Introduction
The metastasis of tumors into bone tissue is a
common clinical event and typically leads to intractable pain and
bone destruction (1). A number of
types of cancer have a predisposition to metastasize to bone and
induce bone destruction. This most commonly occurs in patients with
breast, lung and prostate cancer, in which a metastatic rate of
~80% is observed (2). Astrocytes and
microglia in the spinal cord serve crucial roles in the production
and maintenance of pain. Upon activation, glial cells release a
range of neuroexcitatory substances including pro-inflammatory
cytokines (3) and D-serine (4) that potentiate the transmission of pain
by neurons. Pro-inflammatory cytokines are typically produced in a
cascade and have been demonstrated to participate in the
development of allodynia and hyperalgesia in rat and mouse animal
models of pain (5). Tumor necrosis
factor (TNF) is considered to be a key pro-inflammatory cytokine
during pain nociception, due to its early generation and
stimulation of target cells to produce numerous other cytokines
within a complex cascade, including interleukin (IL)-1β and IL-6
(6).
Within bone tumors, TNF-α has been identified as a
key factor in the development and maintenance of cancer-related
pain (7). In turn, the TNF-α
antagonist etanercept has demonstrated efficacy in reducing pain
and osteoclast-mediated osteolysis in a clinical research and
murine model of bone cancer pain (8–10). The
results of this clinical research involving two patients one with a
diagnosis of non-small cell lung cancer and the other with a
diagnosis of breast cancer metastases to bone, that had
treatment-refractory pain due to cancer, has suggested that
targeted administration of etanercept in anatomical proximity to a
site of bone metastasis may provide rapid, substantial and
prolonged pain relief (8). However,
it remains unknown whether TNF-α in the spinal cord is involved in
sensitization of the central nervous system to bone cancer pain.
Therefore, the current study used a bone cancer model established
in mice to determine if spinal cord TNF-α contributes to bone
cancer-related pain, by evaluating levels of TNF-α mRNA in the
spinal cord and pain-related behaviors. Furthermore, the efficacy
of etanercept in attenuating bone cancer pain was evaluated.
Materials and methods
Cell culture
The osteosarcoma cell line NCTC 2472 [American Type
Culture Collection (ATCC), Manassas, VA, USA, lot no. 2087787] was
used in the current experiments. Cells were incubated in NCTC-135
medium (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) supplemented
with 10% horse serum (Gibco; Thermo Fisher Scientific, Waltham, MA,
USA) at 37°C in 5% CO2, according to ATCC
recommendations.
Animal treatment
Animal experiments were performed according to the
National Institute of Health Guide for the Care and Use of
Laboratory Animals (NIH Publications no. 80-23, revised 1996) and
were approved by the Ethics Review Board for Animal Studies of
Nanjing University (Nanjing, China). The number of animals used and
their suffering were minimized. A total of 150 male C3H/HeJ mice
(Model Animal Research Center of Nanjing University), 4–6 weeks old
and weighing 20–25 g were used. Mice were housed in a
temperature-controlled (21±1°C) room under a 12/12 h light/dark
cycle with food pellets and water provided ad libitum. The
mice were divided into three groups (n=8 in each group): i) NCTC
2472 cell-treated; ii) NCTC 2472 cell + etanercept-treated; and
iii) a control group.
In accordance with a previous method by Schwei et
al (9), 1% pentobarbital sodium
(50 mg/kg, Sigma-Aldrich, Merck KGaA) in normal saline was
intraperitoneally injected into mice. A minimal skin incision was
subsequently made on the right hind leg and the patellar ligaments
were cut to expose the condyles of the distal femur. A 23-gauge
needle (Jiangsu Zhengkang Medical Equipment, Changzhou, China) was
used to perforate the cortex at the level of the intercondylar
notch, then ~2.5×105 NCTC 2472 cells in 20 µl α-Minimal
Essential Medium (Thermo Fisher Scientific, Inc.) was injected
unilaterally into the intramedullary cavity of the femur with a
syringe. The injection site was sealed with Paladur dental acrylic
(Heraeus Kulzer GmbH, Hanau, Germany) to prevent leakage of cells
from the bone. The femurs of control mice were inoculated with 20
µl α-MEM alone. All mice were housed in the same room and
conditions to minimize stress associated with novel environmental
cues. For the behavior experiments, etanercept (100 µg in 0.5 ml
saline, Amgen, Inc., Thousand Oaks, CA, USA) was intraperitoneally
injected into mice prior to femur inoculation, and on days 3 and 6
thereafter.
Pain-related behaviors
Mice were given 30 min to acclimatize prior to each
test. Spontaneous foot lifting (SFL), paw withdrawal mechanical
threshold (PWMT) and paw withdrawal thermal latency (PWTL) were
measured prior to femur inoculation and on days 3, 7, 10 and 14
thereafter. All tests were performed during the light phase of the
mice light/dark cycle.
SFL
Mice were individually placed in a clear plastic
chamber (60×30×15 cm) and the number of mice that accompanied
lifting of their ipsilateral hind foot with aversive behavior
(e.g., foot shaking/licking) was measured over 5 min. Foot lifting
associated with exploratory behavior, locomotion and body
repositioning was excluded.
PWMT
Mechanical hyperalgesia was assessed by applying von
Frey filaments (Stoelting, Co., Wood Dale, IL, USA) to the right
hind paw, as described by Chaplan et al (10). Mice were placed in individual
transparent plexiglass compartments (10×10×15 cm) on a metal mesh
floor (graticule: 0.5×0.5 cm) and mechanical threshold was measured
using a set of von Frey filaments (0.16, 0.4, 0.6, 1.0, 1.4 and 2.0
g). Filaments were poked vertically into the plantar surface of the
right hind paw with sufficient force to cause slight bending of the
filament, then held for 6–8 sec. Brisk withdrawal or paw flinching
were considered to be positive responses. PWMT was determined by
using sequentially larger and smaller filaments, corresponding to
an increase and decrease in stimulus force, respectively (the
‘up-and-down’ method). Each mouse was tested 5 times per stimulus
strength with a 10 min interval between consecutive stimuli. The
minimum von Frey filament that evoked ≥3 positive responses was
regarded as the PWMT.
PWTL
Thermal hyperalgesia was assessed by measuring PWTL
to radiant heat, according to a protocol by Hargreaves et al
(11). Mice were placed on a 3
mm-thick glass floor in individual transparent plexiglass
compartments (10×10×5 cm). A BME410A radiant thermal stimulator
(Institute of Biological Medicine, Academy of Medical Science,
China) was focused onto the plantar surface of the right hind paw
through the glass floor. Characteristic lifting or licking of the
right hind paw were considered to be the nociceptive endpoints of
the test and the time taken to reach the endpoint was defined as
the PWTL. A 20 sec cut-off time was used to avoid tissue damage.
Each mouse was tested 5 times with a 5 min interval between
consecutive stimuli.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
On days 7, 10 and 14 after establishment of the bone
cancer model, mice were decapitated and the L3-L5 lumbar spinal
cord segments were removed immediately, frozen in liquid nitrogen
and stored at −80°C until use. Total RNA was isolated and purified
using an RNeasy Mini kit (Qiagen GmbH, Hilden, Germany), according
to the manufacturer's protocol. The concentration of RNA was
adjusted to ~1 µg/µl prior to use. RT was performed at 37°C for 15
min, 85°C for 5 sec, and 4°C for 7 sec using a PrimeScript RT
reagent kit (Takara Bio, Inc., Otsu, Japan). A total of 2 µl cDNA
in a 20 µl reaction system and a Power SYBR-Green Master mix
(Thermo Fisher Scientific, Inc.) was used for PCR in a StepOnePlus
system (Applied Biosystems, Thermo Fisher Scientific, Inc.). TNF-α
and β-actin, which was used as an internal standard, were amplified
using the following primers (GenScript, Piscataway, NJ, USA):
TNF-α, forward, 5′-GGCAGGTCTACTTTGGAGTCATTGC-3′ and reverse,
5′-ACATTCGAGGCTCCAGTGAATTCGG-3′; β-actin, forward,
5′-GAGACCTTCAACACCCCAGC-3′ and reverse, 5′-CCACAGGATTCCATACCAA-3′.
PCR amplification was performed at 94°C for 5 min, followed by 30
cycles at 94°C for 30 sec, 60°C for 30 sec and then 72°C for 15
sec. This was succeeded by a melt step from 60 to 94°C in
increments of 0.3°C (held at 15 sec each) for subsequent melt curve
analysis. Relative expression was calculated using the ΔΔCq method
provided by the StepOnePlus system (Applied Biosystems) and
optimized with a standard curve to confirm specificity (12). Levels of TNF-α were normalized to
β-actin. Three repetitions were performed per sample. A sample
lacking template DNA was used as negative control. Following PCR, 5
µl amplified cDNA was electrophoresed on a 2% agarose gel and
stained with ethidium bromide.
Statistical analysis
Data are expressed as the mean ± standard deviation.
Results of the SFL, PWMT and PWTL assays were analyzed by repeated
measures analysis of variance (ANOVA) to determine if differences
were significant at each time point. Two-way ANOVA followed by
Fisher's Least Significant Difference post hoc test was used for
multiple group comparisons of TNF-α mRNA levels. All analyses were
performed using SPSS 13.0 software (SPSS, Inc., Chicago, IL, USA)
and P<0.05 was considered to indicate a statistically
significant difference.
Results
Etanercept attenuates bone
cancer-induced SFL
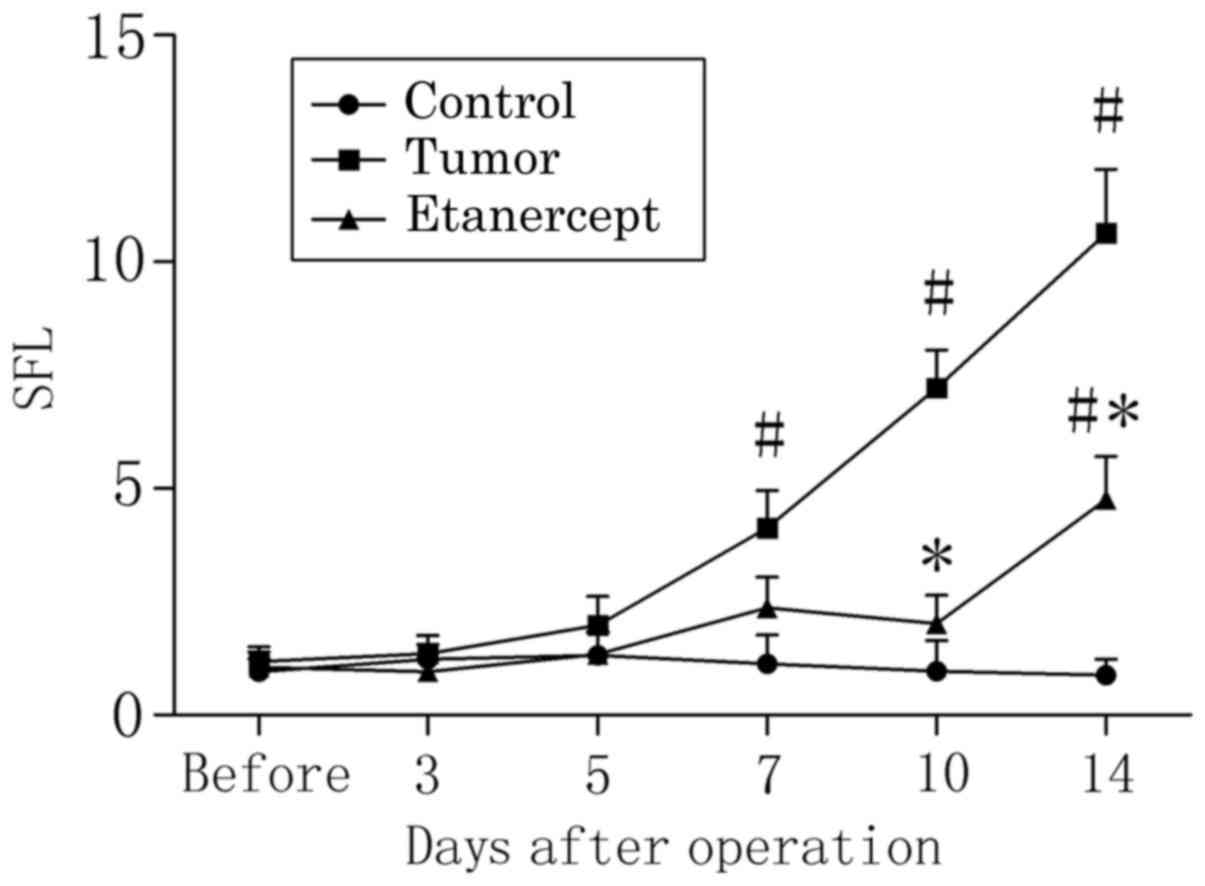
In tumor-bearing mice, the rate of SFL (Fig. 1) was significantly elevated 7 days
after establishment of the bone cancer pain model, relative to
control mice at the same time point (P<0.05; 4.13±0.83 vs.
1.13±0.64, respectively). The SFL rate of tumor-bearing mice was
further elevated by day 14 post-operation, relative to controls
(P<0.05; 10.63±1.41 vs. 0.88±0.35, respectively). By contrast,
the rate of SFL of tumor-bearing mice treated with etanercept did
not significantly differ to that of control mice by days 7
(2.37±0.68) and 10 (2.02±0.72) post-operation. In addition,
treatment with etanercept significantly reduced the increased SFL
rate of tumor-bearing mice on days 10 and 14 post-operation (both
P<0.05; 2.02±0.72 and 4.75±1.66, respectively). However, SFL
rate in etanercept-treated tumor-bearing mice remained
significantly higher than that in control mice on day 14
post-operation (P<0.05).
Etanercept attenuates bone
cancer-induced thermal hyperalgesia
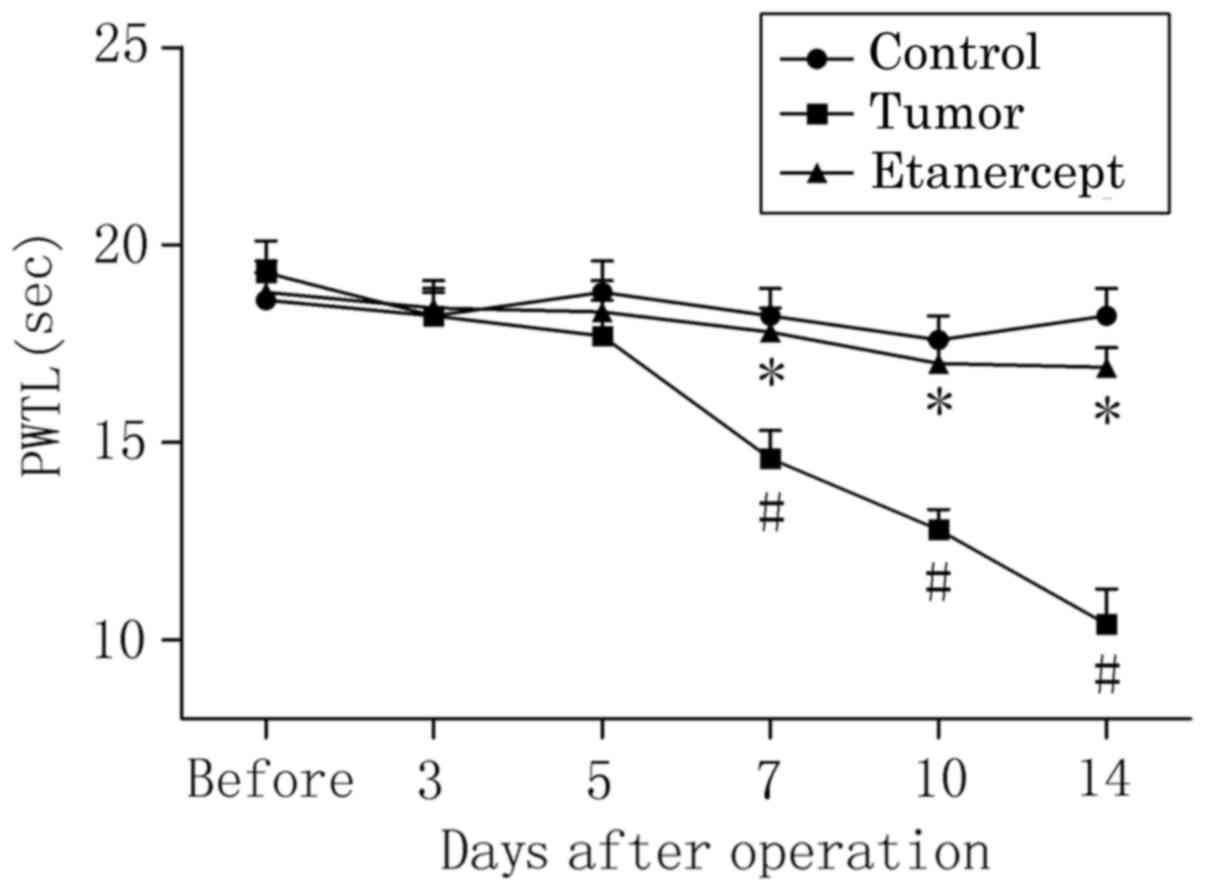
The PWTL of the right hind limb to radiant heat
stimulation in tumor-bearing mice was significantly decreased by
day 7 post-operation, relative to control mice (P<0.05; 14.6±0.7
vs. 18.2±0.7, respectively; Fig. 2).
Relative to controls, this significant decrease in the PWTL of
tumor-bearing mice was consecutively enhanced on days 10
(P<0.05; 12.8±0.5 vs. 17.6±0.6) and 14 (P<0.05; 10.4±0.9 vs.
18.2±0.7) post-operation, suggesting that tumor-bearing mice became
increasingly sensitive to the noxious heat stimulus. In addition,
treatment with etanercept significantly reversed the reduced PWTL
of tumor-bearing mice on days 7 (17.8±0.6 vs. 18.2±0.7), 10
(17.0±0.6 vs. 17.6±0.6) and 14 (16.9±0.5 vs. 18.2±0.7)
post-operation (all P<0.05), indicating that bone cancer-induced
thermal hyperalgesia was attenuated by etanercept.
Etanercept attenuates bone
cancer-induced mechanical allodynia
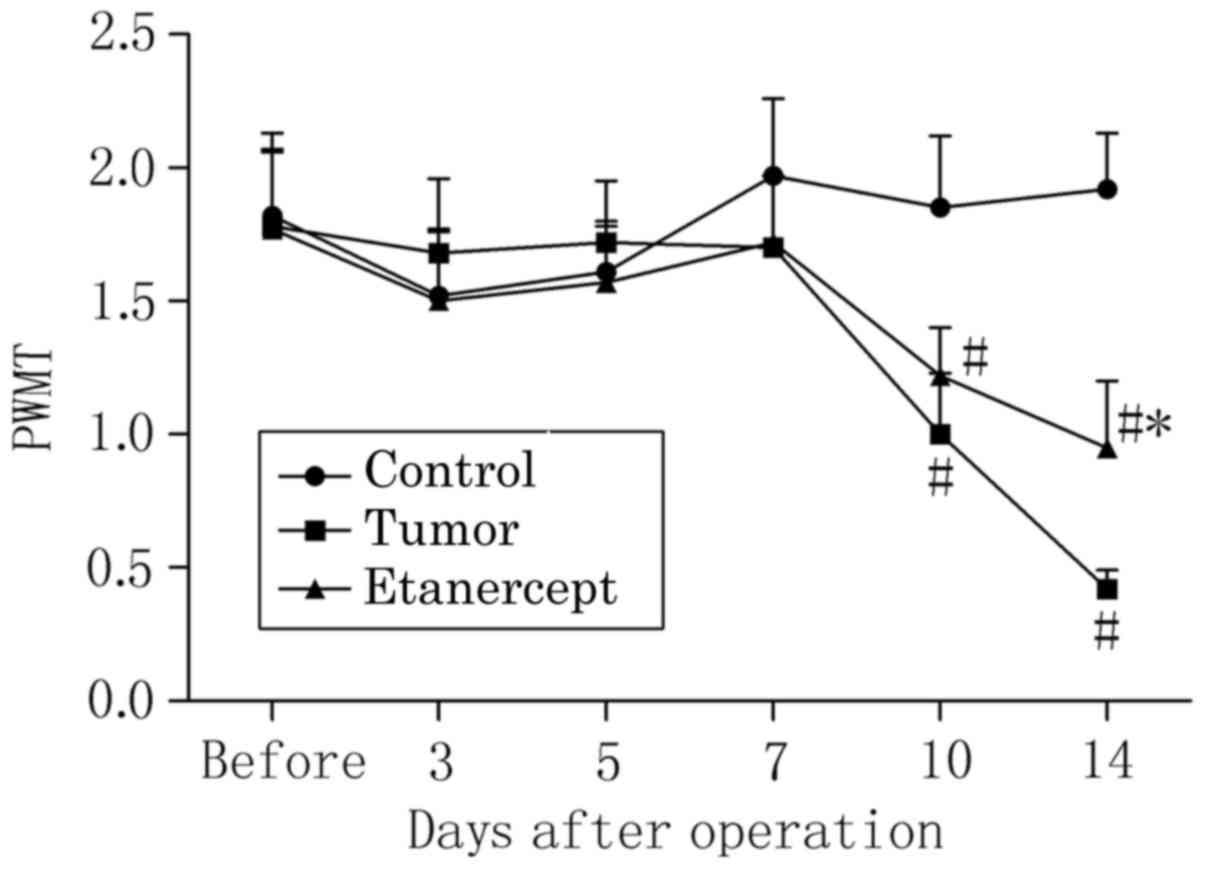
The PWMT of the right hind limb to stimulation by
von Frey filaments in tumor-bearing mice was significantly
decreased by day 10 post-operation, relative to control mice
(P<0.05; 1.02±0.23 vs. 1.78±0.28), and further decreased by day
14, relative to controls (P<0.05; 0.42±0.07 vs. 1.92±0.21;
Fig. 3). Similarly, significant
decreases in the PWMT of mice treated with etanercept were observed
on days 10 (1.22±0.18 vs. 1.85±0.27) and 14 (0.95±0.25 vs.
1.92±0.21), relative to controls (both P<0.05); however,
etanercept treatment significantly elevated the lowered PWMT of
tumor-bearing mice on day 14 (P<0.05). This suggests that
etanercept attenuated bone cancer-induced mechanical allodynia,
though at a different time point to its attenuation of thermal
hyperalgesia.
Elevated TNF-α transcription in the
spinal cord during bone cancer is attenuated by etanercept
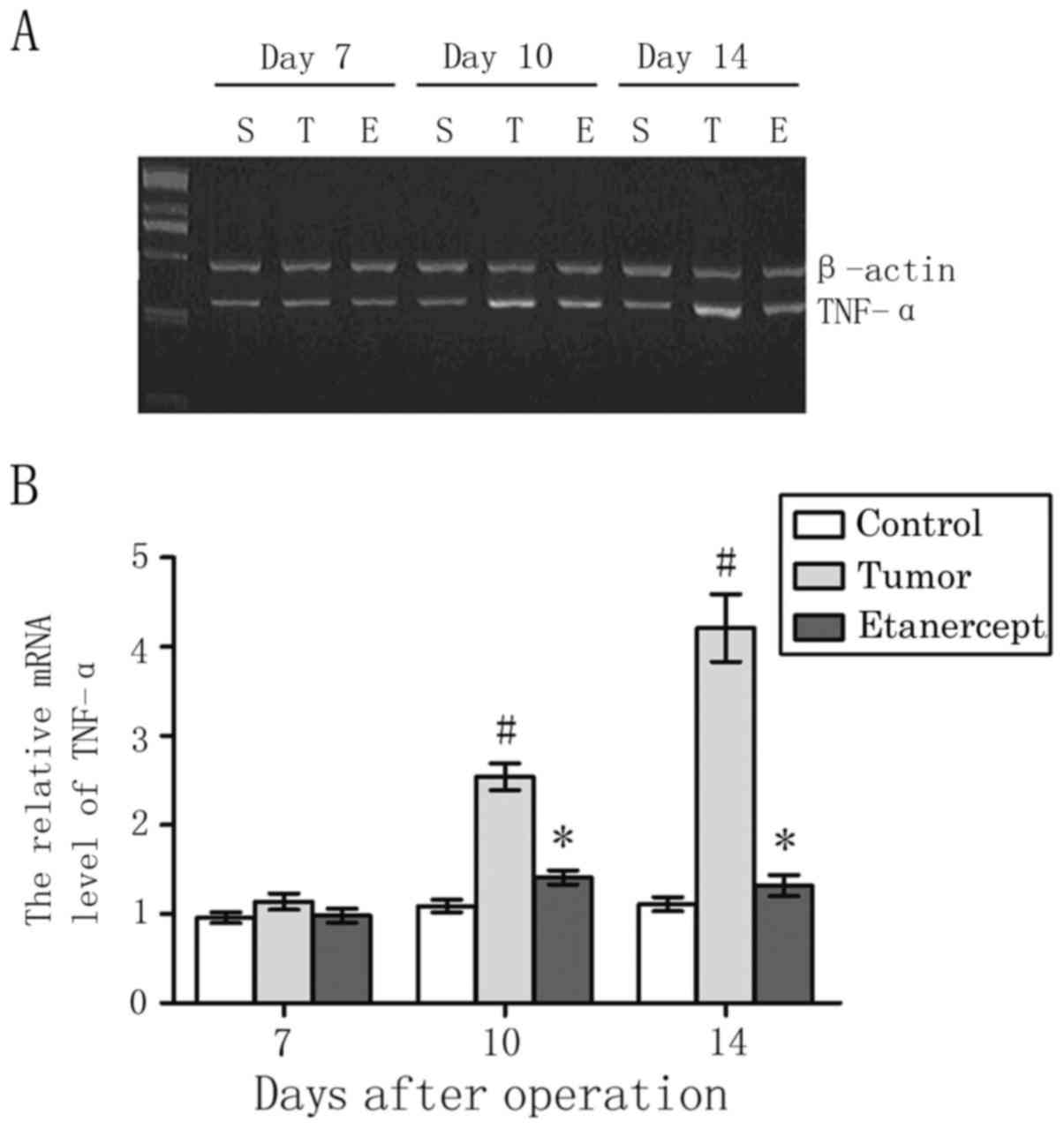
As depicted in Fig.
4, the level of TNF-α mRNA in the L3-L5 lumbar spinal cord
segments in tumor-bearing mice did not significantly differ by day
7, when compared to that in control mice (1.14±0.09 vs. 0.96±0.06,
respectively). β-actin was used as an internal standard. However,
relative to control mice, levels of TNF-α in tumor-bearing mice
gradually elevated to significant levels by day 10 (1.09±0.07 vs.
2.54±0.15) and 14 (1.11±0.08 vs. 4.21±0.38) post-operation (both
P<0.05). In addition, treatment with etanercept significantly
reversed the elevated levels of TNF-α in tumor-bearing mice on days
10 (1.31±0.08) and 14 (1.22±0.12; both P<0.05).
Discussion
In the present study, a mouse model of bone cancer
pain was successfully established by inoculating osteosarcoma
cancer cells into the femoral intramedullary space. Results
demonstrated that bone cancer induced progressive mechanical and
heat hyperalgesia, along with increased levels of TNF-α mRNA in the
spine. Furthermore, it was observed that pain-related behaviors and
elevated TNF-α levels were attenuated by the TNF-α antagonist
etanercept, indicating that TNF-α in the spine serves a key role in
the generation and development of bone cancer pain.
TNF-α has been demonstrated to participate in
peripheral and central nociception in a number of pain models,
including those for inflammatory (13) and neuropathic pain (14). However, neurochemical changes
observed in the spinal cord and sensory neurons of mice due to
cancer pain are distinct from those induced by inflammatory or
neuropathic conditions (15).
Previous in vitro studies have indicated that TNF-α within
tumors contributes to peripheral sensitization of bone
cancer-related pain. Specifically, it has been observed that TNF-α
produced by cancer cells may be responsible for stimulation of
osteoclast activity, which in turn serve a key role in bone
destruction and hyperalgesia (16,17).
Proinflammatory cytokines are typically released from activated
microglia and astrocytes (18) and
subsequently bind to cognate receptors expressed by neurons. In
electrophysiological studies, it has been demonstrated that
injection of exogenous proinflammatory cytokines over the spinal
cord region enhances nociception, by increasing neuronal
excitability in response to noxious stimuli following the cytokine
injection (19). The current study
indicated that TNF-α in the spinal cord also contributes to the
generation of bone cancer pain. Collectively, these results
indicate that nociceptive neurons innervating the tumor area are
sensitized during bone cancer, and that sensory neurons in the
spinal cord may be activated by TNF-α originating from spinal
microglia and astrocytes.
Several studies have suggested that TNF-α inhibitor
may be ineffective in alleviating hypersensitivity if it is
administered long after the development of hyperalgesia (20–22).
Therefore, the present study intraperitoneally administered
etanercept to mice prior to establishment of the bone cancer model
and on days 3 and 6 thereafter, when pain-related behaviors had not
yet been observed. Results demonstrated that this treatment
procedure alleviated hyperalgia and attenuated the increased levels
of TNF-α, thus indicating the key role of TNF-α in initiating a
positive feedback cascade of proinflammatory cytokines.
TNF-α interacts with two distinct membrane
receptors, TNF receptor (R)-1 and TNFR2. The effects of TNF-α occur
through its direct action on neurons via TNFR1 or through its
facilitation of macrophage accumulation in dorsal root ganglion via
a TNFR2-mediated pathway (23). In a
sciatic nerve chronic constriction injury (CCI) model of
neuropathic pain, intra-operative epineural administration of
neutralizing antibodies against TNFR-1 was demonstrated to reduce
mechanical allodynia and thermal hyperalgesia (24), while administration on day 4 post-CCI
reduced thermal, but not mechanical hyperalgesia (25). Constantin et al (25) also observed that TNFR2 knockdown
attenuated heat hyperalgesia in tumor-bearing mice, while TNFR1
knockdown had less of an effect, suggesting that TNFR2 serves the
primary role in the generation of tumor-induced heat hyperalgesia.
In the current study, both mechanical allodynia and thermal
hyperalgesia were alleviated in tumor-bearing mice treated with
etanercept; however, the effect of etanercept on thermal
hyperalgesia appeared earlier and more substantial. This suggests
that TNF-α may participate in bone cancer-related pain through
different signaling pathways, which warrant further
investigation.
In conclusion, the present study demonstrated that
the proinflammatory cytokine TNF-α within the spine was upregulated
in a mouse bone cancer model, and may have contributed to
nociceptive signal processing in the development of bone cancer
pain. In addition, it was observed that neutralization of TNF-α had
a significant beneficial effect on bone cancer-induced mechanical
allodynia and thermal hyperalgesia, thus suggesting that
administration of etanercept in the early stages of bone cancer may
be a potential therapeutic strategy for the prevention and
treatment of cancer-related pain.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant nos. 81171047, 81300950,
81371207 and 81300951).
References
|
1
|
Coleman RE: Skeletal complications of
malignancy. Cancer. 80(8 Suppl): S1588–S1594. 1997. View Article : Google Scholar
|
|
2
|
Janjan N: Bone metastases: Approaches to
management. Semin Oncol. 28(4 Suppl 11): S28–S34. 2001. View Article : Google Scholar
|
|
3
|
Jha MK, Jeon S and Suk K: Glia as a link
between neuroinflammation and neuropathic pain. Immune Netw.
12:41–47. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Petrenko AB, Yamakura T, Baba H and
Shimoji K: The role of N-methyl-D-aspartate (NMDA) receptors in
pain: A review. Anesth Analg. 97:1108–1116. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
McMahon SB, Cafferty WB and Marchand F:
Immune and glial cell factors as pain mediators and modulators. Exp
Neurol. 192:444–462. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Watkins LR and Maier SF: Glia: A novel
drug discovery target for clinical pain. Nat Rev Drug Discov.
2:973–985. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lozano-Ondoua AN, Symons-Liguori AM and
Vanderah TW: Cancer-induced bone pain: Mechanisms and models.
Neurosci Lett. 557(Pt A): 52–59. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tobinick EL: Targeted etanercept for
treatment-refractory pain due to bone metastasis: Two case reports.
Clin Ther. 25:2279–2288. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Schwei MJ, Honore P, Rogers SD,
Salak-Johnson JL, Finke MP, Ramnaraine ML, Clohisy DR and Mantyh
PW: Neurochemical and cellular reorganization of the spinal cord in
a murine model of bone cancer pain. J Neurosci. 19:10886–10897.
1999.PubMed/NCBI
|
|
10
|
Chaplan SR, Bach FW, Pogrel JW, Chung JM
and Yaksh TL: Quantitative assessment of tactile allodynia in the
rat paw. J Neurosci Methods. 53:55–63. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hargreaves K, Dubner R, Brown F, Flores C
and Joris J: A new and sensitive method for measuring thermal
nociception in cutaneous hyperalgesia. Pain. 32:77–88. 1988.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Narita M, Shimamura M, Imai S, Kubota C,
Yajima Y, Takagi T, Shiokawa M, Inoue T, Suzuki M and Suzuki T:
Role of interleukin-1beta and tumor necrosis factor-alpha-dependent
expression of cyclooxygenase-2 mRNA in thermal hyperalgesia induced
by chronic inflammation in mice. Neuroscience. 152:477–486. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Winkelstein BA, Rutkowski MD, Sweitzer SM,
Pahl JL and DeLeo JA: Nerve injury proximal or distal to the DRG
induces similar spinal glial activation and selective cytokine
expression but differential behavioral responses to pharmacologic
treatment. J Comp Neurol. 439:127–139. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Honore P, Rogers SD, Schwei MJ,
Salak-Johnson JL, Luger NM, Sabino MC, Clohisy DR and Mantyh PW:
Murine models of inflammatory, neuropathic and cancer pain each
generates a unique set of neurochemical changes in the spinal cord
and sensory neurons. Neuroscience. 98:585–598. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tumber A, Morgan HM, Meikle MC and Hill
PA: Human breast-cancer cells stimulate the fusion, migration and
resorptive activity of osteoclasts in bone explants. Int J Cancer.
91:665–672. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kitaura H, Sands MS, Aya K, Zhou P,
Hirayama T, Uthgenannt B, Wei S, Takeshita S, Novack DV, Silva MJ,
et al: Marrow stromal cells and osteoclast precursors
differentially contribute to TNF-alpha-induced osteoclastogenesis
in vivo. J Immunol. 173:4838–4846. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Watkins LR, Hansen MK, Nguyen KT, Lee JE
and Maier SF: Dynamic regulation of the proinflammatory cytokine,
interleukin-1beta: Molecular biology for non-molecular biologists.
Life Sci. 65:449–481. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Svensson CI, Schäfers M, Jones TL, Powell
H and Sorkin LS: Spinal blockade of TNF blocks spinal nerve
ligation-induced increases in spinal P-p38. Neurosci Lett.
379:209–213. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Olmarker K, Nutu M and Størkson R: Changes
in spontaneous behavior in rats exposed to experimental disc
herniation are blocked by selective TNF-alpha inhibition. Spine
(Phila Pa 1976). 28:1635–1642. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Murata Y, Olmarker K, Takahashi I,
Takahashi K and Rydevik B: Effects of selective tumor necrosis
factor-alpha inhibition to pain-behavioral changes caused by
nucleus pulposus-induced damage to the spinal nerve in rats.
Neurosci Lett. 382:148–152. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sasaki N, Kikuchi S, Konno S, Sekiguchi M
and Watanabe K: Anti-TNF-alpha antibody reduces pain-behavioral
changes induced by epidural application of nucleus pulposus in a
rat model depending on the timing of administration. Spine (Phila
Pa 1976). 32:413–416. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sommer C, Schmidt C and George A:
Hyperalgesia in experimental neuropathy is dependent on the TNF
receptor 1. Exp Neurol. 151:138–142. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sommer C, Lindenlaub T, Teuteberg P,
Schäfers M, Hartung T and Toyka KV: Anti-TNF-neutralizing
antibodies reduce pain-related behavior in two different mouse
models of painful mononeuropathy. Brain Res. 913:86–89. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Constantin CE, Mair N, Sailer CA,
Andratsch M, Xu ZZ, Blumer MJ, Scherbakov N, Davis JB, Bluethmann
H, Ji RR and Kress M: Endogenous tumor necrosis factor alpha
(TNFalpha) requires TNF receptor type 2 to generate heat
hyperalgesia in a mouse cancer model. J Neurosci. 28:5072–5081.
2008. View Article : Google Scholar : PubMed/NCBI
|