Introduction
The association between physical exercise and the
increase in the production of free radicals has been well
established (1,2). Free radicals are products of normal
metabolism and include mainly reactive oxygen species (ROS), such
as superoxide radical (O2•−), hydroxyl radical (OH•),
and peroxyl radical (RO2•), as well as reactive nitrogen species
(RNS), such as nitric oxide (NO) and the peroxynitrite radical
(ONOO•) (3). The excessive
production of ROS may affect several cell functions, such as the
regulation of signaling pathways and is involved in gene expression
and apoptosis (4). ROS generation is
affected by endogenous sources, such as the mitochondrial
respiratory chain, inflammation and cytochrome P450 activity
(5), or exogenous sources, such as
smoking, air pollution and UV light (6). However, ROS generation during exercise
and in particular during aerobic exercise, is believed to be caused
by the increased uptake of oxygen from the active peripheral
skeletal muscle tissues (2).
Excessive ROS production may lead to a pathological condition known
as oxidative stress (7). For the
determination of oxidative stress levels following exercise, a
number of oxidative stress markers are assessed, such as the levels
and activity of antioxidant enzymes and molecules, oxidative DNA
damage, lipid peroxidation and protein oxidation (8–12).
As regards protein oxidation, this is usually
assessed by measuring protein carbonyl (PC) levels in plasma. The
most abundant protein in plasma (approximately 50% of total
protein) is human serum albumin (HSA) (13,14). HSA
is a multifunctional, non-glycosylated globular protein composed of
585 amino acids with a molecular weight of 66 kDa, and is mainly
synthesized in the liver (15–17). The
structure of the protein contains a center made up of hydrophobic
radicals used as a binding site for ligands, while the outer part
is composed of hydrophilic ligands. More specifically, albumin
binds to and transfers several ligands, such as bilirubin,
hormones, metal ions and xenobiotics (18).
In addition, HSA possesses a free thiol group in
Cys34, and thus it may function as an extracellular antioxidant by
scavenging ROS (19,20). Davies and co-workers published a
series of studies explaining in detail the association between
oxidative damage and the increased proteolytic susceptibility of
bovine serum albumin (BSA) (21–24).
Particularly, albumin residues contain cysteine and methionine
sulfhydryl groups reacting with peroxides, leading to thiol
oxidation (25,26). It is considered that albumin acts as
an antioxidant, since Cys34 of albumin, a cysteine that represents
approximately 80% of the total thiol content in plasma, scavenges
ROS (27). However, under oxidative
stress conditions, albumin is oxidized and Cys34 forms a disulfide
with low molecular weight thiols, such as cysteine. Thus, the
oxidation caused by free radicals may affect the molecule's
conformation and structure (28).
Moreover, albumin dimers have been reported as products of
peroxidation caused by free radicals, and consequently they may be
used as a marker of oxidative stress (29). Furthermore, since a number of studies
have reported the association between the oxidation of albumin and
exercise (20,28,30), the
determination of albumin dimer levels may be a good indicator of
oxidative stress in athletes.
Therefore, the present study focused on the
determination of the levels of albumin dimers in the plasma of
runners participating in an exhaustive mountain marathon race,
‘Olympus Mythical Trail 2015’. This mountain marathon race covers a
distance of 103 km in the mountain of Olympus in Northern Greece.
It is considered to be one of the most demanding routes worldwide,
since it includes a 7,200 m elevation gain and a highest altitude
of 2,906 m, while 40 km of the route take place at an altitude
higher than 2,000 m.
Moreover, in one of our previous studies, protein
oxidation in the plasma of these runners was determined
spectrophotometrically by phosphatidylcholine assay in order to
assess the redox status from 24 to 72 h post-race (31). Thus, we also examined the correlation
between the levels of HSA oxidation with the PC levels of total
plasma protein, as well as with other oxidative stress markers. The
determination of these correlations would help to determine whether
HSA oxidation is a good marker for the assessment of oxidative
stress following exercise.
Materials and methods
Subjects
Twelve adult male runners aged 41.1±3.2 years,
voluntarily participated in the present study (height, 1.78±0.02 m;
weight 72.9±2.0 kg). The subjects were informed not to receive any
anti-inflammatory medicines or nutritional supplements and they
were all familiar with mountain running.
The participants visited the Litohoro Health Center,
located close to the starting point, 8 h before the race in order
to complete a health and activity questionnaire, and their
anthropometric parameters were taken. Moreover, written informed
consent to participate in the study was provided by all the
participants prior to blood collection. Body mass was measured to
the nearest 0.5 kg (beam balance 710; Seca, Birmingham, UK) with
the subjects lightly dressed and barefoot. Standing height was
measured to the nearest 0.5 cm (Stadiometer 208; Seca).
All the performed procedures were in accordance with
the Helsinki declaration of 1975 as revised in 2000 and approval
was received by the human subjects committee of the University of
Thessaly.
Description of the race
The volunteers participated at one of the most
extreme mountain ultra-marathons worldwide known as the ‘Olympus
Mythical Trail’, on July 4-5th 2015 in Olympus Mountain in Northern
Greece. The peculiarity and difficulty of the race lies on the fact
that it is a 103 km ‘loop’-type route with a total ascent (positive
height difference) of 7,200 m (more than twice the altitude of
Olympus Mountain), while approximately 40 km of the route are at an
altitute above 2,000 m. The starting and ending points are placed
at Litohoro town in Greece. The route consists mostly of paths
(95%) and dirt (5%) and is divided into 18 checkpoints. The maximum
time allowed for race completion was 28 h.
Subject performance
Following the completion of the race, 8 out of 12
participants managed to finish the race (individuals no. 1, 2, 3,
4, 5, 6, 9 and 11), while 2 of them gave up at the 70th km
(individuals no. 7 and 10) and the other 2 at the 60th km
(individuals no. 8 and 12). The mean running time of the athletes
was 19.57±1.09 h.
Blood collection and processing
The blood samples (10 ml) were drawn from a forearm
vein with the subjects in the seated position at four different
time points; 8 h before the competition (pre-race sample) and at
24, 48 and 72 h post-race. The samples were stored in
ethylenediamine acid (EDTA) tubes and centrifuged at 1,370 × g for
10 min at 4°C to divide the erythrocytes from the plasma. The
plasma lysates were then stored at −80°C prior to biochemical
analysis.
Albumin determination assay
Albumin was determined spectrophotometrically at 628
nm, based on the formation of a coloured complex with bromocresol
green reagent (BCG) solution in a 0.075 M succinate buffer (pH
4.20) (32).
Partial purification of albumin
For sample preparation, 1 volume of plasma was
diluted in 50 volumes of a 0.1 M HEPES buffer (pH 7.0), containing
1 mM EDTA (Buffer A). The column was equilibrated by 10 ml of
Buffer A using an AKTA prime protein purification system (ΑΚΤΑ
purifier UPC 10; GE Healthcare Bio-Sciences AB, Uppsala, Sweden),
and the diluted sample was then applied to a Blue Sepharose column
(1 ml) (ΗiTrap Blue HP; GE Healthcare Bio-Sciences AB). The column
was washed with 10 ml of Buffer A, and the purified HSA was eluted
and selected in a test tube using a 10 ml solution of 0,15 M KCl
containing Buffer A (Buffer B).
Western blot analysis of albumin and
albumin dimers
Protein concentration in plasma following the
purification of the samples was measured using the Bradford reagent
(Sigma-Aldrich, St. Louis, MO, USA). The calculation of the albumin
concentration was made by using an albumin standard curve. Albumin
monomers and dimers were then determined in the purified plasma
samples by western blot analysis using a non-reducing SDS loading
buffer. A non-reducing SDS loading buffer was used, as non-reducing
conditions allow the visualization of any disulfide-linked dimers
(33). Specifically, non-reducing
buffers do not contain 2-mercaptoethanol (2-ME) or dithiothreitol
(DTT), which can reduce disulphide bridges in proteins. In order to
perform western blot analysis, the purified sample was diluted
until the final concentration of 1 µg of albumin was achieved.
Afterwards, an aliquot containing the diluted purified sample and a
2X non-reducing loading buffer was prepared, heated in boiling
water for 3 min and separated by SDS-PAGE, using a polyacrylamide
gel 8% (w/v). After 1 h of electrophoresis at 150 V, proteins were
transferred onto polyvinylidenedifluoride (PVDF) membranes (Bio-Rad
Laboratories, Hercules, CA, USA) and blocked overnight with 5%
non-fat milk in TBST (13 mmol/l Tris, 150 mmol/l NaCl (pH 7.5)
solution, containing also 0.2% Tween-20 (TBSTMS).
The membranes were then incubated in a shaker for 1
h at room temperature with a goat anti-human albumin antibody
diluted 1:5,000 in TBSTMS. Following extensive washes in TBST (5
times for 5 min) the blots were incubated for 30 min with anti-goat
IgG secondary antibody (1:3,000 dilution). The membranes washed in
TBST (3 times for 15 min) and the labeled protein bands were
visualized by enhanced chemiluminescence (Bio-Rad Laboratories) and
subsequent exposure to XAR 5 film (Fujifilm Corp., Tokyo, Japan).
The protein bands were quantified using Alpha View quantification
software (Alpha Innotech, San Leandro, CA, USA). Each sample was
analyzed in triplicate.
Statistical analysis
The statistical analysis was based on one-way ANOVA
followed by Dunnett's test for multiple pairwise comparisons. The
statistical significance level was set at P<0.05. Correlations
between oxidized albumin and the other oxidative stress markers
were examined by Spearman's correlation analysis. The level of
significance was also set at P<0.05. For all statistical
analyses, SPSS version 20.0 (SPSS, Inc., Chicago, IL, USA) was
used. Data are presented as the means ± standard error of the
mean.
Results
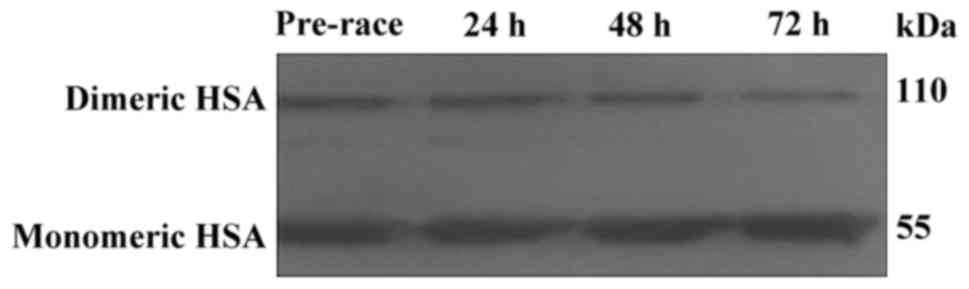
Western blot analysis was used for the assessment of
albumin monomers and dimers. Monomer bands were displayed at ~55
kDa, while dimer formation appeared at approximately 110 kDa
(Fig. 1). The lower molecular weight
of the dimer bands compared to the theoretical one has been
observed previously (29).
In each sample, the percentage ratio of dimers to
monomers was quantified and considered as marker of HSA oxidation.
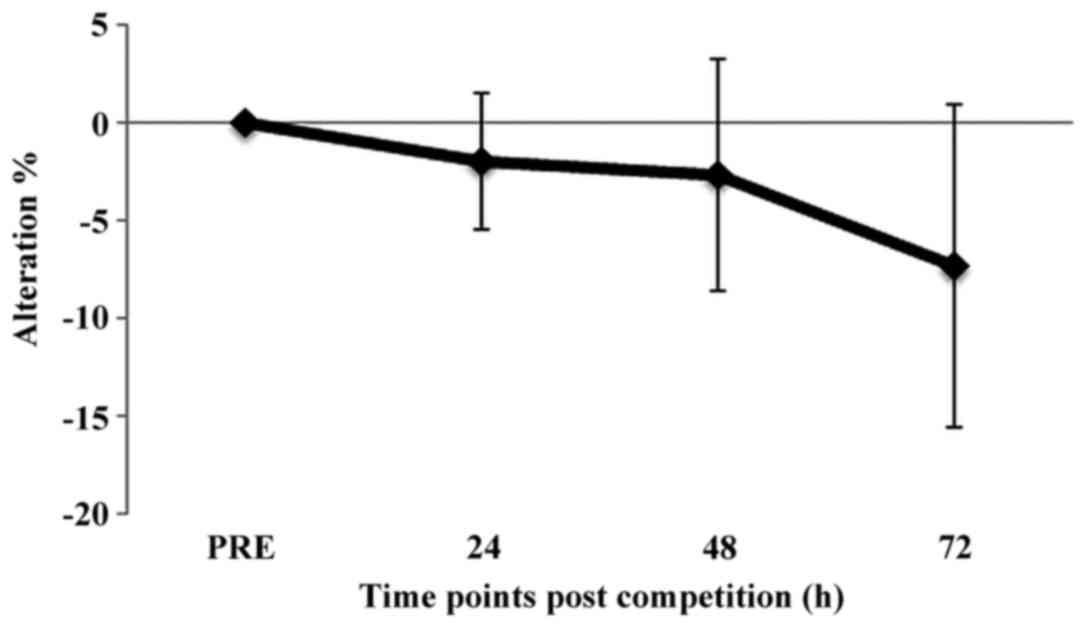
The percentage change of oxidized HSA (i.e., the ratio of dimers to
monomers) at 24, 48 and 72 h post-race compared to pre-race is
shown in Fig. 2. There were not
statistically significant differences between time points post-race
and pre-race (Fig. 2).
Similarly, the percentage ratio of dimers to the
total amount of HSA [i.e., dimers/(dimers + monomers)] was also
quantified in order to obtain a clearer view regarding the changes
of the protein after the race (Table
I). Also, in this case, there were no statistically significant
differences in the values post-race compared to those pre-race.
 | Table I.Percentage of dimer HSA to total HSA
of athletes at all time points. |
Table I.
Percentage of dimer HSA to total HSA
of athletes at all time points.
|
| Oxidized/total HSA
levels (%) |
|---|
|
|
|
|---|
|
| Time point (h) |
|---|
|
|
|
|---|
| Individual | Pre-race | 24 | 48 | 72 |
|---|
| 1 | 36.59 | 34.12 | 30.19 | 27.42 |
| 2 | 39.06 | 34.27 | 37.31 | 44.01 |
| 3 | 45.37 | 44.84 | 38.53 | 29.22 |
| 4 | 28.96 | 37.98 | 30.38 | 37.12 |
| 5 | 36.43 | 31.74 | 28.09 | 22.88 |
| 6 | 36.58 | 36.04 | 43.06 | 34.82 |
| 7 | 38.38 | 35.12 | 33.47 | 39.18 |
| 8 | 21.95 | 22.92 | 25.51 | 24.46 |
| 9 | 25.44 | 24.68 | 27.93 | 15.42 |
| 10 | 39.51 | 41.73 | 40.26 | 39.90 |
| 11 | 30.11 | 32.19 | 36.51 | 37.55 |
| 12 | 35.11 | 33.60 | 32.74 | 35.95 |
| Mean | 34.95±2.02 | 34.60±1.89 | 34.17±1.36 | 32.83±2.53 |
In a previous study, in the samples, we assessed the
percentage changes at 24, 48 and 72 h post-race in the oxidative
stress markers, namely PC levels, thiobarbituric acid reactive
species (TBARS) levels, glutathione (GSH) levels, total antioxidant
capacity (TAC), catalase (CAT) activity, static oxidation-reduction
potential (sORP) and capacity oxidation-reduction potential (cORP)
compared to those at pre-race (31).
In order to determine whether the changes in HSA oxidation are
associated with any other marker, a correlation analysis was
carried out between the percentage changes of HSA at time points
post-race and the percentage changes of the other oxidative stress
biomarkers (Table II). The results
revealed that there was a significantly high correlation between
HSA oxidation and PC at all three time points post-race (Table II). There were also moderate, yet
significant correlations between HSA oxidation and sORP at 24 h
post-race, as well as with cORP at 24 and 48 h post-race (Table II).
 | Table II.Correlation analysis between
percentage changes of HSA oxidation at 24, 48 and 72 h post-race
compared to pre-race and the corresponding percentage changes of
PC, TBARS, GSH, sORP and cORP oxidative stress markers. |
Table II.
Correlation analysis between
percentage changes of HSA oxidation at 24, 48 and 72 h post-race
compared to pre-race and the corresponding percentage changes of
PC, TBARS, GSH, sORP and cORP oxidative stress markers.
|
| Time point (h) | PC | TBARS | GSH | TAC | CAT | sORP | cORP |
|---|
| HSA | 24 | 0.769a | −0.329 |
0.448 | −0.098 | −0.147 |
0.58a |
−0.601a |
|
| 48 | 0.867b |
0.336 |
0.063 | −0.329 | −0.231 |
0.448 |
−0.657a |
|
| 72 | 0.860b | −0.091 | −0.140 | −0.007 | −0.524 | −0.105 |
0.056 |
In our previous study, we demonstrated that there
were great differences in the percentage changes post-race of
oxidative stress markers (including PC) between different
individuals (31). Thus, it was
suggested that some markers should be examined individually in
order to make safer conclusions and apply the appropriate
interventions (25). Similarly, the
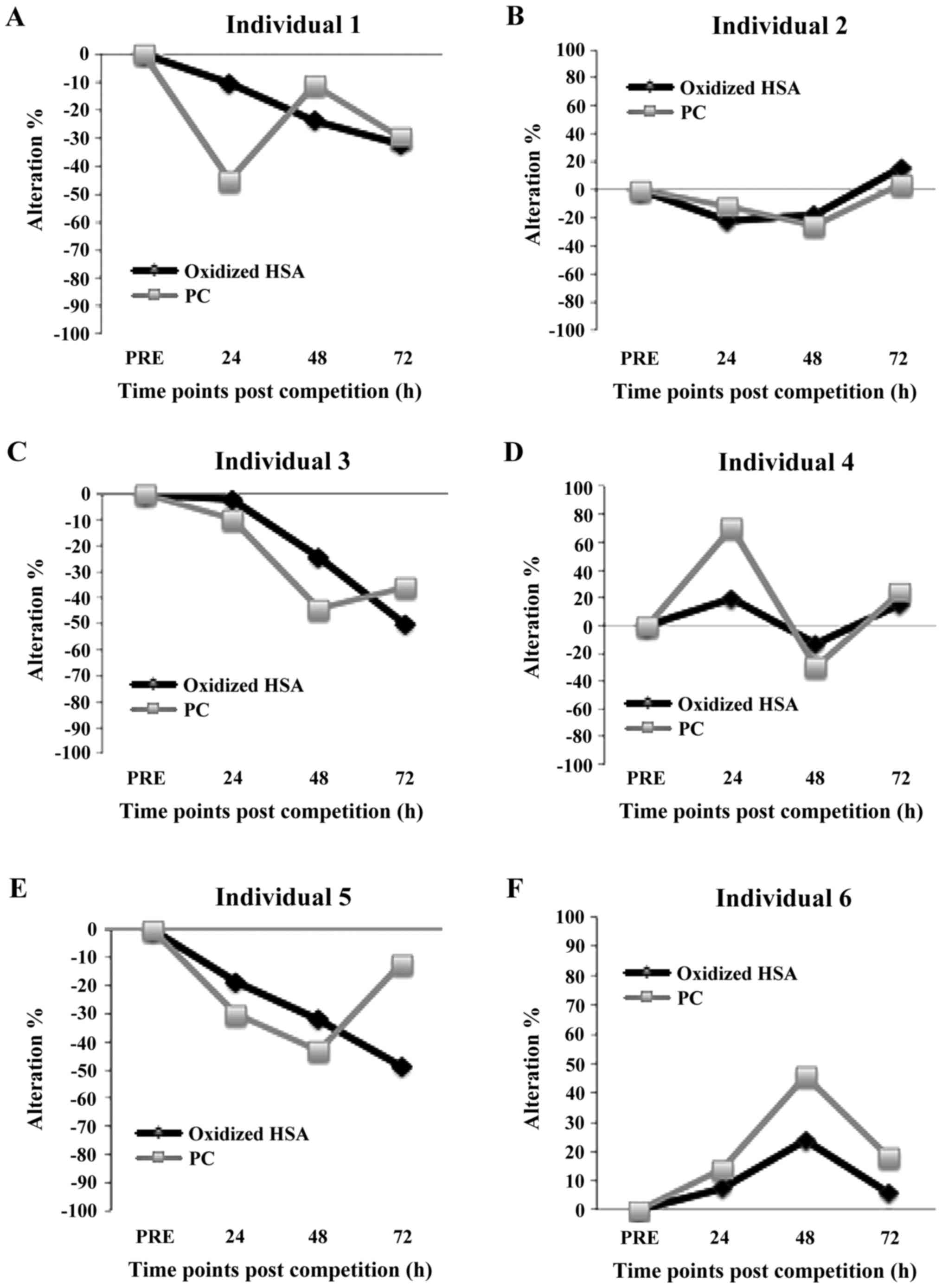
changes in HSA oxidation (i.e., dimer/monomer percentage) post-race
were also examined individually (Figs.
3 and 4). Moreover, since HSA
exhibited a high correlation with PC, showing the oxidation of
total protein in plasma, the individual changes in HSA post-race
were displayed along with the individual changes in PC (Figs. 3 and 4). For this individual analysis, the
criteria used for determining whether there was an increase (or
decrease) in HSA oxidation post-race compared to pre-race were: i)
HSA oxidation should be increased (or decreased) at all time points
post-race compared to pre-race; and ii) HSA oxidation should be
increased (or decreased) >20% at least at one time point
post-race compared to pre-race. This individual analysis indicated
that in some athletes (nos. 6, 8 and 11), HSA oxidation was
increased post-race compared to pre-race (Figs. 3 and 4). However, in other athletes (nos. 1, 3
and 5), HSA oxidation post-race was lower than that at pre-race
(Fig. 3). In 6 athletes (nos. 2, 4,
7, 9, 10 and 12), HSA oxidation exhibited no changes between pre-
and post-exercise (Figs. 3 and
4). The individual comparison
between HSA oxidation and PC indicated that in 6 athletes
(individuals no. 2, 3, 7, 8, 9 and 10) the changes of the two
markers at time points post-race compared to pre-race followed the
same trend (i.e., increase or decrease) (Figs. 3 and 4). The two markers are considered to follow
the same trend if the difference in the percentage change between
them at each time point post-race was <20%. However in 2
athletes (individuals no. 1 and 11), HSA was oxidized more than the
total protein, while in others (individuals no. 4, 5, 6 and 12) HSA
was more protected from ROS compared with the total protein
(Figs. 3 and 4).
Discussion
In the present study, the changes in HSA oxidation
were examined following strenuous exercise, such as a
mountain-marathon race. Specifically, blood samples were collected
from 12 experienced male mountain-marathon runners who participated
in a 103 km mountain marathon race, the ‘Olympus Mythical Trail’.
Blood samples were collected from athletes at four different time
points, pre-race and at 24, 48 and 72 h post-race, in order to
assess the alterations in their redox status by quantifying HSA
dimer formation, that is, HSA oxidation.
HSA is the most abundant protein in plasma, as it
makes up approximately 55% of the total serum protein content
(34). The function of HSA is based
on non-specific binding sites, which allow it to transfer a variety
of molecules throughout the circulatory system. Specifically, it
binds water, cations (e.g., Ca2+, Na+ and
K+), fatty acids, hormones, pharmaceuticals and
vitamins. The main function of albumin is the regulation of the
colloidal osmotic pressure of blood (35). Thus, many of the enzymatic activities
of HSA are connected with the binding of metabolic products, which
affects the related metabolic pathways (36).
HSA contains a total of 35 cysteine residues from
which 34 are involved in intramolecular disulfide bonds and only
cysteine34 (Cys34) remains free (37). It is estimated that approximately 70%
of the total free thiol content in plasma exists in HSA Cys34
(38). This pool of thiol compounds
in plasma gives rise to thiol exchange reactions, leading to a
number of disulfide bonds, and thus to the formation of dimers
acting as antioxidants by scavenging hydroxyl or other radicals
through the reduced sulfhydryl group (39). The dimerization site proved to be the
Cys34 by forming a disulfide bridge between two albumin molecules
(40). According to Ogasawara et
al (29), the formed dimers as a
result to ROS exposure can be used as an oxidative stress marker.
As shown by us and others, the formation of HSA dimers was also
displayed following exhaustive exercise-induced oxidative stress
(20,41). It is noteworthy that the
exercise-induced increase in ROS activates adaptive responses
through signaling pathways regulated by thiol status, including
reduced Cys34 of HSA (28,42–44).
Additionally, changes in the thiol redox status induce the
expression of nuclear factor-κB (NF-κB) and activator protein-1
(AP-1), leading to an increase in the levels of the cytokines, IL-6
and TNF-α (42,45). Βοth of these cytokines not only
affect muscle regeneration, but also the development of tolerance
following ROS-induced muscle damage. In general, the oxidation of a
protein and more specifically HSA following ROS exposure, can lead
to a loss of its structural and catalytic function (46). Oxidation and thus dimerization of HSA
impedes its activity, as the dimer is more rigid, rendering
substrate binding less favorable (47). Specifically, the oxidation of HSA has
been observed to decrease both the ligand binding property of site
II and the esterase-like activity of HSA, most probably due to
conformational changes in subdomain IIIA (47). In addition, HSA has been shown to
exhibit an intristic enolase activity towards dihydrotestosterone
that is reduced upon dimerization (36). It is known that when oxidized
proteins are accumulated in the cells, degradation systems are then
activated (48). Moreover, Kawakami
et al reported that the oxidation of the major antioxidant
thiol group Cys34 of HSA results in reduced scavenging activity
against highly ROS produced after exercise such as hydroxyl
radicals, which may affect athletes' recovery (49). Therefore, it is obvious that
excessive HSA oxidation should be prevented. The significant
interplay between HSA and oxidative stress necessitates the
investigation of HSA oxidation, particularly in athletes undergoing
demanding exercise.
The present results indicated that on average, HSA
oxidation was not increased at any time point post-race compared to
that at pre-race. Although there was no oxidation of thiols of HSA
post-race compared to pre-race, thiol groups of glutathione of the
same samples were oxidized as previously demonstrated (31). Therefore, HSA seems to be more
protected against oxidation than GSH, since the latter may be
involved more in ROS scavenging. Our results were in agreement with
those of our previous study on the same samples, which have shown
that there was no difference in the oxidation of total protein in
plasma between pre and post-race (31). However, other studies have shown an
increase in HSA oxidation following exercise (20,28,41). As
we have reported previously, this discrepancy between different
studies regarding protein oxidation may be explained by the
inter-individual variation of protein oxidation after strenuous
exercise (31). Likewise, HSA
oxidation exhibited great variability, since it was increased in 3
athletes, decreased in 3 athletes and had no change in 6 athletes
post-race compared to pre-race.
The samples used in the present study were also
analyzed in one of our previous studies by measuring other
oxidative stress markers, such as PC, TBARS, TAC, GSH, CAT, sORP
and cORP (31). Thus, HSA oxidation
was assessed by quantifying dimers, so as to compare its levels
with the other performed assays and make conclusions about its
usefulness as a biomarker for strenuous exercise-induced oxidative
stress. The results revealed a significantly high correlation
between HSA oxidation and PC (i.e., oxidation of total protein) at
all time points post-race. The aforementioned correlation was
expected, since the increased concentration of PC following
exercise has been suggested to be mainly derived from the oxidation
of HSA making up approximately 55% of total serum protein, as well
as of other major proteins (50,51). In
addition, there was a significant positive correlation between HSA
oxidation and sORP at 24 h post-race, and a negative correlation
with cORP at 48 and 72 h post-race, respectively. These two novel
markers have been used previously in several of our studies for
assessing exercise-induced oxidative stress (8–11). The
above correlations were meaningful, since sORP increased values
correspond to higher oxidative stress levels, as it represents the
integrated balance of oxidants and reductants, while cORP is a
measure of antioxidant reserve available in the body's system.
As mentioned above, HSA oxidation exhibited a great
variation between different athletes. Moreover, HSA oxidation had a
high correlation with PC, that is, with the oxidation of total
plasma protein. Thus, each athlete was examined separately in order
to compare the changes in HSA oxidation and PC levels, since HSA
constitutes approximately 55% of the total protein content in
plasma as mentioned before. Several studies have demonstrated that
albumin, as well as fibrinogen are the main protein targets of
oxidative stress in plasma (50–53).
Moreover, since HSA represents about the half of the total protein
content in plasma, a similar trend of PC and HSA oxidation levels
in each individual was expected post-race. However, our results
indicated that protein oxidation post-race in several athletes
exhibited great differences compared to PC levels. Specifically,
the results revealed that similar oxidation levels between PC and
HSA at all time points post-race were displayed in only 6 out of 12
runners. Thus, in these individuals, it seems that HSA oxidation
post-race was not affected differently than the other plasma
proteins. However, the remaining 6 athletes exhibited great
differences in the changes between PC and HSA oxidation at one or
more time points post-race. It was remarkable, that in 4 athletes,
HSA was more protected from oxidation than the other plasma
proteins. These results supported the hypothesis of Madian and
Regnier (54), who suggested that
despite the abundance of HSA in plasma, it is not so vulnerable to
oxidation as other proteins, such as fibrinogen (53). However, in 2 athletes, HSA oxidation
was higher compared to total plasma proteins. To sum up, all these
findings suggested that the measurement of only PC is not
sufficient to make conclusions about HSA oxidation, and thus it is
needed to be examined separately after exercise.
Moreover, in a previous study, we demonstrated that
in some of these athletes, instead of protein oxidation post-race,
there was protein reduction (31).
Similarly, albumin in some of these athletes (individuals no. 1, 3
and 5) exhibited a decrease in oxidation and not an increase
post-race. The manifestation of reductive stress instead of
oxidative one, particularly after eccentric exercise, has been
reported by us, as well as by others (8,55). This
intriguing effect can be explained by the high complexity of the
regulation of redox homeostasis in human, since many genetic,
physiological, biochemical or dietary factors may affect the final
outcome of oxidant stimuli (56–58).
The changes in the ratio of dimer to total HSA
post-exercise compared to pre-exercise showed the same trend with
the ratio of dimer to monomer HSA, that is, in each athlete both
ratios either decreased or increased post exercise. Similar to the
ratio of dimer to monomer HSA, the ratio of dimer to total HSA
exhibited great variability, from 15.42 to 45.37%, between
different individuals at all time points post exercise. This
variability in HSA oxidation may indicate differences in its
functionality, that is, individuals with higher HSA oxidation
levels are likely to have lower HSA activity and vice versa. As
mentioned above, lower HSA functionality may affect its binding
capacity for several ligands, and thus there may be need for a
dietary intervention in order to improve the athletes' redox status
particularly following strenuous exercise.
In conclusion, the present results support the
notion that the assessment of HSA dimers, that is, HSA oxidation
may be used as a complementary marker of oxidative stress after
exhaustive exercise, particularly as regards the effects on
proteins. This inference is supported by the correlation between
HSA oxidation and other oxidative stress markers, such as PC, sORP
and cORP. In general, thiol levels have been suggested as a marker
of oxidative stress (35). However,
the assessment of oxidative stress using low molecular weight
thiols is difficult, as they are susceptible to oxidative damage
and their measurement, particularly in blood, is not easy. Thus,
the measurement of stable oxidized thiol groups, such as albumin
dimers, is more practical, taking into account that 70% of the
total free thiol content in plasma exists in HSA (29). Moreover, the fact that in some
athletes the changes in HSA oxidation post-exercise did not follow
the changes of PC, suggested the need for assessing both of these
markers in order to reach a more confident conclusion about protein
oxidation in plasma and make the appropriate dietary interventions.
Finally, to the best of our knowledge, this study demonstrated for
the first time that in some athletes, HSA was reduced instead of
being oxidized post-exercise, highlighting the need for
investigating further the individual impact on HSA oxidation and
generally in redox status. The understanding of the
inter-individual variability of HSA oxidation may prove to be
useful for applying the appropriate interventions through nutrition
and supplementation to athletes participating in demanding exercise
such as mountain marathon race (7,12).
The need for a separate analysis of HSA oxidation is
supported by its abundance in plasma and its important
physiological roles, which are disturbed after oxidation. This
individual approach to HSA oxidation may help athletes to better
improve immediate recovery process and consequently health status
and performance.
Glossary
Abbreviations
Abbreviations:
|
CAT
|
catalase
|
|
EDTA
|
ethylenediamine tetraacetic acid
|
|
GSH
|
glutathione
|
|
HSA
|
human serum albumin
|
|
PC
|
protein carbonyls
|
|
ROS
|
reactive oxygen species
|
|
sORP
|
static oxidation-reduction
potential
|
|
TAC
|
total antioxidant capacity
|
|
TBARS
|
thiobarbituric acid reactive
substances
|
References
|
1
|
Alessio HM, Hagerman AE, Fulkerson BK,
Ambrose J, Rice RE and Wiley RL: Generation of reactive oxygen
species after exhaustive aerobic and isometric exercise. Med Sci
Sports Exerc. 32:1576–1581. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sen CK: Oxidants and antioxidants in
exercise. J Appl Physiol (1985). 79:675–686. 1995.PubMed/NCBI
|
|
3
|
Halliwell B: The wanderings of a free
radical. Free Radic Biol Med. 46:531–542. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ghosh J and Myers CE: Inhibition of
arachidonate 5-lipoxygenase triggers massive apoptosis in human
prostate cancer cells. Proc Natl Acad Sci USA. 95:13182–13187.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Valko M, Leibfritz D, Moncol J, Cronin
MTD, Mazur M and Telser J: Free radicals and antioxidants in normal
physiological functions and human disease. Int J Biochem Cell Biol.
39:44–84. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Orient A, Donkó A, Szabó A, Leto TL and
Geiszt M: Novel sources of reactive oxygen species in the human
body. Nephrol Dial Transplant. 22:1281–1288. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Veskoukis AS, Tsatsakis AM and Kouretas D:
Dietary oxidative stress and antioxidant defense with an emphasis
on plant extract administration. Cell Stress Chaperones. 17:11–21.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Stagos D, Goutzourelas N, Ntontou A-M,
Kafantaris I, Deli CK, Poulios A, Jamurtas AZ, Bar-Or D and
Kouretas D: Assessment of eccentric exercise-induced oxidative
stress using oxidation-reduction potential markers. Oxid Med Cell
Longev. 2015:2046152015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Stagos D, Goutzourelas N, Bar-Or D,
Ntontou AM, Bella E, Becker AT, Statiri A, Kafantaris I and
Kouretas D: Application of a new oxidation-reduction potential
assessment method in strenuous exercise-induced oxidative stress.
Redox Rep. 20:154–162. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Spanidis Y, Goutzourelas N, Stagos D,
Mpesios A, Priftis A, Bar-Or D, Spandidos DA, Tsatsakis AM, Leon G
and Kouretas D: Variations in oxidative stress markers in elite
basketball players at the beginning and end of a season. Exp Ther
Med. 11:147–153. 2016.PubMed/NCBI
|
|
11
|
Spanidis Y, Mpesios A, Stagos D,
Goutzourelas N, Bar-Or D, Karapetsa M, Zakynthinos E, Spandidos DA,
Tsatsakis AM, Leon G, et al: Assessment of the redox status in
patients with metabolic syndrome and type 2 diabetes reveals great
variations. Exp Ther Med. 11:895–903. 2016.PubMed/NCBI
|
|
12
|
Kerasioti E, Kiskini A, Veskoukis A,
Jamurtas A, Tsitsimpikou C, Tsatsakis AM, Koutedakis Y, Stagos D,
Kouretas D and Karathanos V: Effect of a special
carbohydrate-protein cake on oxidative stress markers after
exhaustive cycling in humans. Food Chem Toxicol. 50:2805–2810.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Farrugia A: Albumin usage in clinical
medicine: Tradition or therapeutic? Transfus Med Rev. 24:53–63.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Malik A, Al-Senaidy A, Skrzypczak-Jankun E
and Jankun J: Isolation and characterization of serum albumin from
Camelus dromedarius. Exp Ther Med. 6:519–524. 2013.PubMed/NCBI
|
|
15
|
Kragh-Hansen U, Pedersen AO, Galliano M,
Minchiotti L, Brennan SO, Tárnoky AL, Franco MH and Salzano FM:
High-affinity binding of laurate to naturally occurring mutants of
human serum albumin and proalbumin. Biochem J. 320:911–916. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Pawar SK, Punith R, Naik RS and
Seetharamappa J: Spectroscopic and molecular modeling approaches to
investigate the binding of proton pump inhibitors to human serum
albumin. J Biomol Struct Dyn. Nov 18–2016.(Epub ahead of print).
View Article : Google Scholar
|
|
17
|
Ishida K, Sawada N and Yamaguchi M:
Expression of albumin in bone tissues and osteoblastic cells:
Involvement of hormonal regulation. Int J Mol Med. 14:891–895.
2004.PubMed/NCBI
|
|
18
|
Doweiko JP and Nompleggi DJ: Role of
albumin in human physiology and pathophysiology. JPEN J Parenter
Enteral Nutr. 15:207–211. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Halliwell B: Albumin - an important
extracellular antioxidant? Biochem Pharmacol. 37:569–571. 1988.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Imai H, Hayashi T, Negawa T, Nakamura K,
Tomida M, Koda K, Tajima T, Koda Y, Suda K and Era S: Strenuous
exercise-induced change in redox state of human serum albumin
during intensive kendo training. Jpn J Physiol. 52:135–140. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Davies KJ: Protein damage and degradation
by oxygen radicals. I. general aspects. J Biol Chem. 262:9895–9901.
1987.PubMed/NCBI
|
|
22
|
Davies KJ, Delsignore ME and Lin SW:
Protein damage and degradation by oxygen radicals. II. Modification
of amino acids. J Biol Chem. 262:9902–9907. 1987.PubMed/NCBI
|
|
23
|
Davies KJ and Delsignore ME: Protein
damage and degradation by oxygen radicals. III. Modification of
secondary and tertiary structure. J Biol Chem. 262:9908–9913.
1987.PubMed/NCBI
|
|
24
|
Davies KJ, Lin SW and Pacifici RE: Protein
damage and degradation by oxygen radicals. IV. Degradation of
denatured protein. J Biol Chem. 262:9914–9920. 1987.PubMed/NCBI
|
|
25
|
Alvarez B, Ferrer-Sueta G, Freeman BA and
Radi R: Kinetics of peroxynitrite reaction with amino acids and
human serum albumin. J Biol Chem. 274:842–848. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Carballal S, Radi R, Kirk MC, Barnes S,
Freeman BA and Alvarez B: Sulfenic acid formation in human serum
albumin by hydrogen peroxide and peroxynitrite. Biochemistry.
42:9906–9914. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gutteridge JM: Antioxidant properties of
the proteins caeruloplasmin, albumin and transferrin. A study of
their activity in serum and synovial fluid from patients with
rheumatoid arthritis. Biochim Biophys Acta. 869:119–127. 1986.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lamprecht M, Greilberger JF, Schwaberger
G, Hofmann P and Oettl K: Single bouts of exercise affect albumin
redox state and carbonyl groups on plasma protein of trained men in
a workload-dependent manner. J Appl Physiol 1985. 104:1611–1617.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ogasawara Y, Namai T, Togawa T and Ishii
K: Formation of albumin dimers induced by exposure to peroxides in
human plasma: A possible biomarker for oxidative stress. Biochem
Biophys Res Commun. 340:353–358. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Veskoukis AS, Kyparos A, Stagos D and
Kouretas D: Differential effects of xanthine oxidase inhibition and
exercise on albumin concentration in rat tissues. Appl Physiol Nutr
Metab. 35:244–250. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
31
|
Spanidis Y, Stagos D, Orfanou M,
Goutzourelas N, Bar-Or D, Spandidos D and Kouretas D: Variations in
Oxidative Stress Levels in 3 Days Follow-up in Ultramarathon
Mountain Race Athletes. J Strength Cond Res. 31:582–594. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Dumas BT, Watson WA and Biggs HG: Albumin
standards and the measurement of serum albumin with bromcresol
green. 1971. Clin Chim Acta. 258:21–30. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Sheffield WP, McCurdy TR and Bhakta V:
Fusion to albumin as a means to slow the clearance of small
therapeutic proteins using the Pichia pastoris expression system: A
case study. Methods Mol Biol. 308:145–154. 2005.PubMed/NCBI
|
|
34
|
Anderson NL and Anderson NG: The human
plasma proteome: History, character, and diagnostic prospects. Mol
Cell Proteomics. 1:845–867. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Quinlan GJ, Martin GS and Evans TW:
Albumin: Biochemical properties and therapeutic potential.
Hepatology. 41:1211–1219. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Drmanovic Z, Voyatzi S, Kouretas D,
Sahpazidou D, Papageorgiou A and Antonoglou O: Albumin possesses
intrinsic enolase activity towards dihydrotestosterone which can
differentiate benign from malignant breast tumors. Anticancer Res.
19:(5B). 4113–4124. 1999.PubMed/NCBI
|
|
37
|
Theodore Peters Jr: All About Albumin:
Biochemistry, Genetics, and Medical Applications. 1st. Academic
Press; San Diego, CA: 1997
|
|
38
|
Turell L, Carballal S, Botti H, Radi R and
Alvarez B: Oxidation of the albumin thiol to sulfenic acid and its
implications in the intravascular compartment. Braz J Med Biol Res.
42:305–311. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Roche M, Rondeau P, Singh NR, Tarnus E and
Bourdon E: The antioxidant properties of serum albumin. FEBS Lett.
582:1783–1787. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Naldi M, Baldassarre M, Nati M, Laggetta
M, Giannone FA, Domenicali M, Bernardi M, Caraceni P and Bertucci
C: Mass spectrometric characterization of human serum albumin
dimer: A new potential biomarker in chronic liver diseases. J Pharm
Biomed Anal. 112:169–175. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Veskoukis AS, Nikolaidis MG, Kyparos A,
Kokkinos D, Nepka C, Barbanis S and Kouretas D: Effects of xanthine
oxidase inhibition on oxidative stress and swimming performance in
rats. Appl Physiol Nutr Metab. 33:1140–1154. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Ji LL, Gomez-Cabrera M-C and Vina J:
Exercise and hormesis: Activation of cellular antioxidant signaling
pathway. Ann N Y Acad Sci. 1067:425–435. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Melikoglu MA, Kaldirimci M, Katkat D, Sen
I, Kaplan I and Senel K: The effect of regular long term training
on antioxidant enzymatic activities. J Sports Med Phys Fitness.
48:388–390. 2008.PubMed/NCBI
|
|
44
|
Zembron-Lacny A, Slowinska-Lisowska M and
Ziemba A: Integration of the thiol redox status with cytokine
response to physical training in professional basketball players.
Physiol Res. 59:239–245. 2010.PubMed/NCBI
|
|
45
|
Kerksick C and Willoughby D: The
antioxidant role of glutathione and N-acetyl-cysteine supplements
and exercise-induced oxidative stress. J Int Soc Sports Nutr.
2:38–44. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Levine RL and Stadtman ER: Oxidative
modification of proteins during aging. Exp Gerontol. 36:1495–1502.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Anraku M, Yamasaki K, Maruyama T,
Kragh-Hansen U and Otagiri M: Effect of oxidative stress on the
structure and function of human serum albumin. Pharm Res.
18:632–639. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Jung T, Höhn A and Grune T: The proteasome
and the degradation of oxidized proteins: Part II-protein oxidation
and proteasomal degradation. Redox Biol. 2:99–104. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Kawakami A, Kubota K, Yamada N, Tagami U,
Takehana K, Sonaka I, Suzuki E and Hirayama K: Identification and
characterization of oxidized human serum albumin. A slight
structural change impairs its ligand-binding and antioxidant
functions. FEBS J. 273:3346–3357. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Margonis K, Fatouros IG, Jamurtas AZ,
Nikolaidis MG, Douroudos I, Chatzinikolaou A, Mitrakou A,
Mastorakos G, Papassotiriou I, Taxildaris K, et al: Oxidative
stress biomarkers responses to physical overtraining: Implications
for diagnosis. Free Radic Biol Med. 43:901–910. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Michailidis Y, Jamurtas AZ, Nikolaidis MG,
Fatouros IG, Koutedakis Y, Papassotiriou I and Kouretas D: Sampling
time is crucial for measurement of aerobic exercise-induced
oxidative stress. Med Sci Sports Exerc. 39:1107–1113. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Shacter E, Williams JA, Lim M and Levine
RL: Differential susceptibility of plasma proteins to oxidative
modification: Examination by western blot immunoassay. Free Radic
Biol Med. 17:429–437. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Michelis R, Gery R, Sela S, Shurtz-Swirski
R, Grinberg N, Snitkovski T, Shasha SM and Kristal B: Carbonyl
stress induced by intravenous iron during haemodialysis. Nephrol
Dial Transplant. 18:924–930. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Madian AG and Regnier FE: Profiling
carbonylated proteins in human plasma. J Proteome Res. 9:1330–1343.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Margaritelis NV, Kyparos A, Paschalis V,
Theodorou AA, Panayiotou G, Zafeiridis A, Dipla K, Nikolaidis MG
and Vrabas IS: Reductive stress after exercise: The issue of redox
individuality. Redox Biol. 2:520–528. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Simoneau JA and Bouchard C: Human
variation in skeletal muscle fiber-type proportion and enzyme
activities. Am J Physiol. 257:E567–E572. 1989.PubMed/NCBI
|
|
57
|
Kant AK and Graubard BI: Ethnic and
socioeconomic differences in variability in nutritional biomarkers.
Am J Clin Nutr. 87:1464–1471. 2008.PubMed/NCBI
|
|
58
|
Rankinen T and Bouchard C: Gene-physical
activity interactions: Overview of human studies. Obesity (Silver
Spring). 16:(Suppl 3). S47–S50. 2008. View Article : Google Scholar : PubMed/NCBI
|