, VATS required more surgical
manipulation and therefore, resulted in a longer time under
anesthesia. This may explain the result observed in the present
study; that the serum concentration of CRP in the VATS group was
significantly higher compared with the open group on day 3
postoperation, and that no differences were observed between the
two groups at other time points.
YKL-40 is not only an APP, but also a biomarker
closely associated with respiratory function. Elevated plasma
YKL-40 is a prognostic indicator in patients with idiopathic
pulmonary arterial hypertension (35), predicts bronchiolitis obliterans
development in lung transplant recipients (36), predicts poor prognosis in
hepatocellular carcinoma (37) and
is elevated in patients with chronic obstructive pulmonary disease
and activated alveolar macrophages (38). Therefore, although YKL-40 is not as
sensitive as CRP and SAA in detecting surgical trauma and
inflammation, the changing concentration of YKL-40 reflects the
condition of the respiratory system. In the present study, the
change in YKL-40 was similar to the changes in CRP and SAA as a
result of body stress and inflammation; no difference was observed
between the two groups, indicating that VATS did not have a greater
effect on the respiratory system than open thoracotomy.
Thoracic cavity surgery causes pulmonary tissue
damage and a decrease in respiratory function, which is reflected
by the change in blood gases. There was a slight change in blood
gases in all animals in the current study as a result of mechanical
ventilation. Significant changes in blood gases during laparoscopic
surgery have been reported owing to pneumoperitoneum; therefore,
use of the endoscope in the thoracic cavity in the current study
did not cause the complication of pneumoperitoneum.
Standard pleural drainage was not used in the
present study. Leakage or pneumothorax was not observed in any
dogs. Chest drain use remains controversial, as thoracotomy
incisions may damage the intercostal nerves and lead to chronic
neuropathy (39). Satherley et
al (40) reported that the use
of an intercostal chest drain following lung biopsy increased the
period of hospitalization and Nakashima et al (41) reported that postoperative morbidity
did not increase following thoracoscopic lung wedge resection
without a chest tube. Luckraz et al (42) demonstrated that there was no
requirement for an intercostal chest drain in patients that had
received VATS lung resection if no air leakage was noted during
surgery. Intraoperative blood loss was not recorded in the current
study, as there was very minimal intraoperative bleeding in both
groups; however, the blood loss observed in the VATS group was
subjectively less than in the open group. The reason for this may
be the improved visual field and accuracy of surgery due to the
magnified image provided by the endoscope in VATS.
A number of previous studies have demonstrated the
feasibility of the natural orifice transluminal endoscopic surgery
(NOTES) approach (transgastric, transumbilical, transoral,
transvaginal) applied in thoracic surgery and indicate that NOTES
caused less stress than the open approach (43–46). The
NOTES approach reportedly resulted in improved cosmetic outcomes
compared with VATS, laparoscopic surgery and open surgery (47). However, a number of NOTES approaches
in the abdominal cavity (including gastric, uterine, and umbilical)
damaged the diaphragm and the surgical path was affected by the
liver (48–51). Further investigations are required to
compare the difference between NOTES and VATS in thoracic
surgery.
In conclusion, no significant difference between
VATS and open pneumonectomy was observed in dogs following an
analysis in the change to APPs and hemodynamics. Animals in the
VATS group experienced a longer surgical time and a smaller
incision scar. No complication was observed in either group.
Therefore, VATS is a feasible and safe approach for pneumonectomy
in dogs.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant nos. 31272617 and
31472245).
References
|
1
|
Liu L, Zhang LJ, Chen B, Cao JM, Lu GM,
Yuan L, Li K and Xu J: Novel CT-guided coil localization of
peripheral pulmonary nodules prior to video-assisted thoracoscopic
surgery: A pilot study. Acta Radiol. 55:699–706. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Panagopoulos N, Papavasileiou G, Koletsis
E, Kastanaki M and Anastasiou N: VATS bullectomy and apical
pleurectomy for spontaneous pneumothorax in a young patient with
Swyer-James-Mc Leod syndrome: Case report presentation and
literature review focusing on surgically treated cases. J
Cardiothorac Surg. 9:132014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Liu CY, Chu Y, Wu YC, Yuan HC, Ko PJ, Liu
YH and Liu HP: Transoral endoscopic surgery versus conventional
thoracoscopic surgery for thoracic intervention: Safety and
efficacy in a canine survival model. Surg Endosc. 27:2428–2435.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jackson J, Richter KP and Launer DP:
Thoracoscopic partial pericardiectomy in 13 dogs. J Vet Intern Med.
13:529–533. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fujimoto T, Zaboura G, Fechner S, Hillejan
L, Schröder T, Marra A, Krbek T, Hinterthaner M, Greschuchna D and
Stamatis G: Completion pneumonectomy: Current indications,
complications and results. J Thorac Cardiovasc Surg. 121:484–490.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mcgovern EM, Trastek VF, Pairolero PC and
Payne WS: Completion pneumonectomy: Indications, complications, and
results. Ann Thoracic Surg. 46:141–146. 1988. View Article : Google Scholar
|
|
7
|
Gonzalez-Zamora JF, Perez-Guille B,
Soriano-Rosales RE, Jimenez-Bravo-Luna MA, Gutierrez-Castrellon P,
Ridaura-Sanz C and Alvarez FV: Video-assisted thoracoscopy for
diaphragmatic plication: Experimental study in a canine model. J
Laparoendosc Adv Surg Tech A. 15:661–666. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Case JB, Mayhew PD and Singh A: Evaluation
of video-assisted thoracic surgery for treatment of spontaneous
pneumothorax and pulmonary bullae in dogs. Vet Surg. 44 Suppl
1:S31–S38. 2015. View Article : Google Scholar
|
|
9
|
Bodner J: Video-assisted thoracoscopic
(VATS) sublobar anatomic resections for lung cancer. Zentralbl
Chirur. 139:102–107. 2014.(In German).
|
|
10
|
Lyscov A, Obukhova T, Ryabova V,
Sekhniaidze D, Zuiev V and Gonzalez-Rivas D: Double-sleeve and
carinal resections using the uniportal VATS technique: A single
centre experience. J Thorac Dis. 8 Suppl 3:S235–S241.
2016.PubMed/NCBI
|
|
11
|
Nivy R, Caldin M, Lavy E, Shaabon K, Segev
G and Aroch I: Serum acute phase protein concentrations in dogs
with spirocercosis and their association with esophageal
neoplasia-a prospective cohort study. Vet Parasitol. 203:153–159.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
The Animal Welfare Acts: US PL 89–544.
91–579, 94-279; and PL 99–198.
|
|
13
|
American Veterinary Medical Association
Guidelines for the Euthanasia of Animals: 2013 Edition. American
Veterinary Medical Association; Schaumburg, IL, USA:
|
|
14
|
Lewis RJ, Sisler GE and Caccavale RJ:
Imaged thoracic lobectomy: Should it be done? Ann Thorac Surg.
54:80–83. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kirby TJ, Mack MJ, Landreneau RJ and Rice
TW: Initial experience with video-assisted thoracoscopic lobectomy.
Ann Thorac Surg. 56:1248–1253. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tsang FH, Chung SS and Sihoe AD:
Video-assisted thoracic surgery for bronchopulmonary sequestration.
Interact Cardiovasc Thorac Surg. 5:424–426. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Shiraishi T, Shirakusa T, Miyoshi T,
Hiratsuka M, Yamamoto S and Iwasaki A: A completely thoracoscopic
lobectomy/segmentectomy for primary lung cancer-technique,
feasibility, and advantages. Thorac Cardiovasc Surg. 54:202–207.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Oda M, Ishikawa N, Tsunezuka Y, Matsumoto
I, Tamura M, Kawakami K and Watanabe G: Closed three-port anatomic
lobectomy with systematic nodal dissection for lung cancer. Surg
Endosc. 21:1464–1465. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tajiri M, Maehara T, Nakayama H and
Sakamoto K: Decreased invasiveness via two methods of thoracoscopic
lobectomy for lung cancer, compared with open thoracotomy.
Respirology. 12:207–211. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yim AP, Wan S, Lee TW and Arifi AA: VATS
lobectomy reduces cytokine responses compared with conventional
surgery. Ann Thorac Surg. 70:243–247. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Phan K and Yan TD: VATS segmentectomy for
pulmonary metastasis. Ann Cardiothorac Surg. 3:192–193.
2014.PubMed/NCBI
|
|
22
|
Cao C, Manganas C, Ang SC, Peeceeyen S and
Yan TD: Video-assisted thoracic surgery versus open thoracotomy for
non-small cell lung cancer: A meta-analysis of propensity
score-matched patients. Interact Cardiovasc Thorac Surg.
16:244–249. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Scott WJ, Matteotti RS, Egleston BL, Oseni
S and Flaherty JF: A comparison of perioperative outcomes of
video-assisted thoracic surgical (VATS) lobectomy with open
thoracotomy and lobectomy: Results of an analysis using propensity
score based weighting. Ann Surg Innov Res. 4:12010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yang X, Wang S and Qu J: Video-assisted
thoracic surgery (VATS) compares favorably with thoracotomy for the
treatment of lung cancer: A five-year outcome comparison. World J
Surg. 33:1857–1861. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sawada S, Komori E, Yamashita M, Nakata M,
Nishimura R, Teramoto N, Segawa Y and Shinkai T: Comparison in
prognosis after VATS lobectomy and open lobectomy for stage I lung
cancer: Retrospective analysis focused on a histological subgroup.
Surg Endosc. 21:1607–1611. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Aujesky R, Neoral C, Kral V, Bohanes T,
Vrba R and Vomackova K: Video-assisted laparoscopic resection of
the esophagus for carcinoma after neoadjuvant therapy.
Hepatogastroenterology. 56:1035–1038. 2009.PubMed/NCBI
|
|
27
|
Chung YG, Won YS, Kwon YJ, Shin HC, Choi
CS and Yeom JS: Comparison of serum CRP and procalcitonin in
patients after spine surgery. J Korean Neurosurg Soc. 49:43–48.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Planellas M, Cuenca R, Tabar MD, Bertolani
C, Poncet C, Closa JM, Lorente J, Cerón JJ and Pastor J: Clinical
assessment and C-reactive protein (CRP), haptoglobin (Hp) and
cardiac troponin I (cTnI) values of brachycephalic dogs with upper
airway obstruction before and after surgery. Can J Vet Res.
79:58–63. 2015.PubMed/NCBI
|
|
29
|
Syeda T, Hashim AS, Rizvi HA and Hadi SM:
Pre- and post-operative values of serum CRP in patients undergoing
surgery for brain tumour. J Pak Med Assoc. 64:271–274.
2014.PubMed/NCBI
|
|
30
|
Neumaier M, Metak G and Scherer MA:
C-reactive protein as a parameter of surgical trauma: CRP response
after different types of surgery in 349 hip fractures. Acta Orthop.
77:788–790. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kim SR, Kondo F, Otono Y, Imoto S, Ando K,
Hirakawa M, Fukuda K, Sasaki M, Kim SK, Komaki T, et al: Serum
amyloid A and C-reactive protein positive nodule in alcoholic liver
cirrhosis, hard to make definite diagnosis. Hepatol Res.
44:584–590. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Pomorska-Mól M, Kwit K, Pejsak Z and
Markowska-Daniel I: Analysis of the acute-phase protein response in
pigs to clinical and subclinical infection with H3N2 swine
influenza virus. Influenza Other Respir Viruses. 8:228–234. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Christensen MB, Langhorn R, Goddard A,
Andreasen EB, Moldal E, Tvarijonaviciute A, Kirpensteijn J,
Jakobsen S, Persson F and Kjelgaard-Hansen M: Comparison of serum
amyloid A and C-reactive protein as diagnostic markers of systemic
inflammation in dogs. Can Vet J. 55:161–168. 2014.PubMed/NCBI
|
|
34
|
Jitpean S, Holst BS, Höglund OV,
Pettersson A, Olsson U, Strage E, Södersten F and Hagman R: Serum
insulin-like growth factor-I, iron, C-reactive protein, serum
amyloid a for prediction of outcome in dogs with pyometra.
Theriogenology. 82:43–48. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Chen G, Yang T, Gu Q, Ni XH, Zhao ZH, Ye
J, Meng XM, Liu ZH, He JG and Xiong CM: Elevated plasma YKL-40 as a
prognostic indicator in patients with idiopathic pulmonary arterial
hypertension. Respirology. 19:608–615. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Jaksch P, Taghavi S, Klepetko W and Salama
M: Pretransplant serum human chitinase-like glycoprotein YKL-40
concentrations independently predict bronchiolitis obliterans
development in lung transplant recipients. J Thorac Cardiovasc
Surg. 148:273–281. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhu CB, Chen LL, Tian JJ, Su L, Wang C,
Gai ZT, Du WJ and Ma GL: Elevated serum YKL-40 level predicts poor
prognosis in hepatocellular carcinoma after surgery. Ann Surg
Oncol. 19:817–825. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Létuvé S, Kozhich A, Arouche N,
Grandsaigne M, Reed J, Dombret MC, Kiener PA, Aubier M, Coyle AJ
and Pretolani M: YKL-40 is elevated in patients with chronic
obstructive pulmonary disease and activates alveolar macrophages. J
Immunol. 181:5167–5173. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Lin TY, Chu Y, Wu YC, Liu CY, Yeh CJ,
Hsieh MJ, Yuan HC, Ko PJ, Liu YH and Liu HP: Feasibility of
transumbilical lung wedge resection in a canine model. J
Laparoendosc Adv Surg Tech A. 23:684–692. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Satherley LK, Luckraz H, Rammohan KS,
Phillips M, Kulatilake NE and O'Keefe PA: Routine placement of an
intercostal chest drain during video-assisted thoracoscopic
surgical lung biopsy unnecessarily prolongs in-hospital length of
stay in selected patients. Eur J Cardiothorac Surg. 36:737–740.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Nakashima S, Watanabe A, Mishina T, Obama
T, Mawatari T and Higami T: Feasibility and safety of postoperative
management without chest tube placement after thoracoscopic wedge
resection of the lung. Surg Today. 41:774–779. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Luckraz H, Rammohan KS, Phillips M, Abel
R, Karthikeyan S, Kulatilake NE and O'Keefe PA: Is an intercostal
chest drain necessary after video-assisted thoracoscopic (VATS)
lung biopsy? Ann Thorac Surg. 84:237–239. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Boylu U, Oommen M, Joshi V, Thomas R and
Lee BR: Natural orifice translumenal endoscopic surgery (NOTES)
partial nephrectomy in a porcine model. Surg Endosc. 24:485–489.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Zhu LH, Chen L, Yang S, Liu D, Zhang J,
Cheng X and Chen W: Embryonic NOTES thoracic sympathectomy for
palmar hyperhidrosis: Results of a novel technique and comparison
with the conventional VATS procedure. Surg Endosc. 27:4124–4129.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Nemani A, Sankaranarayanan G, Olasky JS,
Adra S, Roberts KE, Panait L, Schwaitzberg SD, Jones DB and De S: A
comparison of NOTES transvaginal and laparoscopic cholecystectomy
procedures based upon task analysis. Surg Endosc. 28:2443–2451.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Voermans RP, Sheppard B, van Berge
Henegouwen MI, Fockens P and Faigel DO: Comparison of Transgastric
NOTES and laparoscopic peritoneoscopy for detection of peritoneal
metastases. Ann Surg. 250:255–259. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Freeman LJ, Rahmani EY, Al-Haddad M,
Sherman S, Chiorean MV, Selzer DJ, Snyder PW and Constable PD:
Comparison of pain and postoperative stress in dogs undergoing
natural orifice transluminal endoscopic surgery, laparoscopic, and
open oophorectomy. Gastrointest Endosc. 72:373–380. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Wen CT, Chu Y, Yeh CJ, Liu CY, Yuan HC, Ko
PJ, Liu YH and Liu HP: Feasibility and safety of endoscopic
transumbilical thoracic surgical lung biopsy: A survival study in a
canine model. J Surg Res. 183:47–55. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
de Palma GD, Siciliano S, Addeo P,
Salvatori F, Persico M, Masone S, Rega M, Maione F, Bottazzi E
Coppola, Serrao E, et al: A NOTES approach for thoracic surgery:
Transgastric thoracoscopy via a diaphragmatic incision in a
survival porcine model. Minerva Chir. 65:11–15. 2010.PubMed/NCBI
|
|
50
|
Chu Y, Liu CY, Wu YC, Hsieh MJ, Chen TP,
Chao YK, Wu CY, Yuan HC, Ko PJ, Liu YH and Liu HP: Comparison of
hemodynamic and inflammatory changes between transoral and
transthoracic thoracoscopic surgery. PLoS One. 8:e503382013.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Voermans RP, Faigel DO, van Berge
Henegouwen MI, Sheppard B and Fockens P: Comparison of transcolonic
NOTES and laparoscopic peritoneoscopy for the detection of
peritoneal metastases. Endoscopy. 42:904–909. 2010. View Article : Google Scholar : PubMed/NCBI
|