Introduction
Diabetes affects patients worldwide, and is
characterized by absolute or relative insulin insufficiency,
causing high blood and urine glucose levels (1). Long-term high blood sugar levels can
damage cardiovascular, kidney, eye and nervous tissues, and can
cause great harm to the patient. Treatments for diabetes include
insulin therapy, islet cell transplantation, gene therapy, oral
hypoglycemic drugs, exercise and diet therapy (2). Furthermore, the current oral
hypoglycemic drugs have three major mechanisms: i) Promoting
insulin secretion, ii) increasing insulin sensitivity and iii)
inhibiting α-glucosidase (3).
Hypoglycemic drugs from traditional Chinese herbal remedies are
continually emerging. These have multiple functions, including
reducing blood sugar levels, reducing blood fat and improving blood
viscosity (4–6).
Adenosine monophosphate-activated protein kinase
(AMPK), which is activated by metabolic stressors including
hypoxia, low glucose and nutrient deprivation, regulates cellular
and systemic energy homeostasis in mammalian cells (7). Activation of AMPK in skeletal muscle,
liver and adipose tissue enhances metabolism, improves insulin
sensitivity, and may be favorable for the treatment of diabetes
(8). AMPK is an attractive drug
target that serves a key role in the regulation of whole-body
energy homeostasis (9). Activation
of hepatic AMPK leads to increased fatty acid oxidation and,
simultaneously, inhibition of hepatic glucose production, as well
as lipogenesis and cholesterol synthesis (9,10). A
number of previous studies found that AMPK may adjust key enzymes
[phosphoenolpyruvate carboxykinase (PEPCK) and
glucose-6-phosphatase (G6Pase)] involved in hepatic
gluconeogenesis. The transcription factors cyclic-AMP response
element binding protein (CREB), hepatocyte nuclear factor 4α
(HNF4α) and forkhead box protein O1 (FOXO1) are the key regulatory
factors of the AMPK channel. TORC2, a signaling protein that
modulates CREB activity, is phosphorylated by AMPK. Through this
mechanism, CREB expression is inhibited by AMPK. AMPK can adjust
SHP, HNF4α and FOXO1 to achieve the inhibition of hepatic
gluconeogenesis, which has the potential to reduce blood glucose
(10).
Ginsenoside Rb3, one of the major active components
of protopanaxdiol type ginsenosides, has received much attention
due to its various bioactivities, including antioxidant activity
and microcirculatory improvement (11), neuroprotection (12), cardiovascular protection (13,14),
prevention of obesity (15) and
prevention of diabetes (16,17). Previous research has indicated that
ginsenoside Rb3 showed antidiabetic activity in alloxan-induced
diabetic mice, and increased glucose consumption in C2C12 myotubes
(16). Furthermore, our group
previously demonstrated that ginsenoside Rb3 can decrease blood
glucose, increase blood glucose tolerance and antioxidants, improve
serum lipid disorders, and improve insulin sensitivity and
resistance in diabetic mice (17).
Numerous animal studies and clinical trials have ascertained that
ginsenoside Rb3 has significant hypoglycemic effects (15–17).
Although the hypoglycemic effects of ginsenoside Rb3 appear
promising, it has not yet been used clinically as an antidiabetic
drug, predominantly due to the fact that the molecular mechanism
has yet to be fully elucidated. The current study assessed the
effect of ginsenoside Rb3 on hepatic gluconeogenesis mediated by
AMPK in HepG2 cells, and provided a theoretical basis for research
on the molecular regulatory mechanism of gluconeogenesis
inhibition.
Materials and methods
Materials
Ginsenoside Rb3 standards were isolated and purified
in our laboratory (the Chinese Herbal Medicine Laboratory of Jilin
Agricultural University, Changchun, China). HepG2 cells were
purchased from the Shanghai Cell Institute of Chinese Academy of
Science (Shanghai, China). AICAR (an AMPK activator), trypsin, and
Dulbecco's modified Eagle's medium (DMEM) culture medium were
purchased from Sigma-Aldrich; Merck Millipore (Darmstadt, Germany).
Antibodies against AMPK (cat. no. sc-25792), P-AMPK (cat. no.
sc-33524), PEPCK (cat. no. sc-32879), G6Pase (cat. no. sc-25840),
FOXO1 (cat. no. sc-C29H4), HNF4α (cat. no. sc-8987) and goat
anti-rabbit IgG-horseradish peroxidase (HRP) secondary antibodies
(cat. no. sc-2004) were purchased from Santa Cruz Biotechnology,
Inc. (Dallas, TX, USA). Dimethyl sulfoxide (DMSO) and the fetal
bovine serum (FBS) and trypsin were purchased from Roche Applied
Science (Penzberg, Germany). MTT was purchased from the Tianjin Lu
Xin Chemical Technology Company (Tianjin, China). Compound C (an
AMPK selective inhibitor) was purchased from North Kangtai Clinical
Reagent Company (Beijing, China). The glucose uptake was measured
using the Amplex Red Glucose/Glucose Oxidase Assay kit (cat. no.
A22189; Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA,
USA). The enhanced chemiluminescence (ECL) kit (cat. no. 32109) was
purchased from Guangzhou Baotaike Biological Company (Guangzhou,
China). X-ray film was from the Eastman Kodak Company (Rochester,
NY, USA).
Cell culture
HepG2 cells, which were frozen with liquid nitrogen,
were placed in a water bath at 37°C and then transferred to a
sterile tube (10 ml). Culture medium (3 ml) was added to cells and
evenly mixed. The pelleted cells were collected after
centrifugation at 1,000 × g for 5 min and were cultured with DMEM
(supplemented with 15% FBS). The culture medium was replaced the
following day. The recovered cells were grown in DMEM (10% FBS),
treated with 0.25% trypsin at an 80% confluency and then passaged
to a 1:3 ratio. The cells used in subsequent experiments were in
the logarithmic growth phase.
Cell experiments
HepG2 cells (1×105 cells/ml) were seeded
into 6-well tissue culture plates in the logarithmic growth phase
and cultured with DMEM (supplemented with 10% FBS). HepG2 cells
were cultured for 12 h at 37°C with 5% CO2, then the
medium was removed and replaced with medium containing 25 µM
ginsenoside Rb3 and/or 1 mM AICAR and/or 10 µM Compound C was
added. Total protein and nucleoprotein were extracted after a 24-h
incubation by cell lysis. The total protein was immunoblotted with
antibodies specific for AMPK, p-AMPK, PEPCK and G6Pase, while
nucleoprotein was immunoblotted with FOXO1 and HNF4α. This Western
blotting procedure is described in a subsequent section.
MTT assay
The cell viability of HepG2 cells was assessed using
an MTT assay following treatment in the absence or presence of
ginsenoside Rb3 (0, 3.125, 6.25, 12.5, 25 and 50 µM) for 24 h. The
cells were treated with MTT in accordance with the manufacturer's
protocol, and the supernatant was removed and DMSO added to
dissolve the blue crystals. The optical density (OD) values were
measured with a microplate reader at 490 nm, and then the cell
viability rate was calculated [cell viability rate(%)=treatment
group average OD/control group average ODx100]. Finally, the
ginsenoside Rb3 concentration that resulted in no damage to HepG2
cells was determined to be the optimum concentration for glucose
production and hepatic gluconeogenesis in the subsequent
experimental analysis.
Glucose production assay
According to the method reported previously
(14,15), the experiment was set up with three
treatment groups [0 (control), 12.5 and 25 µM ginsenoside Rb3] in
triplicate. The procedures were as follows: HepG2 cells were
cultured to the exponential phase; 1-ml cell suspensions in 24-well
plates were cultured with 10% FBS DMEM (5% CO2
concentration) at 37°C for 24 h; then, the medium was replaced with
the glucose production buffer solution (5% CO2
concentration) at 37°C for 4 h. Finally, the medium culture was
transferred into an Eppendorf tube, and glucose production from
HepG2 cells was measured with the Amplex Red Glucose/Glucose
Oxidase Assay kit.
Analysis of protein content
HepG2 cells were lysed with 4°C lysis buffer (50 mM
Tris-HCl, pH 7.4, 1% NP-40, 0.25% Na-deoxycholate, 150 mM NaCl, 1
mM EDTA) supplemented with a protease inhibitor (1 mM
phenylmethylsulfonyl fluoride) and phosphatase inhibitors (50 mM
NaF, 0.1 mM sodium vanadate, 10 mM sodium molybdate, 20 mM
3-glycerol phosphate, 10 mM 4-nitropyrophosphate). Protein
concentration was measured using the Bradford assay (cat. no.
5000002; Bio-Rad Laboratories Inc., Hercules, CA, USA). The
immunoblotting analysis was performed by western blot. Total
protein extracts (40 µg) were separated by 12% SDS-PAGE. The
polyvinylidene difluoride (Merck Millipore, Darmstadt, Germany)
protein hybridization membranes were first soaked with methanol for
15 sec to activate the membrane and then placed in the pre-cooled
transmembrane buffer. The fibrin glue and transmembrane device were
placed in the correct orientation, and then the proteins were
transferred to membranes at 100 V at 4°C for 2 h. The membranes
were then washed with Tris buffered saline-Tween (TBST) three times
for 10 min each. Next, the membranes were blocked with 5% non-fat
milk powder for 2 h. The primary and secondary antibody incubations
were performed with an antibody hybridization process using
standard hybridization techniques (18). The blocked membranes were incubated
overnight in TBST with the primary antibodies against AMPK (1:500),
p-AMPK (1:500), PEPCK (1:1,000), G6Pase (1:1,000), PGC-1α (1:500),
HNF4α (1:1,000), FOXO1 (1:1,000), and β-actin (1:2,000) at 4°C.
Membranes were then incubated and hybridized in TBST with
anti-rabbit IgG-HRP secondary antibodies (1:1,000) at 37°C for 1 h.
Subsequently, membranes were washed with TBST three times for 10
min each. Finally, the membranes were treated with ECL reagent and
X-ray film was used to obtain the hybrid picture. X-ray films were
scanned, and the results were analyzed with Quantity One software
v. 4.6 (Bio-Rad Laboratories, Inc.). The ratio of the target
protein expression to the reference protein expression was
calculated as relative expression, and different treatments were
compared with one-way analysis of variance.
Statistical analysis
Data are expressed as the mean ± standard error of
the mean. Significant differences from the control group were
determined by Student's t-test. All statistics were calculated with
SPSS statistical software (version 13.0; SPSS, Inc., Chicago, IL,
USA). P<0.05 was considered to indicate a statistically
significant difference.
Results
Effect of ginsenoside Rb3 on cell
viability of HepG2 cells
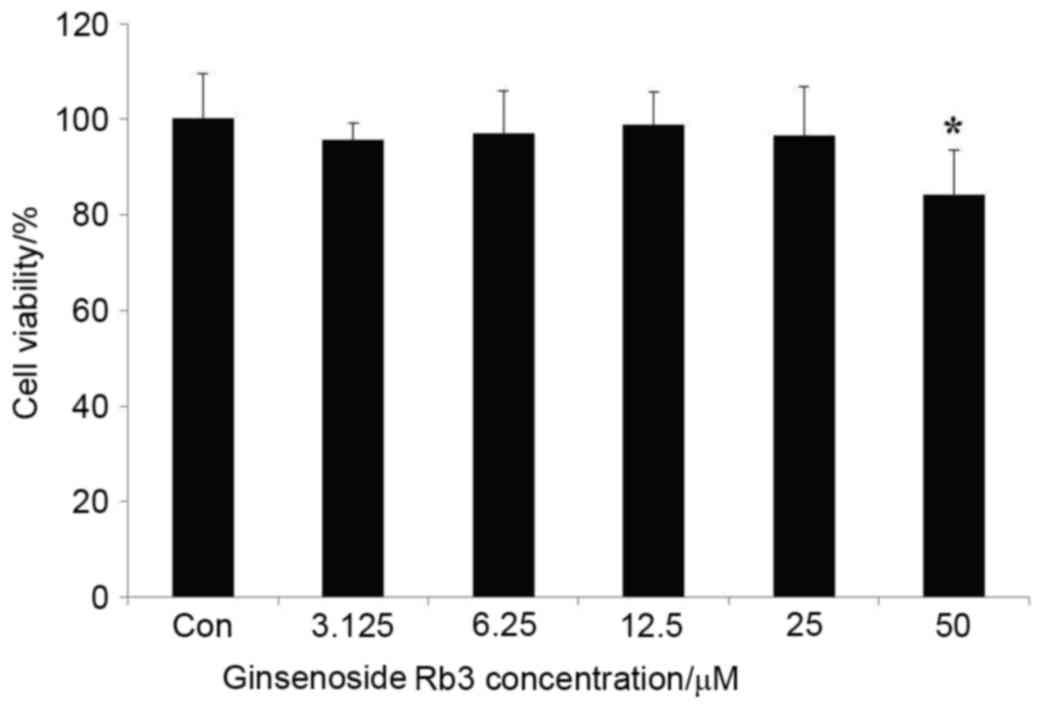
Fig. 1. indicates
that 3.125, 6.25, 12.5, and 25 µM ginsenoside Rb3 treatments caused
limited damage to HepG2 cells after ginsenoside Rb3 incubation for
24 h; however, 50 µM ginsenoside Rb3 led to significant damage to
HepG2 cells, as indicated by a significant reduction in cell
viability (P<0.05). With use of the latter dose, the cells
exhibited blebbing and apoptosis. Therefore, 12.5 and 25 µM
ginsenoside Rb3 were determined to be suitable concentrations for
further experiments.
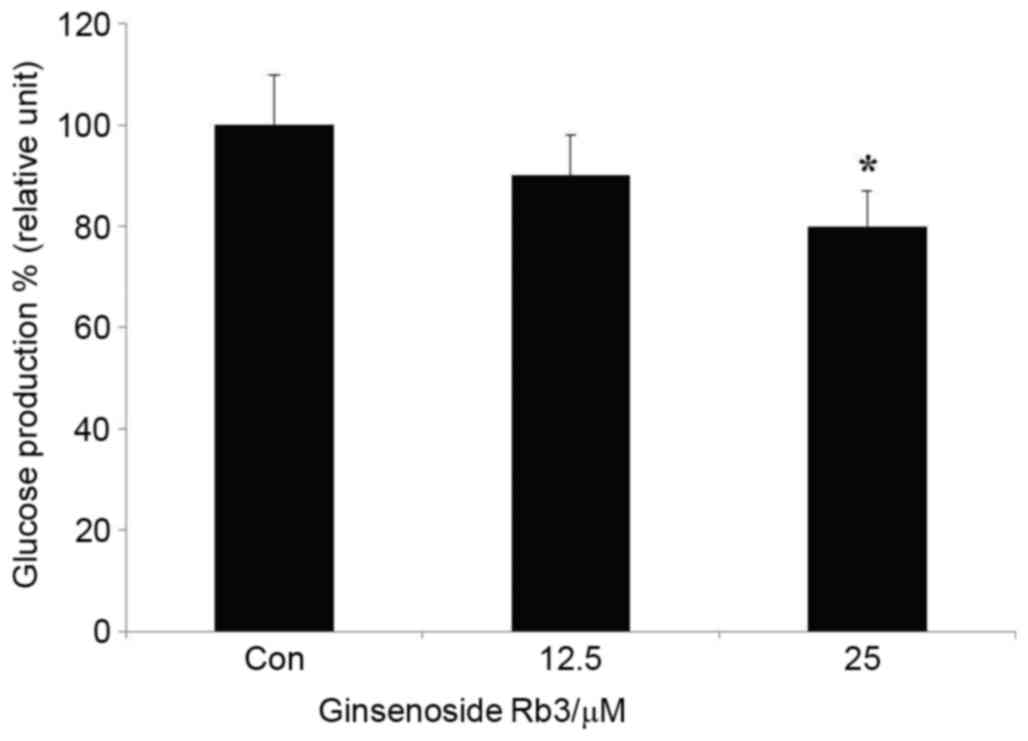
Effect of ginsenoside Rb3 on glucose
production of HepG2 cells
Subsequent to the determination of a suitable
ginsenoside Rb3 dose, the HepG2 cells were rinsed with PBS,
dissociated with protease, and then the protein was extracted.
Finally, the measured glucose content was compared with the
extracted total protein content on the basis of quantitative
protein, so that the ratio could more accurately reflect the
inhibitory effect of ginsenoside Rb3 on the glucose production
ability of HepG2 cells. Fig. 2
indicates that both doses of ginsenoside Rb3 had an inhibitory
effect on gluconeogenesis in HepG2 cells. However, only 25 µM
ginsenoside Rb3 significantly inhibited gluconeogenesis
(P<0.05).
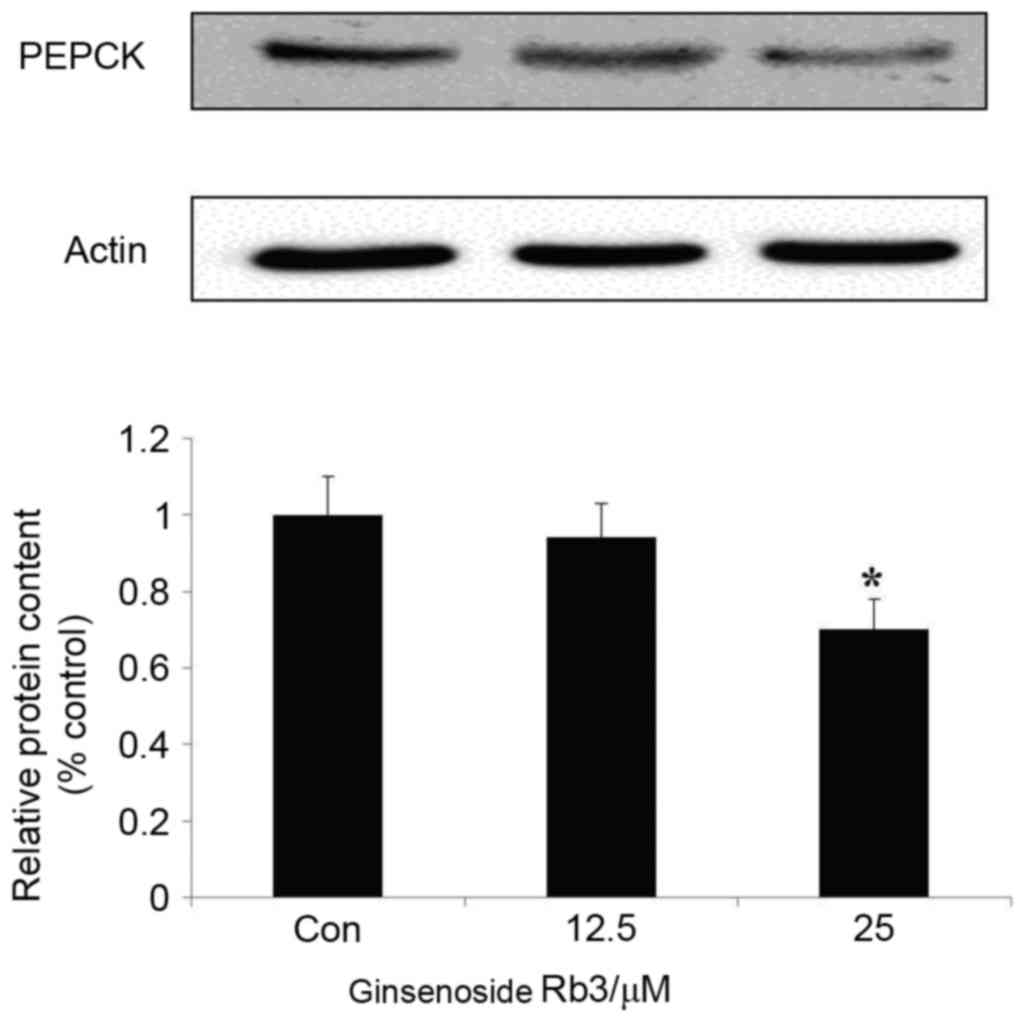
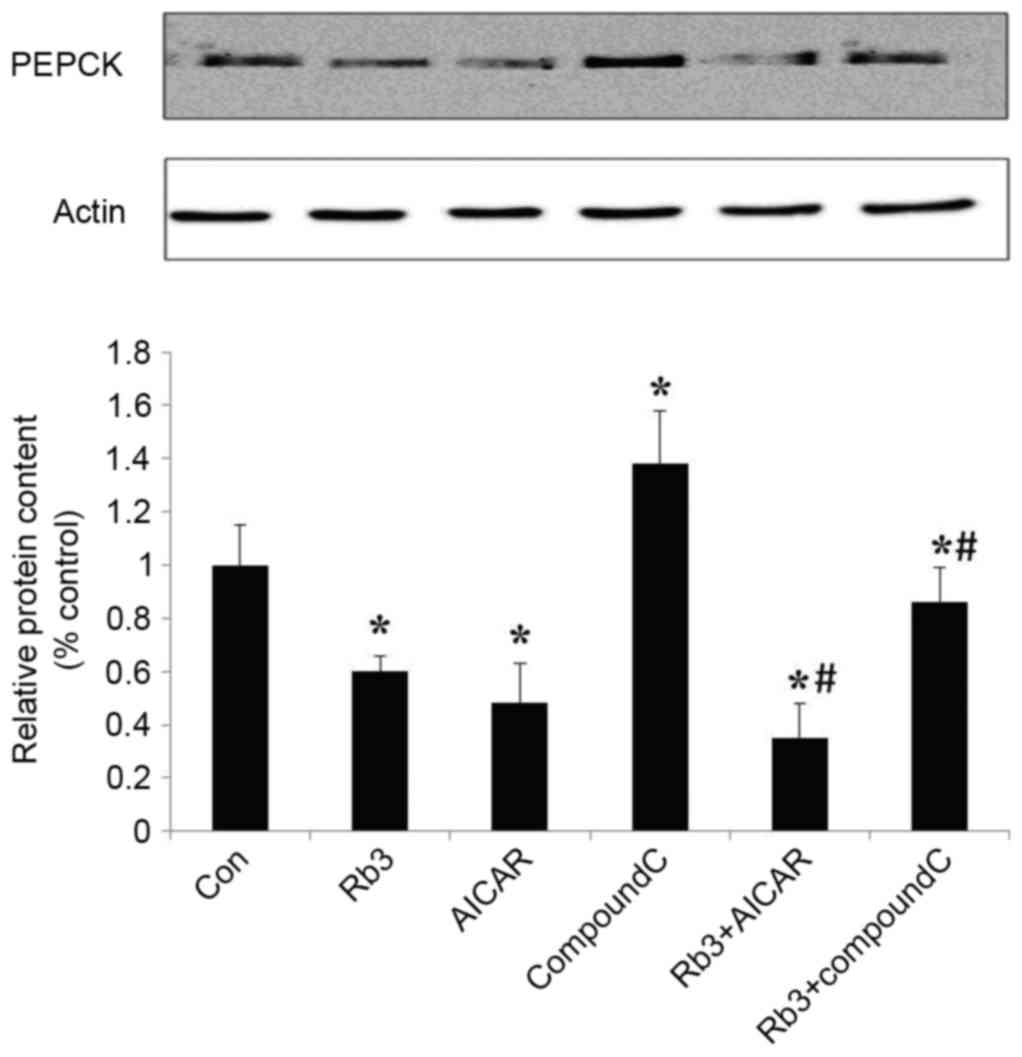
Effect of ginsenoside Rb3 on PEPCK
expression
To further investigate the molecular mechanism of
ginsenoside Rb3 on gluconeogenesis, expression levels of PEPCK, a
key enzyme of the gluconeogenesis pathway, were analyzed by western
blot analysis. Fig. 3 indicates that
treatment with ginsenoside Rb3 after 24 h inhibited the expression
of PEPCK. Inhibition of PEPCK expression by 25 µM ginsenoside Rb3
treatment reached a significant level (P<0.05), which may be
consistent with the inhibitory effect on glucose production of
HepG2 cells.
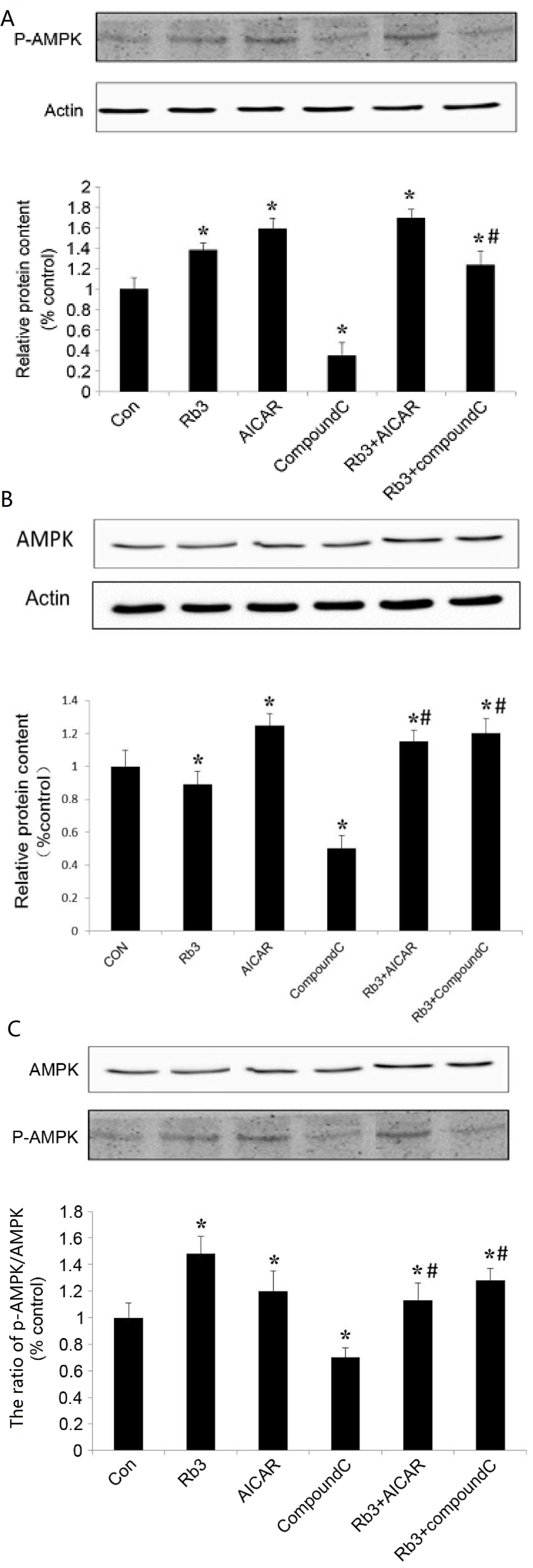
Effect of ginsenoside Rb3 on the
expression levels of AMPK and p-AMPK in HepG2 cells
AMPK is an attractive target that serves a key role
in the regulation of energy homeostasis at the whole body level
(9). Activation of hepatic AMPK
leads to increased fatty acid oxidation and simultaneously inhibits
hepatic glucose production, in addition to lipogenesis and
cholesterol synthesis (10). To
further investigate the regulation of gluconeogenesis and AMPK
expression by ginsenoside Rb3, analysis with AICAR (AMPK agonists)
and Compound C (AMPK inhibitors) was performed. In the experiment,
6 groups were used: i) Control group, an untreated group, ii) 25 µM
ginsenoside Rb3 group, iii) 1 mM AICAR group, iv) 10 µM Compound C
group, v) 25 µM ginsenoside Rb3+1 mM AICAR group, and vi) 25 µM
ginsenoside Rb3+10 µM Compound group.
The results show that ginsenoside Rb3 and AICAR
activated AMPK, and that the expression levels of p-AMPK
significantly increased (P<0.05; Fig.
4B). In addition, ginsenoside Rb3 and AICAR demonstrated a
synergistic effect on p-AMPK expression levels compared with each
group alone (P<0.05), whilst the p-AMPK expression levels
following treatment with a combination of the two treatments was
significantly higher than that of either alone (P<0.05).
Compound C, an AMPK inhibitor, was able to
significantly inhibit expression of total AMPK and activated p-AMPK
in HepG2 cells (P<0.05; Fig. 4A and
B), and the ratio of p-AMPK to total AMPK was significantly
reduced (Fig. 4C). The results
indicated that Compound C had a marked inhibitory effect on p-AMPK
expression. In the group in which ginsenoside Rb3 and Compound C
were used at the same time, the expression of p-AMPK is higher than
that of Compound C treatment alone (P<0.05). In particular, the
ratio of p-AMPK to AMPK was significantly higher compared with that
following the use of Compound C alone. Therefore, ginsenoside Rb3
significantly increased the expression and activation of AMPK
(P<0.05), which was likely associated with the mechanism causing
the partial reduction of the inhibitory effect by Compound C on
AMPK expression. In addition, the combined use of ginsenoside Rb3
and Compound C caused the expression of p-AMPK and the ratio of
p-AMPK/total AMPK to be significantly lower than that of the
ginsenoside Rb3 single treatment (P<0.01), which indicated that
Compound C could partly block the effects of ginsenoside Rb3 on
AMPK activation.
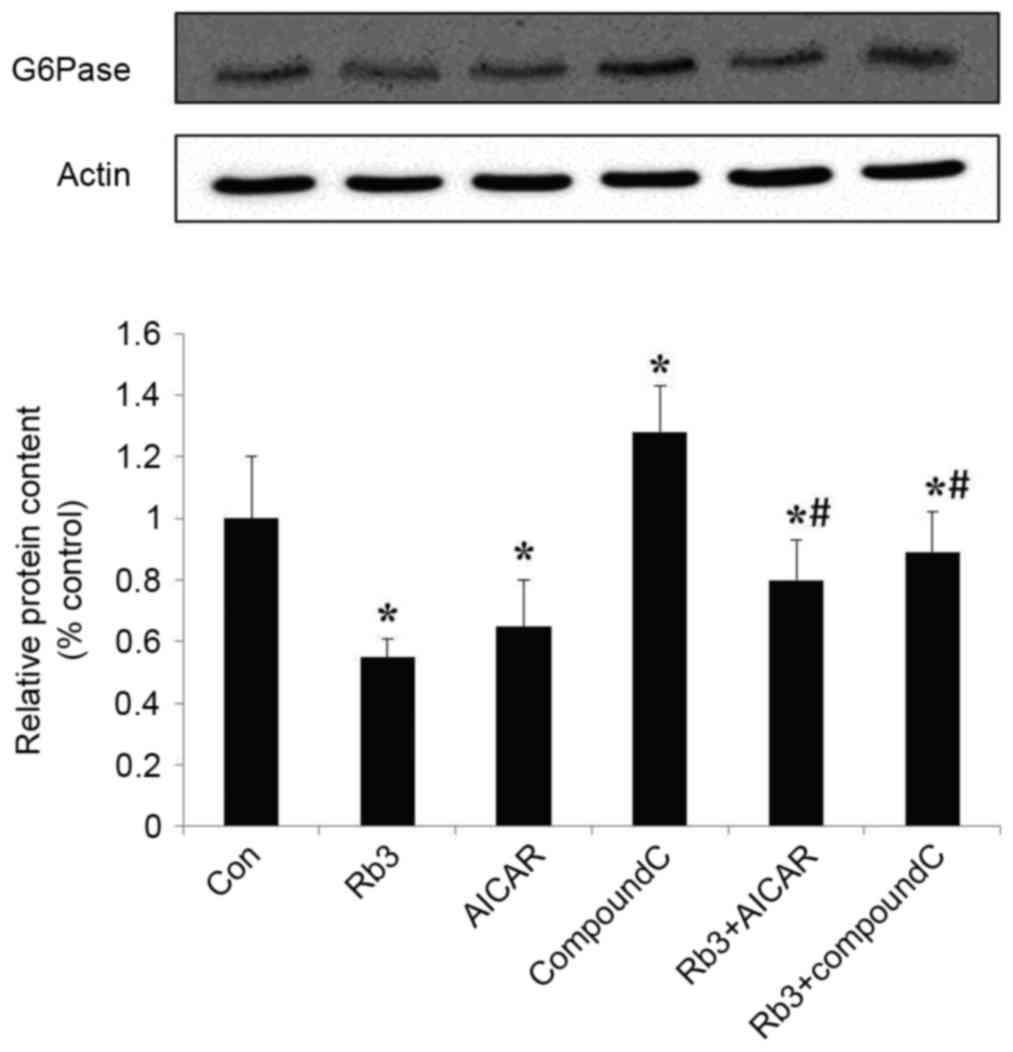
Effect of ginsenoside Rb3 on the
expression of PEPCK and G6Pase
The expression levels of PEPCK and G6Pase, which are
the key gluconeogenic enzymes, were analyzed by western blot
analysis in HepG2 cells. These data indicated that ginsenoside Rb3,
AICAR (an AMPK activator) and a combinatorial treatment with these
significantly inhibited the expression of G6Pase (Fig. 5) and PEPCK (Fig. 6) in comparison with the control group
(P<0.05). The expression levels of PEPCK and G6Pase were
significantly increased following the use of Compound C
(P<0.05). In addition, the expression levels of PEPCK and G6Pase
were significantly lower in the control group compared with the
combination of ginsenoside Rb3 and Compound C (P<0.05); however,
the expression levels of PEPCK and G6Pase following a single
treatment with Compound C were significantly higher compared with
those subsequent to treatment with ginsenoside Rb3 only
(P<0.05), which indicates that the inhibitory effect of
ginsenoside Rb3 on PEPCK and G6Pase is partially blocked by
Compound C. Overall, the inhibition of gluconeogenesis by
ginsenoside Rb3 was demonstrated to be associated with AMPK
activation.
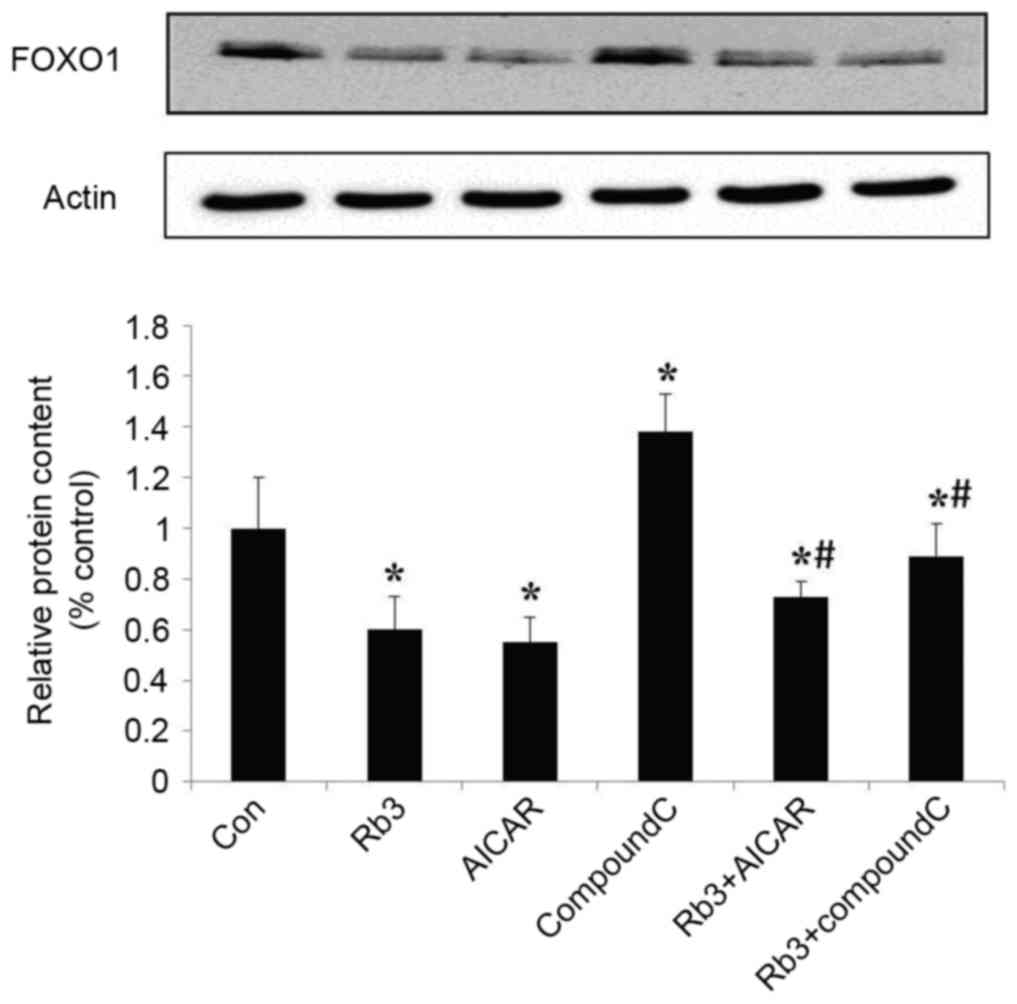
Effect of ginsenoside Rb3 on
gluconeogenesis nuclear transcription factors in HepG2 cells
Transcription factors are crucial in the regulation
of eukaryotic gene expression (9).
To further explore the molecular mechanism underlying the effect of
ginsenoside Rb3 on gluconeogenesis, the expression levels of FOXO1
and HNF4α, two transcription factors, were analyzed by protein
hybridization (Figs. 7 and 8). The expression levels of FOXO1 were
assessed by western blot analysis (Fig.
7). Ginsenoside Rb3 and AICAR had inhibitory effects on the
expression levels of FOXO1, and a combination of the two had a
significant inhibitory effect on the expression levels of FOXO1
(P<0.05). The expression of transcription factor FOXO1 was
significantly lower when both were combined, compared with the use
of either alone (P<0.05). Notably, although AICAR did not
enhance the inhibitory effects of ginsenoside Rb3 on the expression
of FOXO1, the expression levels of FOXO1 were significantly higher
when Compound C and ginsenoside Rb3 were combined, compared with
the use of ginsenoside Rb3 alone (P<0.05). Compound C lessened
the inhibitory effect of ginsenoside Rb3 on FOXO1 expression.
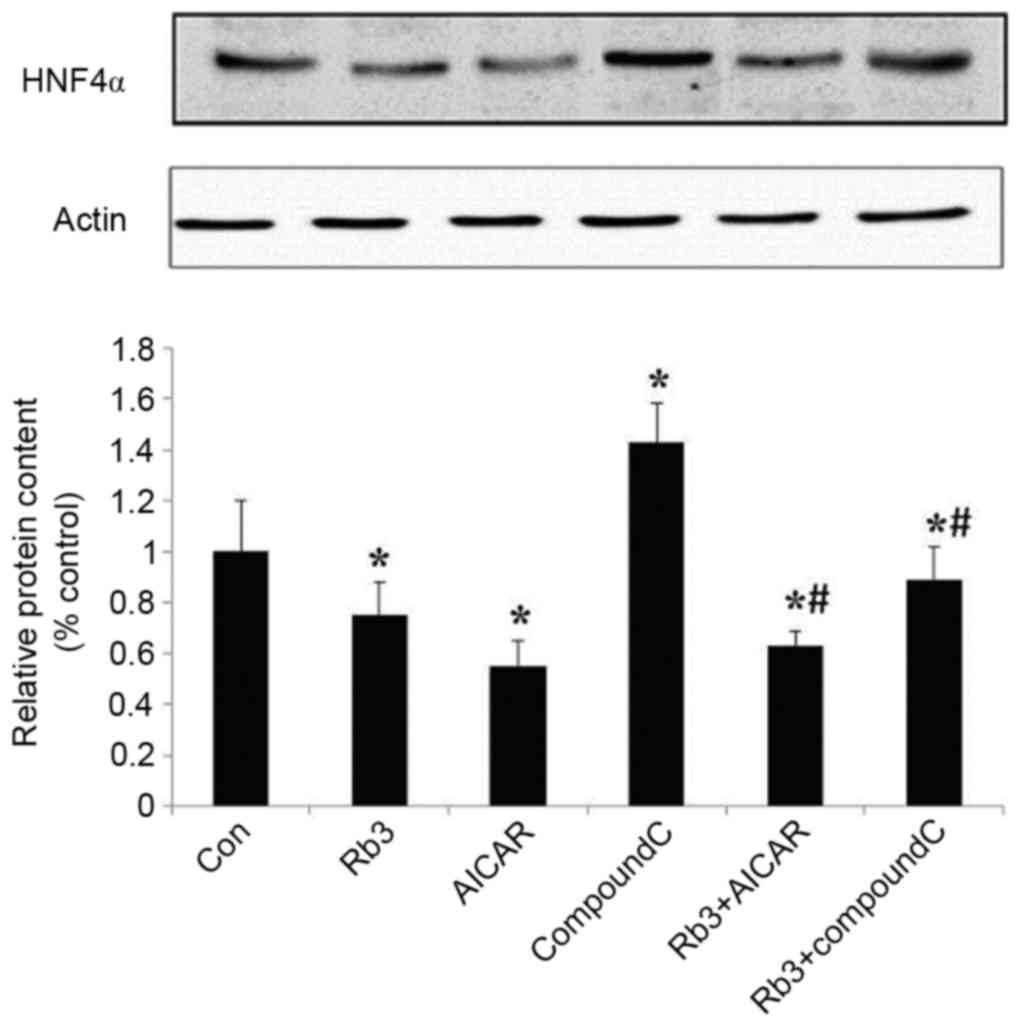
The expression levels of HNF4α were analyzed
(Fig. 8) and revealed that the
expression of HNF4α was significantly inhibited by ginsenoside Rb3
and AICAR alone, or by their combination (P<0.05), and that the
expression levels of HNF4α were significantly lower when both were
combined, compared with the use of ginsenoside Rb3 alone
(P<0.05). HNF4α was markedly (but not significantly) higher
compared with the use of AICAR alone, and Compound C significantly
reduced the inhibitory effect of ginsenoside Rb3 on HNF4α
expression levels (P<0.05).
Discussion
Previous reports indicated that ginsenoside Rb3
exerted antidiabetic activity in alloxan-induced diabetic mice, and
increased glucose consumption in C2C12 myotubes (16,19). In
addition, our previous study demonstrated that ginsenoside Rb3
decreases blood glucose, increases blood glucose tolerance and
antioxidants, improves serum lipid disorders and improves insulin
sensitivity and resistance in diabetic mice (17). Numerous animal studies and clinical
trials have ascertained that ginsenoside Rb3 has a significant
hypoglycemic effect (15–17). To analyze the regulatory mechanism
underlying the effect of ginsenoside Rb3 on AMPK, the present study
used AMPK inhibitor Compound C (20–22) and
AMPK activator AICAR (23–26).
The HepG2 cell line is a human hepatocellular
carcinoma cell line, and has several of the biological
characteristics of normal liver cells. It is commonly used to
conduct experiments regarding hepatic gluconeogenesis (27,28).
During these experiments, the level of glucose synthesis can be
measured in HepG2 cells to reflect gluconeogenesis and its effect
on the treatment of type 2 diabetes mellitus. Therefore, the HepG2
cell line is often used for drug-carrier screenings for diabetes
treatment, and allows for thorough investigation of the
hypoglycemic effect (27,28).
Previous results obtained using various animal
models of type 2 diabetes confirm the physiological importance of
hepatic AMPK in glucose homeostasis (9). The AMPK pathway has been reported to
regulate the phosphorylation and nuclear exclusion of
CREB-regulated transcription coactivator 2 (TORC2) (29). In response to fasting stimuli, TORC2
is dephosphorylated and transported from the cytoplasm to the
nucleus, where it enhances the transcriptional activation of
gluconeogenic genes. The transcriptional coactivator mediates
CREB-dependent transcription (PGC-1α). Expression of the
coactivator PGC-1α further induces the transcription of key
gluconeogenic enzymes such as PEPCK and G6Pase in association with
the factors HNF4α and FOXO1. AMPK is able to phosphorylate TORC2
and lead to the inhibition of gluconeogenesis in the cytoplasm
(18,30).
The current results show that ginsenoside Rb3 and
AICAR influence the activity of AMPK in HepG2 cells, in addition to
PEPCK and G6Pase, which are key rate-limiting enzymes in the
pathway of gluconeogenesis, and had significant inhibitory effects
when used in combination. This may be due to the ability of
ginsenoside Rb3 to inhibit gluconeogenesis. In addition, the
present study also revealed that the combined use of ginsenoside
Rb3 and AICAR dramatically enhanced the inhibition of PEPCK, and
showed strong synergistic effects. Compound C could effectively
block the inhibitory effect of ginsenoside Rb3 on PEPCK and G6Pase,
which indicated that AMPK was an important target site of action of
ginsenoside Rb3. In addition, FOXO1 and HNF4α, two key nuclear
transcription factors in the gluconeogenic pathway, and analysis of
the expression levels performed in the present study indicated that
ginsenoside Rb3 had significant inhibitory effects on their
expression, and that Compound C could partially block this
inhibitory effect. The current study therefore suggests that the
inhibitory effect of ginsenoside Rb3 on these two transcription
factors may be associated with the activation of AMPK. In the
regulation of HNF4α protein expression, the use of a combination of
the two drugs also demonstrated a reduced inhibitory effect of
AICAR on HNF4α expression. Therefore, ginsenoside Rb3 and AICAR
both disrupt the regulation of certain transcription factors. These
results suggest that ginsenoside Rb3 had a significant inhibitory
effect on gluconeogenesis, which is associated, at least partly,
with the activation of AMPK and its downstream signaling pathway in
HepG2 cells.
Several antidiabetic drugs, such as metformin,
regulate blood glucose by transcriptional inhibition of the
gluconeogenic program (31,32). The glucose-lowering effect of
metformin has been predominantly attributed to its ability to
suppress hepatic gluconeogenesis through the AMPK signaling pathway
(9). Additional studies are required
to compare the use of ginsenoside Rb3 with that of metformin.
In conclusion, the current results provide further
insight into the mechanism of hepatic gluconeogenesis and its
modulation by ginsenoside Rb3 in HepG2 cells in vitro.
Furthermore, ginsenoside Rb3 suppresses hepatic gluconeogenesis, at
least in part, by activating AMPK. These data support the
hypothesis that preparations of ginsenoside Rb3 have potential to
prevent and treat type 2 diabetes.
Acknowledgements
The present study was supported by the Jilin Science
and Technology Development Plan (grant no. 20060902).
Glossary
Abbreviations
Abbreviations:
|
AMPK
|
adenosine monophosphate-activated
protein kinase
|
|
PEPCK
|
phosphoenolpyruvate carboxykinase
|
|
G6Pase
|
glucose-6-phosphatase
|
|
FOXO1
|
forkhead transcription factor 1
|
|
HNF4α
|
hepatic nuclear receptor 4α
|
References
|
1
|
Biadgo B, Melku M, Abebe SM and Abebe M:
Hematological indices and their correlation with fasting blood
glucose level and anthropometric measurements in type 2 diabetes
mellitus patients in Gondar, Northwest Ethiopia. Diabetes Metab
Syndr Obes. 9:91–99. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Budinsky A, Wolfram R, Oguogho A,
Efthimiou Y, Stamatopoulos Y and Sinzinger H: Regular ingestion of
opuntia robusta lowers oxidation injury. Prostaglandins Leukot
Essent Fatty Acids. 65:45–50. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Li Q, Zhou JP and Zhang HB: Research
progresses in anti-diabetic drugs. Progress in Pharmaceutical
Sciences. 37:417–427. 2013.
|
|
4
|
Lai Yu, Chai Dandan, Niu Rui and Xiaodong
S: Study the mechanism of potentilla discolor Bunge extract on
blood sugar in diabetic rats. Aisa-Pacific Traditional Medicine.
12:17–19. 2016.
|
|
5
|
Wan Y, Wu J and Wu Q: A review of the
hypoglycemic activity of Siraitia Grosvenorii. Food Research and
Development. 37:188–191. 2016.
|
|
6
|
Zhang M and Shen Y: Research advances in
pharmacological effects of oleanolic acid in hypoglycemia and
antidiabetic complications. Anti-infection Pharmacy. 12:801–806.
2015.
|
|
7
|
Ryu GR, Lee MK, Lee E, Ko SH, Ahn YB, Kim
JW, Yoon KH and Song KH: Activation of AMP-activated protein kinase
mediates acute and severe hypoxic injury to pancreatic beta cells.
Biochem Biophys Res Commun. 386:356–362. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Magnoni LJ, Vraskou Y, Palstra AP and
Planas JV: AMP-activated protein kinase plays an important
evolutionary conserved role in the regulation of glucose metabolism
in fish skeletal muscle cells. PLoS One. 7:e312192012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Viollet B, Guigas B, Leclerc J, Hébrard S,
Lantier L, Mounier R, Andreelli F and Foretz M: AMP-activated
protein kinase in the regulation of hepatic energy metabolism: From
physiology to therapeutic perspectives. Acta Physiol (Oxf).
196:81–98. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Koo SH, Flechner L, Qi L, Zhang X,
Screaton RA, Jeffries S, Hedrick S, Xu W, Boussouar F, Brindle P,
et al: The CREB coactivator TORC2 is a key regulator of fasting
glucose metabolism. Nature. 437:1109–1111. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shi Y, Han B, Yu X, Qu S and Sui D:
Ginsenoside Rb3 ameliorates myocardial ischemia-reperfusion injury
in rats. Pharm Biol. 49:900–906. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cui J, Jiang L and Xiang H: Ginsenoside
Rb3 exerts antidepressant-like effects in several animal models. J
Psychopharmacol. 26:697–713. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liu X, Jiang Y, Yu X, Fu W, Zhang H and
Sui D: Ginsenoside-Rb3 protects the myocardium from
ischemia-reperfusion injury via the inhibition of apoptosis in
rats. Exp Ther Med. 8:1751–1756. 2014.PubMed/NCBI
|
|
14
|
Wang T, Yu X, Qu S, Xu H, Han B and Sui D:
Effect of ginsenoside Rb3 on myocardial injury and heart function
impairment induced by isoproterenol in rats. Eur J Pharmacol.
636:121–125. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Rui L, Yi-Nan Z and Wen-Cong L: Inhibitory
activity of ginsenoside Rb3 on pancreatic lipase. J Xidian Univ.
23:522–525. 2011.
|
|
16
|
Bu QT, Zhang WY, Chen QC, Zhang CZ, Gong
XJ, Liu WC, Li W and Zheng YN: Anti-diabetic effect of ginsenoside
Rb(3) in alloxan-induced diabetic mice. Med Chem. 8:934–941. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Meng FL, Su XT and Zheng YN: Effects of
Ginsenoside Rb3 on antihyperglycemia and antioxidation in diabetic
mice. J South Chin Agr Univ. 34:553–557. 2013.
|
|
18
|
Wei S, Li W, Yu Y, Yao F, A L, Lan X, Guan
F, Zhang M and Chen L: Ginsenoside Compound K suppresses the
hepatic gluconeogenesis via activating adenosine-5′monophosphate
kinase: A study in vitro and in vivo. Life Sci. 139:8–15. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lee MS, Hwang JT, Kim SH, Yoon S, Kim MS,
Yang HJ and Kwon DY: Gimenoside Rc, an active component of Panax
ginseng, stimulates glucose uptake in C2C12 myotubes through an
AMPK-dependent mechanism. J Ethnopharmacol. 127:771–776. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jin J, Mullen TD, Hou Q, Bielawski J,
Bielawska A, Zhang X, Obeid LM, Hannun YA and Hsu YT: AMPK
inhibitor Compound C stimulates ceramide production and promotes
Bax redistribution and apoptosis in MCF7 breast carcinoma cells. J
Lipid Res. 50:2389–2397. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kim YM, Kim MY, Kim HJ, Roh GS, Ko GH, Seo
HG, Lee JH and Chang KC: Compound C independent of AMPK inhibits
ICAM-1 and VCAM-1 expression in inflammatory stimulants-activated
endothelial cells in vitro and in vivo. Atherosclerosis. 219:57–64.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Vucicevic L, Misirkic M, Janjetovic K,
Vilimanovich U, Sudar E, Isenovic E, Prica M, Harhaji-Trajkovic L,
Kravic-Stevovic T, Bumbasirevic V and Trajkovic V: Compound C
induces protective autophagy in cancer cells through AMPK
inhibition-independent blockade of Akt/mTOR pathway. Autophagy.
7:40–50. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Martinez-Martin N, Blas-García A, Morales
JM, Marti-Cabrera M, Monleón D and Apostolova N: Metabolomics of
the effect of AMPK activation by AICAR on human umbilical vein
endothelial cells. Int J Mol Med. 29:88–94. 2012.PubMed/NCBI
|
|
24
|
Lee H, Kang R, Bae S and Yoon Y: AICAR, an
activator of AMPK, inhibits adipogenesis via the WNT/β-catenin
pathway in 3T3-L1 adipocytes. Int J Mol Med. 28:65–71.
2011.PubMed/NCBI
|
|
25
|
Guo D, Hildebrandt IJ, Prins RM, Soto H,
Mazzotta MM, Dang J, Czernin J, Shyy JY, Watson AD, Phelps M, et
al: The AMPK agonist AICAR inhibits the growth of
EGFRvIII-expressing glioblastomas by inhibiting lipogenesis. Proc
Natl Acad Sci USA. 106:12932–12937. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Langelueddecke C, Jakab M, Ketterl N,
Lehner L, Hufnagl C, Schmidt S, Geibel JP, Fuerst J and Ritter M:
Effect of the AMP-kinase modulators AICAR, metformin and compound C
on insulin secretion of INS-1E rat insulinoma cells under standard
cell culture conditions. Cell Physiol Biochem. 29:75–86. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Fei F, Xin-rong W, Ming L and Huan L:
Effect of bioactive components in mulberry leaves on glucose
metabolism in insulinresistant HepG2 cells. J Guang Pharm Col.
27:637–639. 2011.
|
|
28
|
Li XL, He SM, Zhu Y, Feng B, Huang XQ,
Chen T and Zheng GJ: Establishment and identify of HepG2 cells
model of insulin resistance. Chin J Exp Trad Med Formul.
19:203–207. 2013.
|
|
29
|
Shaw RJ, Lamia KA, Vasquez D, Koo SH,
Bardeesy N, Depinho RA, Montminy M and Cantley LC: The kinase LKB1
mediates glucose homeostasis in liver and therapeutic effects of
metformin. Science. 310:1642–1646. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lin J, Tarr PT, Yang R, Rhee J, Puigserver
P, Newgard CB and Spiegelman BM: PGC-1beta in the regulation of
hepatic glucose and energy metabolism. J Biol Chem.
278:30843–30848. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
He L, Sabet A, Djedjos S, Miller R, Sun X,
Hussain MA and Radovick S: Metformin and insulin suppress hepatic
gluconeogenesis through phosphorylation of CREB binding protein.
Cell. 137:635–646. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Caton PW, Kieswich J, Yaqoob MM, Holness
MJ and Sugden MC: Metformin opposes impaired AMPK and SIRT1
function and deleterious changes in core clock protein expression
in white adipose tissue of genetically-obese db/db mice. Diabetes
Obes Metab. 13:1097–1104. 2011. View Article : Google Scholar : PubMed/NCBI
|