Introduction
Stem cells are cells that possess self-renewal
capacity and multi-directional differentiation potential in human
body. Stem cells are classified by the stage of development into
embryonic stem cells (ES) and adult stem cells (ASCs). The
differentiation and proliferation of ES is the basis for the
development of an organism, namely the development of fertilized
eggs into individuals with various tissues and organs. Given that
isolation of ES causes damage to the early embryo, this method is
not advocated (1). ASC are
classified into hematopoietic stem cells and mesenchymal stem cells
(MSC) (2). The study of
hematopoietic stem cell started in the 1950s. Hematopoietic stem
cells mainly originate from bone marrow, adult peripheral blood and
umbilical cord blood. Since hematopoietic stem cells can
differentiate into mature blood cells (erythrocyte, leukocyte and
platelet), they have been widely clinically used in the treatment
of hematological diseases (3). In
recent years it was found that MSCs also exist in the peripheral
blood and umbilical cord blood (4).
MSCs can self-proliferate and differentiate into a variety of
mature cells, including osteoblast, chondrocyte, adipose cell,
myocyte, cardiomyocyte, hepatocyte, neuron, melanocyte, desmocyte
and epidermal cells (5–8).
Since the 21st century, MSCs have been used to treat
some special diseases related to cell destruction such as
neurodegeneration, spinal cord injury, osteoporosis, cardiovascular
disease, diabetes, cirrhosis and skin reconstruction. The stem-cell
therapy discussed in this study is also focusing on this aspect.
Autologous bone marrow was initially the major source of MSCs, and
the source has expanded to allogeneic umbilical cord blood in
recent years (9–11).
Based on existing studies, this investigation aimed
at exploring the molecular control of stem cell differentiation and
regulation, and searching for a breakthrough in the directional
induced differentiation of stem cells into cellular components of
skin tissue such as desmocyte, melanocyte, hair follicle, sebaceous
glands and epidermis. Moreover, it is expected that this
investigation can provide important theoretical evidence for the
treatment of various skin diseases or cosmetic dermatology such as
the treatment of melanocyte transplantation for vitiligo, the
restoration of damaged skin or scar, the removal of facial
wrinkles, breast implants and breast reconstruction.
Materials and methods
Materials
Cells: Human umbilical cord blood MSCs: Cyagen
Biosciences Inc., Guangzhou, China (art. no. HUXUB-01001).
Reagents: Dulbecco's modified Eagle's medium (DMEM) (HyClone,
Logan, UT, USA); culture medium for human umbilical cord blood stem
cells: Cyagen Biosciences Inc. (art. no. HUXUB-90011); fetal bovine
serum (Gibco, Grand Island, NY, USA); 10 mg/l of insulin, 10 mg/l
of hydrocortisone, 10 mg/l of glutamine (Sigma, St. Louis, MO,
USA); 0.25% of trypsin (Jrdun Biotechnology Corp., New York, NY,
USA); antibodies for flow cytometry: FITC anti-mouse/human CD44
antibody (art. no. 103021; BioLegend, Inc., San Diego, CA, USA); PE
anti-human CD29 antibody (art. no. 303003; BioLegend, Inc.).
Preparation of solution
Phosphate-buffered saline (PBS) solution: 8 g of
NaCl, 0.2 g of KCl, 1.44 g of Na2HPO4 and
0.24 g of KH2PO4 were dissolved in 600 ml of
ddH2O; HCl was used to adjust the pH to 7.4 and the
final volume was brought to 1 liter with ddH2O; the
solution was filtered, autoclaved and stored at room
temperature.
Instruments and consumable
supplies
CO2 incubator (Thermo Fisher Scientific,
Waltham, MA, USA); biosafety cabinet (Suzhou Jinjing Purifying
Equipment Technology Co., Ltd., Suzhou, China); pipette (Eppendorf
AG, Hamburg, Germany); microscope (Shanghai Caikang Optical
Instrument Co., Ltd., Shanghai, China); consumable supplies for
cell culture (Corning Inc., Acton, MA, USA).
Isolation and culture of rat hair
follicles
The skin in the whisker region of male Wistar rat
was acquired under sterile conditions and cleaned several times in
PBS containing 100 U/l of penicillin and 100 mg/l of streptomycin.
Ophthalmic scissors were used to cut through dermis subcutaneously.
Microsurgical forceps were used to make blunt dissection of hair
follicle and peripheral tissues. The hair follicle was carefully
drawn from subcutaneous tissue without damaging the tissues such as
hair papilla, and dermal sheath. The adipose tissue around the hair
follicle was removed. The complete hair follicles were selected
using a microscope. The selected hair follicles were placed in
24-well culture plate, and each well contained one hair follicle.
With the addition of 0.5 ml of DMEM culture medium (10% of fetal
bovine serum, 10 mg/l of insulin, 10 µg/l of hydrocortisone, 2
mmol/l of glutamine, 100 U/l of penicillin and 100 mg/l of
streptomycin), the culture was carried out in 5% CO2
incubator at 37°C.
Preparation of induction medium
After the first day of hair follicle organ culture,
supernatant was collected every day by means of 10-min
centrifugation at 150 × g. The supernatant was then sterilized
through a 0.22 µm filter and stored at 4°C.
Induced differentiation of human
umbilical cord blood MSCs
Human umbilical cord blood MSCs were placed in
6-well culture plate. Filtered induction medium was added after the
stem cells completely fused for two days. The culture was carried
out in 5% CO2 incubator at 37°C. Induction medium was
changed every 3 days, and the induction lasted for 21 days. In
control group, culture medium for human umbilical cord blood MSCs
was used for cell culture.
Immunophenotypic analysis of umbilical
cord blood stem cells
Cell suspension of umbilical cord blood MSCs
containing 1×109 cells/l was prepared and fluorescent
labeled mouse anti-human antibodies FITC-CD29 and FITC-CD44 were
added to the cells. One test tube of cell suspension without added
antibodies was used as blank control. Flow cytometry was used to
detect the expression of cell surface antigen. The results were
analyzed by Image-Pro Plus software (version X; Media Cybernetics,
Silver Springs, MD, USA) to calculate the percentage of cells for
each surface marker.
RT-PCR method
Cells were placed in a 1 ml TRIzol® to
isolate RNA which was stored at −80°C after purification. The PCR
amplification of K15 was done with forward primer sequence:
5′-TTAGCCCTCCACCATTAC-3′; and reverse primer sequence:
5′-TAACTCCACCTCGTTCAG-3′. To amplify GAPDH cDNA, the forward primer
sequence: 5′-CACCCACTCCTCCACCTTTG-3′; and the reverse primer
sequence: 5′-CCACCACCCTGTTGCTGTAG-3′ were used.
Results
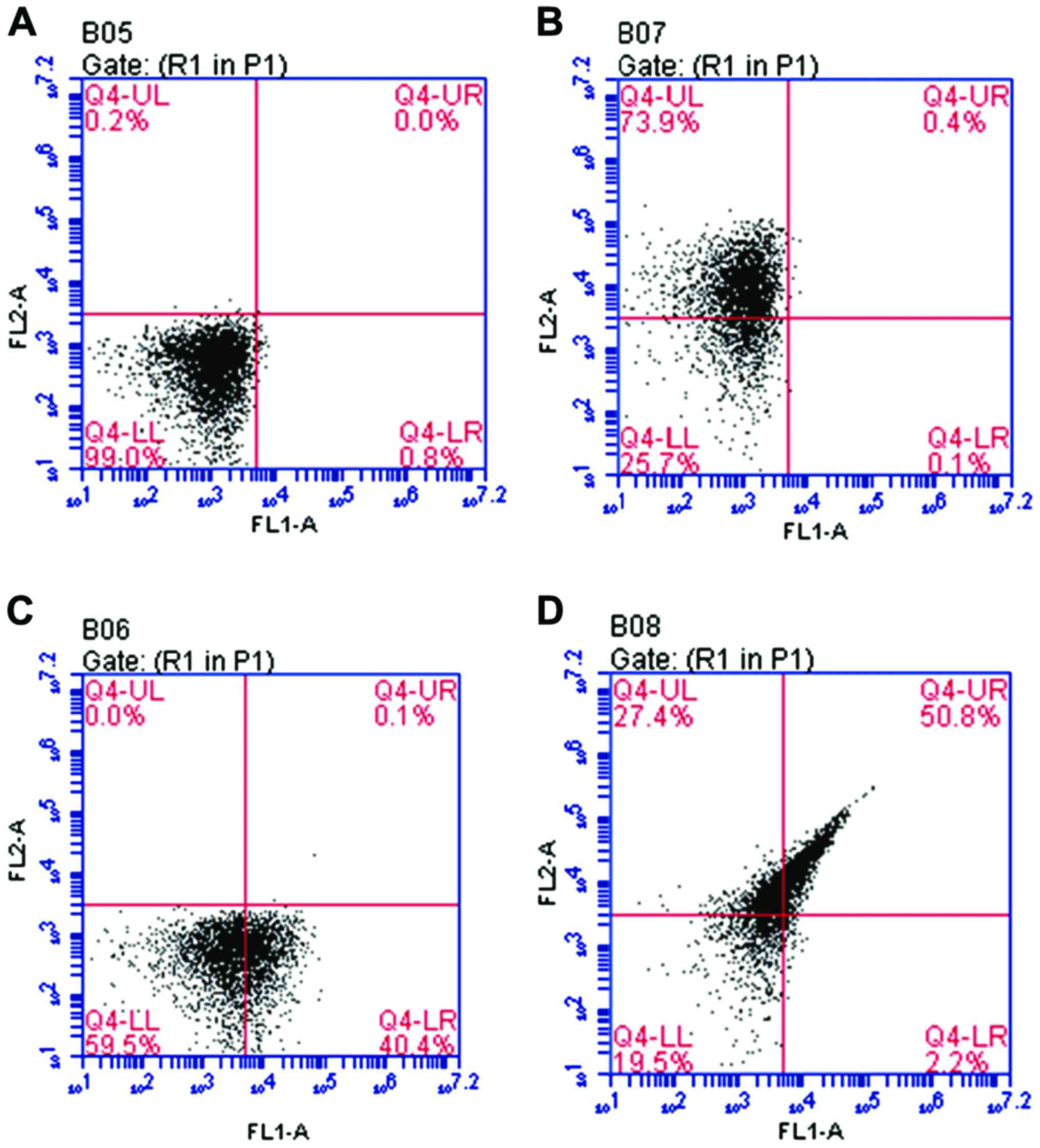
Detection and characterization of
umbilical cord blood MSCs using flow cytometry
Flow cytometry was used to isolate and purify the
samples. Results indicated that CD44+CD29+
double-labeled cells accounted for 50.8% of all the samples of
umbilical cord blood MSCs as shown in Fig. 1.
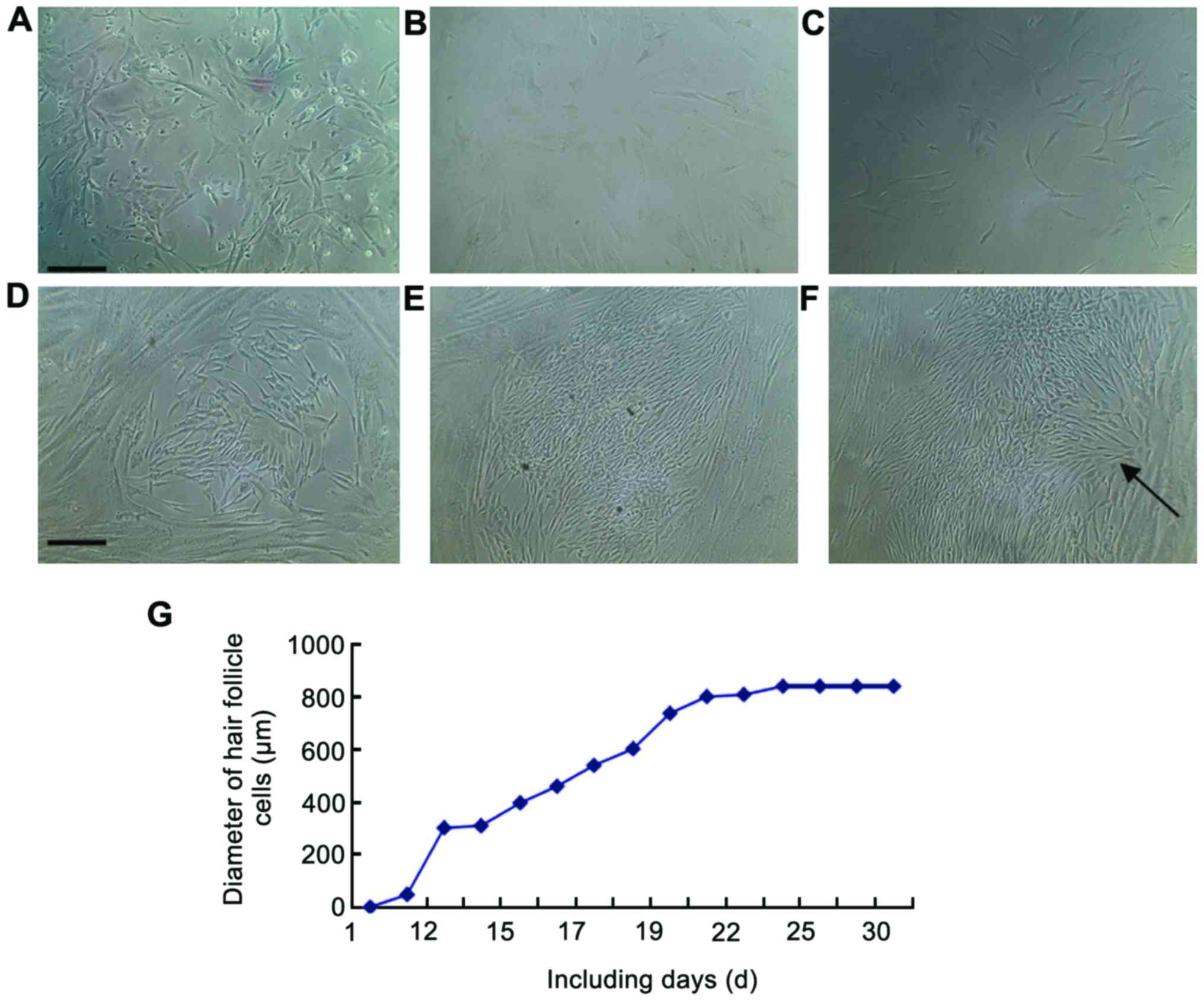
Light microscopy images showing the
process of induced differentiation of umbilical cord blood
MSCs
In order to document the entire process of induced
differentiation of stem cells, the entire process was photographed.
The results can be seen in Fig.
2A-F.
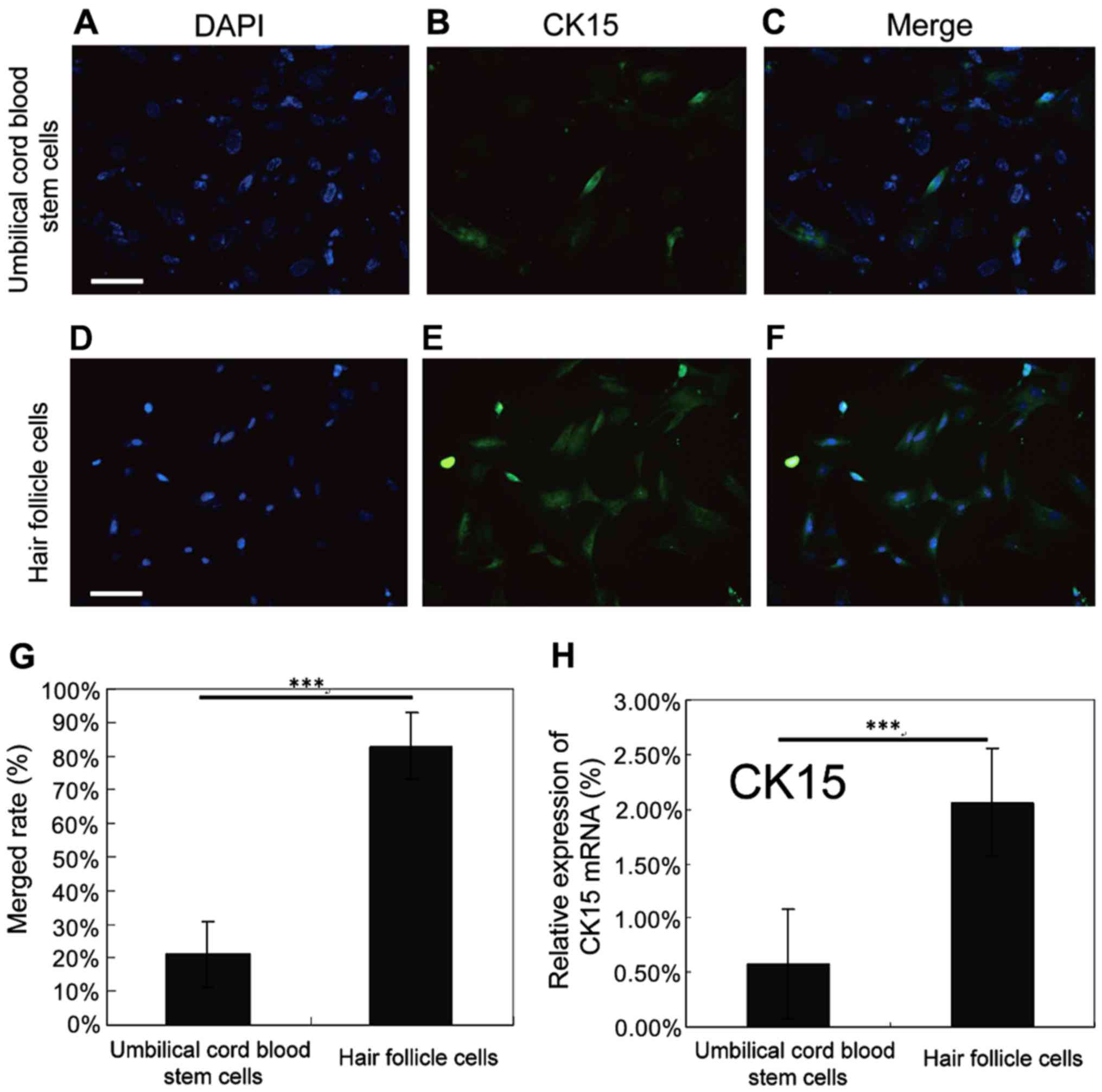
Identification of hair follicle
cells
In order to validate the success of induced
differentiation, hair follicle cells were identified. The nucleus
was marked with blue fluorescence and CK15 with green fluorescence.
The detection and colocalization of surface antigen CK15 expression
were carried out using fluorescence microscopy. Results indicated
that CK15 in the hair follicle cells was able to colocalize with
the nucleus (Fig. 3A-F), and the
expression level of CK15 in hair follicle cells was remarkably
higher than that in human umbilical cord blood stem cells, and the
difference had statistical significance (P<0.05; Fig. 3G). RT-PCR method was used to detect
the expression level of CK15 in human umbilical cord blood stem
cells and hair follicle cells. It was found that the expression
level of CK15 in hair follicle cells was remarkably higher than
that in human umbilical cord blood stem cells, and the difference
had statistical significance (P<0.05; Fig. 3H). These findings suggested that hair
follicle cells were successfully acquired from differentiation.
Discussion
At present, there are two challenges in the
application of stem cells in the clinical treatment: i) The content
of MSCs in human body is very low (12). Due to the lack of specific surface
marker (antigen) to identify the MSCs, it is difficult to purify
this type of cells; ii) in clinical treatment it is essential to
culture limited number of MSCs in vitro for effective
amplification. Nevertheless, during the amplification of MSCs in
vitro, they will spontaneously differentiate and age, which
result in the loss of tissue regenerative capacity (13). Moreover, it will also increase the
risk of stem cell degeneration and transformation of stem cells
into tumor cells. Therefore, these factors strongly inhibit the
clinical application of MSCs.
In this study, the techniques for purification and
rapid amplification of MSCs were improved, which benefit the
further development of induced differentiation and clinical
application of stem cells. The advantages of ECM-centered MSC
culture system we developed in this study are: i) ECM is completely
secreted and built by cells, which is close to the
three-dimensional structure of the human body. It can be amplified
into numerous MSCs in a short time, and preserve the primitiveness
of cells. Therefore, ECM can provide numerous high-performance
(all-purpose) MSCs for the study and clinical application of stem
cells; ii) since the tissue microenvironment (mainly composed of
ECM) determines the direction of MSC differentiation, ECM sourced
from different tissues such as muscle, fat, skin and pancreas will
effectively induce myocyte, adipose cell, skin cell and islet cell,
and provide MSCs with the suitable microenvironment for growth.
Because our ECM product source is from the cells in different
tissues, the ECM that is composed of cells possesses tissue
specificity. These two advantages solved the fundamental problems
of quantity and quality of the cells in the clinical application of
stem cells in China. Due to the lack of specific marker of hair
follicle stem cells at present, the studies of surface marker of
hair follicle stem cells mainly focus on the surface markers such
as integrin (α6, β1 and β4), keratin (CK14, CK15 and CK19), CD34,
CD200, monoclonal antibody C8/144B, transcription factor p63, CD71
and CX43 (14–16). In this study, we selected CK15 as the
surface marker of hair follicle cells to distinguish from umbilical
cord blood stem cells. Results indicated that we successfully
induced the differentiation of umbilical cord blood into hair
follicle cells. Using RT-PCR method to detect the transcription
level of CK15, it was also found that the expression level of CK15
in hair follicle cells was remarkably higher than that in human
umbilical cord blood stem cells, and the difference had statistical
significance (P<0.05). This is also evidence that by using
inducing liquid of hair follicle cells we can successfully induce
the differentiation of umbilical cord blood into hair follicle
cells. This method can be used for high-speed induced
differentiation with high purity, which is promising for clinical
application (17–19).
The finding of transdifferentiation of stem cells
not only rewrote the classic concept that the tissue-specific stem
cells could only differentiate directionally, but also provided the
opportunity for the application of adult-stem-cell therapy.
Artificial skin is the earliest tissue-engineered product. Single
layer artificial skin was developed in the late 1970s in USA. In
1998, bi-layered artificial skin Apligraft was developed by
Organogenesis (USA) and received FDA approval. It is the first
human living cell based tissue-engineered product that received FDA
approval. Apligraft acts like autologous skin, which possesses the
features such as good healing capacity, blood vessel forming
capacity, no immune rejection, and so on. It has been used in the
treatment of skin burns and venous ulcer, and the cure rate is
>80% (20).
Although there are a few precedents for adult stem
cell therapy, the study and application of ASCs are still in the
initial phase. There are still some difficulties in keeping their
clonal growth in vitro while inducing them to differentiate
into the functional cells required by the treatment. The major
reason is that we are not familiar with the microenvironment where
the stem cells are present, namely stem-cell niche (21). At present, the stem cell
transplantation carried out in laboratories around the world are
mainly direct transplantation of stem cells. There exist potential
risks for direct transplantation of stem cells in the long-term,
because in injured tissue microenvironment, stem cells may generate
the cell types that are harmful for tissue repair. Moreover, we
cannot exclude the possibility that ASCs may generate tumors.
Hence, it is clinically significant to directionally induce the
stem cells into required functional cells before transplantation,
which is also the emphasis in the future study of MSCs.
In conclusion, using inducing liquid of hair
follicle cells, we can successfully induce the differentiation of
umbilical cord blood into hair follicle cells. This method can be
used for high-speed induced differentiation with high purity, which
is promising for clinical application. This induction approach is
fast and efficient, and it is able to build up excellent
theoretical basis for the future clinical application.
Acknowledgements
This study was supported by the Hangzhou Science and
Technology Development Project (20110833B03).
References
|
1
|
Codinach M, Blanco M, Ortega I, Lloret M,
Reales L, Coca MI, Torrents S, Doral M, Oliver-Vila I,
Requena-Montero M, et al: Design and validation of a consistent and
reproducible manufacture process for the production of
clinical-grade bone marrow-derived multipotent mesenchymal stromal
cells. Cytotherapy. 18:1197–1208. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kumar K, Agarwal P, Das K, Mili B,
Madhusoodan AP, Kumar A and Bag S: Isolation and characterization
of mesenchymal stem cells from caprine umbilical cord tissue
matrix. Tissue Cell. 48:653–658. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Del Valle-Echevarria AR, Sanseverino W,
Garcia-Mas J and Havey MJ: Pentatricopeptide repeat 336 as the
candidate gene for paternal sorting of mitochondria (Psm) in
cucumber. Theor Appl Genet. 129:1951–1959. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
de Moor JS, Dowling EC, Ekwueme DU, Guy GP
Jr, Rodriguez J, Virgo KS, Han X, Kent EE, Li C, Litzelman K, et
al: Employment implications of informal cancer caregiving. J Cancer
Surviv. 11:48–57. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sigurjónsson OE, Guðmundsson KO and
Guðmundsson S: Mesenchymal stem cells. A review. Laeknabladid.
87:627–632. 2001.(In Icelandic). PubMed/NCBI
|
|
6
|
Maranda EL, Rodriguez-Menocal L and
Badiavas EV: Role of mesenchymal stem cells in dermal repair in
burns and diabetic wounds. Curr Stem Cell Res Ther. 12:61–70. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Astori G, Amati E, Bambi F, Bernardi M,
Chieregato K, Schäfer R, Sella S and Rodeghiero F: Platelet lysate
as a substitute for animal serum for the ex-vivo expansion of
mesenchymal stem/stromal cells: Present and future. Stem Cell Res
Ther. 7:93–100. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Dyrna F, Herbst E, Hoberman A, Imhoff AB
and Schmitt A: Stem cell procedures in arthroscopic surgery. Eur J
Med Res. 21:29–36. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jiang LH, Hao Y, Mousawi F, Peng H and
Yang X: Expression of P2 purinergic receptors in mesenchymal stem
cells and their roles in extracellular nucleotide regulation of
cell functions. J Cell Physiol. 232:287–297. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Morris AD, Chen J, Lau E and Poh J:
Domperidone-associated QT interval prolongation in non-oncologic
pediatric patients: A review of the literature. Can J Hosp Pharm.
69:224–230. 2016.PubMed/NCBI
|
|
11
|
Kavosi Z, Khorrami M Sarikhani, Keshavarz
K, Jafari A, Meshkini A Hashemi, Safaei HR and Nikfar S: Is
taurolidine-citrate an effective and cost-effective hemodialysis
catheter lock solution? A systematic review and cost-effectiveness
analysis. Med J Islam Repub Iran. 30:347–358. 2016.PubMed/NCBI
|
|
12
|
Shi X, Lv S, He X, Liu X, Sun M, Li M, Chi
G and Li Y: Differentiation of hepatocytes from induced pluripotent
stem cells derived from human hair follicle mesenchymal stem cells.
Cell Tissue Res. 366:89–99. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Maruyama CL, Leigh NJ, Nelson JW, McCall
AD, Mellas RE, Lei P, Andreadis ST and Baker OJ: Stem cell-soluble
signals enhance multilumen formation in SMG cell clusters. J Dent
Res. 94:1610–1617. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Son S, Liang MS, Lei P, Xue X, Furlani EP
and Andreadis ST: Magnetofection mediated transient NANOG
overexpression enhances proliferation and myogenic differentiation
of human hair follicle derived mesenchymal stem cells. Bioconjug
Chem. 26:1314–1327. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Maleki M, Ghanbarvand F, Behvarz M Reza,
Ejtemaei M and Ghadirkhomi E: Comparison of mesenchymal stem cell
markers in multiple human adult stem cells. Int J Stem Cells.
7:118–126. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Dong L, Hao H, Xia L, Liu J, Ti D, Tong C,
Hou Q, Han Q, Zhao Y, Liu H, et al: Treatment of MSCs with
Wnt1a-conditioned medium activates DP cells and promotes hair
follicle regrowth. Sci Rep. 4:5432–5440. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wu M, Guo X, Yang L, Wang Y, Tang Y, Yang
Y and Liu H: Mesenchymal stem cells with modification of junctional
adhesion molecule a induce hair formation. Stem Cells Transl Med.
3:481–488. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang Y, Liu J, Tan X, Li G, Gao Y, Liu X,
Zhang L and Li Y: Induced pluripotent stem cells from human hair
follicle mesenchymal stem cells. Stem Cell Rev. 9:451–460. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gola M, Czajkowski R, Bajek A, Dura A and
Drewa T: Melanocyte stem cells: Biology and current aspects. Med
Sci Monit. 18:RA155–RA159. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
DiDomenico L, Landsman AR, Emch KJ and
Landsman A: A prospective comparison of diabetic foot ulcers
treated with either a cryopreserved skin allograft or a
bioengineered skin substitute. Wounds. 23:184–189. 2011.PubMed/NCBI
|
|
21
|
Liang MS and Andreadis ST: Engineering
fibrin-binding TGF-β1 for sustained signaling and contractile
function of MSC based vascular constructs. Biomaterials.
32:8684–8693. 2011. View Article : Google Scholar : PubMed/NCBI
|