Introduction
In June 2009, the concept of transient ischemic
attack (TIA) was newly defined by the American Heart
Association/American Stroke Association, as brief episodes of
neurologic dysfunction that are induced by ischemia of a focal
brain region, or retina, spinal cord, without causing any acute
infarction for the body 1). According to previous studies, minor
stroke has been defined according to various criteria. Clinical
studies on minor stroke use <3 of the current National
Institutes of Health Stroke Scale (NIHSS) score as a common
criterion. In China, there are 3 million new patients with onset
stroke each year, of which 30% are minor strokes (2,3). In
addition, 2 million individuals are diagnosed with TIA (1,4).
TIA/minor stroke patients bear high-risk recurrent and disabling
cerebral stroke at an early stage. Studies have demonstrated that
stroke has a recurrence rate of 10–20% within 3 months and usually
occurs within the first 2 days after the initial onset (5,6).
Therefore, early intervention is critical for TIA/minor stroke
patients.
Antiplatelets taken at an early stage can reduce the
risk of ischemic events. The latest American guidelines suggest
taking an initial oral dose of 325 mg of aspirin (ASA) (class I;
level of evidence A within 24–48 h. However, the efficacy of
clopidogrel (Clop) requires further study (class IIb; level of
evidence C) (7). In 2010, Hankey and
Eikelboom meta-analysis demonstrated that treatment with Clop + ASA
was immediately more effective than treatment with ASA alone, and
the risk of bleeding is not significantly increased when acute
ischemic stroke and TIA patients are at the highest risk of
recurrent strokes (8).
Based on the Cochrane system, this study aimed to
explore the efficacy of Clop + ASA in large scale clinical studies
published in recent years for preventing major ischemic vascular
events and to characterize the associated risk of potential
hemorrhagic events when taken early by TIA/minor stroke patients
with a high-risk stroke recurrence and low-risk bleeding.
Materials and methods
Experimental procedures
As a systematic review and meta-analysis of
published research, no patient consent or ethical approval was
required for the study. The design and implementation of this study
conformed to the criteria of Preferred Reporting Items for
Systematic Reviews and Meta-Analyses (PRISMA) (9).
Eligibility criteria
Research that met the requirements below was
included in the study: ⅰ) the study design of the reviewed research
is a randomized controlled trial (RCT); ⅱ) patients: TIA/minor
stroke patients aged ≥18; ⅲ) interventions: the trial group
received Clop + ASA, while the control group received ASA alone;
and iv) outcomes: incidence of recurrent stroke, vascular
mortalities, myocardial infarction (MI), and major hemorrhagic
events.
Exclusion criteria for the study were: ⅰ)
unpublished studies; ⅱ) studies without assessment of the
measurement indexes; ⅲ) studies with missing data for which
statistical analysis cannot be performed; and ⅳ) repeatedly
published studies or general reviews.
Search strategy
Databases listed as below were searched
independently by two researchers J.T. and M.Z.Z. (PubMed, Cochrane,
EMBase, Medline, and Web of Science) to confirm eligible studies.
The following keywords were used: aspirin, antiplatelet therapy,
cerebral ischemia, clopidogrel, minor stroke, randomized controlled
trial and transient ischemic attack. The screening study was
conducted on the screened full-text in order to evaluate additional
possible eligible trials.
The studies were independently screened and verified
in accordance with the inclusion as well as exclusion criteria.
Titles and abstracts were first reviewed to exclude studies that
did not comply with the inclusion criteria. For the studies that
were potentially eligible, a second screening was conducted by
reading the whole text. Any disagreement was solved by a third
reviewer (CKH) through consultation.
Data extraction and measurement of
outcome
A piloted extraction datasheet was employed with the
following information covered: research title, number of patients,
average age of patients, percentage of male patients, received drug
dose of the trial group, received drug dose of the control group,
time interval from onset of symptoms to entering studies, treatment
duration, and percentage of patients lost to follow-up.
The investigators extracted all data
from the reports
Any disagreement was settled through consultation.
When necessary, we contacted the corresponding authors by e-mail to
gain accurate data. The primary outcome encompassed recurrent
stroke, and MI and vascular mortalities were the secondary
outcomes, and major hemorrhagic events were for the safety
outcome.
Evaluation of risk of bias and the
assessment of the quality of included research
With reference to version 5.1.0 of the Cochrane
Handbook for Systematic Reviews of Interventions, risk of bias was
assessed for this study (10). After
reviewing all included articles, a level of unclear, low or high
was assigned to the following areas: ⅰ) random sequence generation;
ⅱ) blinding of personnel and participants; ⅲ) allocation
concealment; ⅳ) blinding of outcome assessment; ⅴ) selective
reporting; and ⅵ) incomplete outcome data, and other sources of
bias.
The assessment of the quality of the research was
conducted using the GRADE form (11). Evidence was divided into the
following levels: high quality (our confidence in the estimated
effect will unlikely be changed by further research), moderate
quality (our confidence in the estimated effect is likely to be
changed by further research), low quality (our confidence in the
estimated effect is very likely to be changed by further research),
very low quality of the evidence (we remain quite uncertain about
the estimated effect).
Statistical analysis
The relative risk was estimated with a 95%
confidence interval for binary outcomes. I2 was used in tests for
homogeneity. I2 above 50% suggested significant heterogeneity and
that the random effects model was expected to be employed, and if
not the fixed effect model was to be employed (12). When significant heterogeneity was
present, sensitivity analysis was used to identify potential
sources of heterogeneity. If a study was included and the total RR
changed substantially, this suggested that the study had a large
bias and the result should be interpreted carefully. Based on the
sample size, cumulative meta-analysis was used to evaluate the
influence of citing studies with large sample size on the
incorporated result. The Eggers test and Eggers funnel plot were
performed for assessing any publication bias in the studies
included (13). Results were assumed
statistically significant when P<0.05. RevMan 5.2 and R 3.1.1
were used to perform the statistical analyses.
Results
Selection of studies and their
characteristics
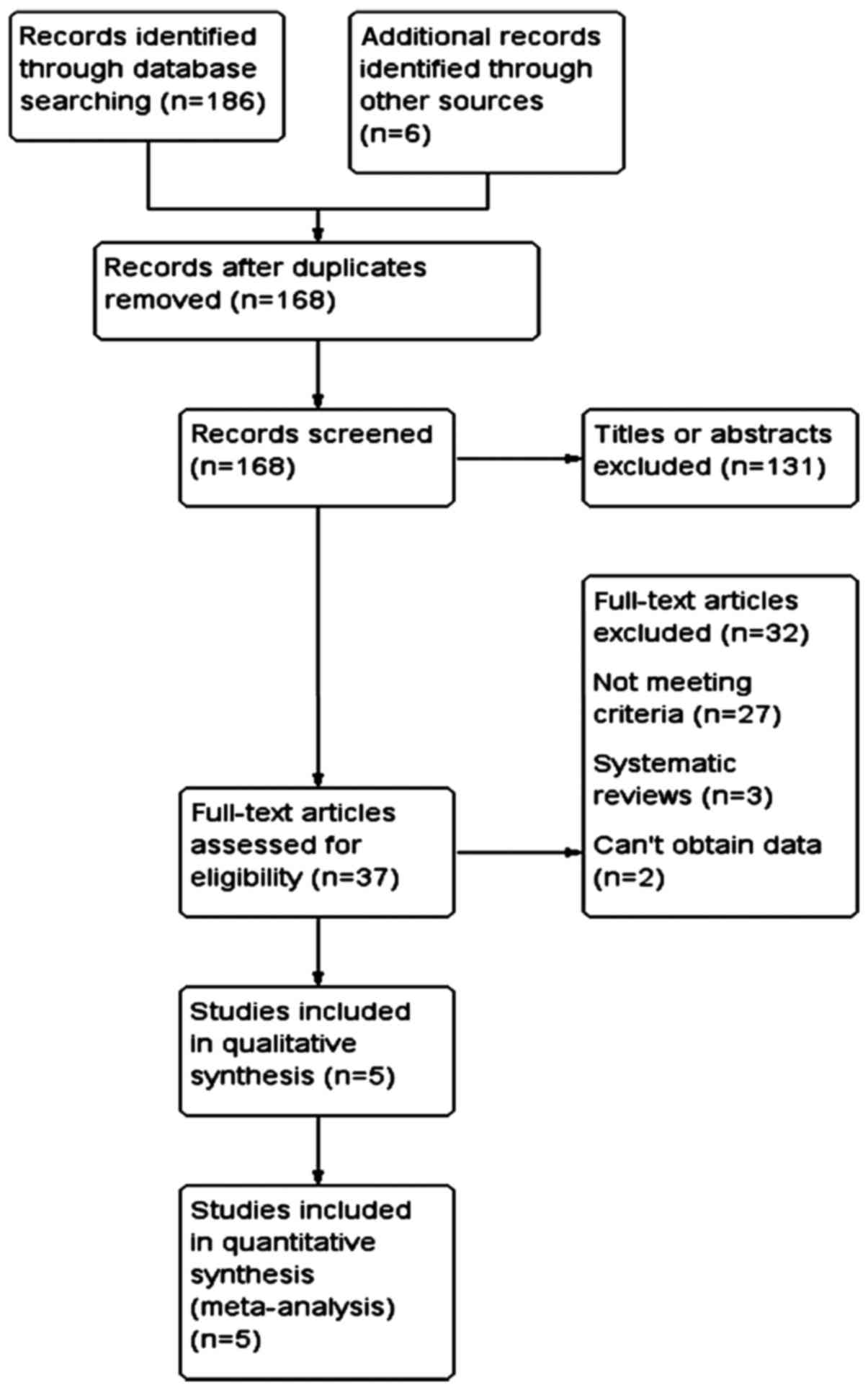
The retrieval and screening results of this study
are shown in Fig. 1. A total of 168
relevant potential studies were obtained through a preliminary
screening, while 131 studies were excluded by reading of their
titles and abstracts. The full contents of the remaining 37 studies
were reviewed, with 32 of them eliminated (27 did not conform to
the criteria, 3 were systematic reviews, and 2 were absent of
data). Five RCT studies conforming to the criteria were selected
for the meta-analysis.
Main characteristics of the 5 RCT studies used for
the meta-analysis are shown in Table
I. The studies were published from 2005 to 2014; 3 were carried
out in Canada, 1 in China, and 1 in the UK. The sample sizes ranged
from 107 to 5,170 (the total sample size was 9,527 cases). The
average age of patients in these 5 RCT studies was 62–68 years,
with a male predominance (53–69%). The duration from onset of
symptoms to inclusion into studies (start of treatment) ranged from
24 h to 3 months (three articles in ≤72 h; two articles within 3
months). The treatment durations ranged from 7 days to 3.5 years
(three articles in ≤3 months; two articles in ≤3.5 years).
 | Table I.Design features and baseline
characteristics of participants. |
Table I.
Design features and baseline
characteristics of participants.
| Study name | No. | Mean age (years) | Men (%) | Treatment group and
dose | Comparison group and
dose | Treatment onset | Treatment
duration | Lost to follow-up
(%) | Refs. |
|---|
| CHANCE | 5,170 | 62 | 66 | Asp (76–300 mg load
then 75 mg od first 12 days) + Clop (300 mg load then 75 mg
od) | Asp (76–300 mg load
then 75 mg od) | 72 h | 90 days | 0.7 | (14) |
| PASTER | 392 | 68 | 53 | Asp (81 mg od) + Clop
(300 mg load then 75 mg od) | Asp (81 mg od) | 24 h | 90 days | 1.79 | (15) |
| CARESS | 197 | 65 | 69 | Asp (75 mg load) +
Clop (300 mg load then 75 mg od) | Asp (75 mg od) | 72 h | 7 days | None | (16) |
| Failure | 888 | 66 | 65 | Asp (325 mg od) +
Clop (75 mg od) | Asp (325 mg od) | 150 days | 3.5 years | NR | (17) |
| SPS3 | 3,020 | 63 | 65 | Asp (325 mg od) +
Clop (75 mg od) | Asp (325 mg od) | 150 days | 3.5 years | NR | (18) |
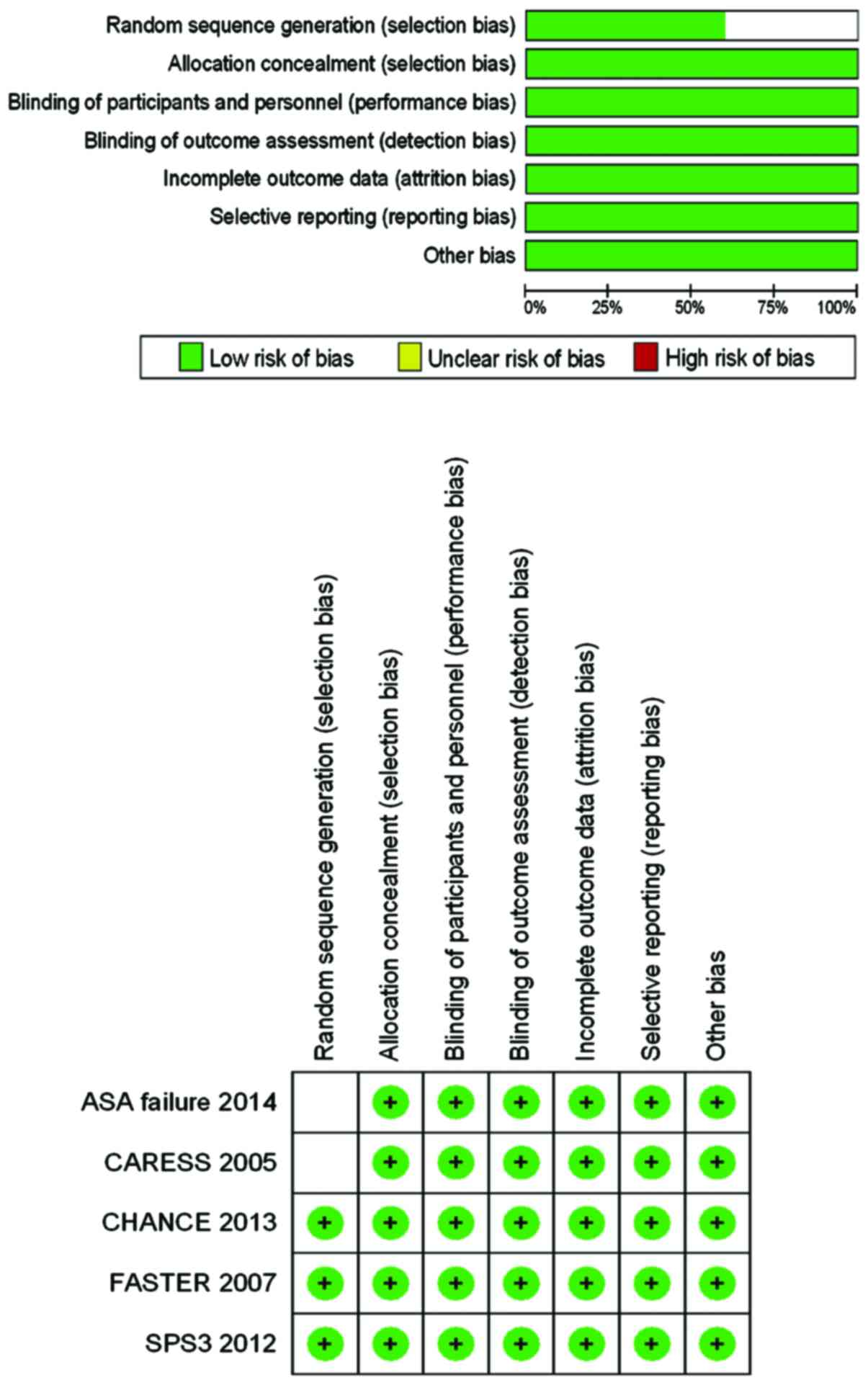
Risk of bias and evidence level
The risks of bias of the five aforementioned RCT
studies are shown in Fig. 2.
Randomized sequence generation was unclear in ASA failure and Clop
+ ASA for reduction of emboli in symptomatic carotid stenosis
because these studies only described randomization instead of the
process of random sequence generation. The remaining studies were
assumed to have low risk of bias.
GRADE was employed to evaluate the quality of
evidence and recommended level (Table
II). The results showed that recurrent strokes, MIs, and
vascular mortalities were high quality evidence, while major
hemorrhagic events were moderate quality.
 | Table II.Clop + ASA compared with ASA for
TIA/minor stroke patients. |
Table II.
Clop + ASA compared with ASA for
TIA/minor stroke patients.
|
| Illustrative
comparative risk (95% CI) |
|
|
|
|---|
|
|
|
|
|
|
|---|
| Outcomes | Assumed risk ASA | Corresponding risk
Clop + ASA | Relative effect (95%
CI) | No. of participants
(studies) | Quality of evidence
(GRADE) |
|---|
| MI and vascular
mortality | Study population |
|
|
|
|
| 21/1,000 | 23/1,000 | RR=1.08 | 9,328 | ++++ |
|
|
| (18–30) | (0.83–1.41) | (5 studies) | High |
|
| Moderate |
|
|
|
|
| 38/1,000 | 41/1,000 |
|
|
|
|
|
| (32–54) |
|
|
|
| Stroke
recurrence | Study population |
|
|
|
|
| 110/1,000 | 84/1,000 | RR=0.76 | 9,328 | ++++ |
|
|
| (74–96) | (0.67–0.87) | (5 studies) | High |
|
| Moderate |
|
|
|
|
| 117/1,000 | 89/1,000 |
|
|
|
|
|
| (78–102) |
|
|
|
| Major
hemorrhage | Study
population |
|
|
|
|
| 2/1,000 | 3/1,000 | RR=1.55 | 9,527 | +++− |
|
|
| (2–7) | (0.72–3.36) | (5 studies) | Moderate |
|
| Moderate |
|
|
|
|
| 2/1,000 | 3/1,000 |
|
|
|
|
|
| (1–7) |
|
|
|
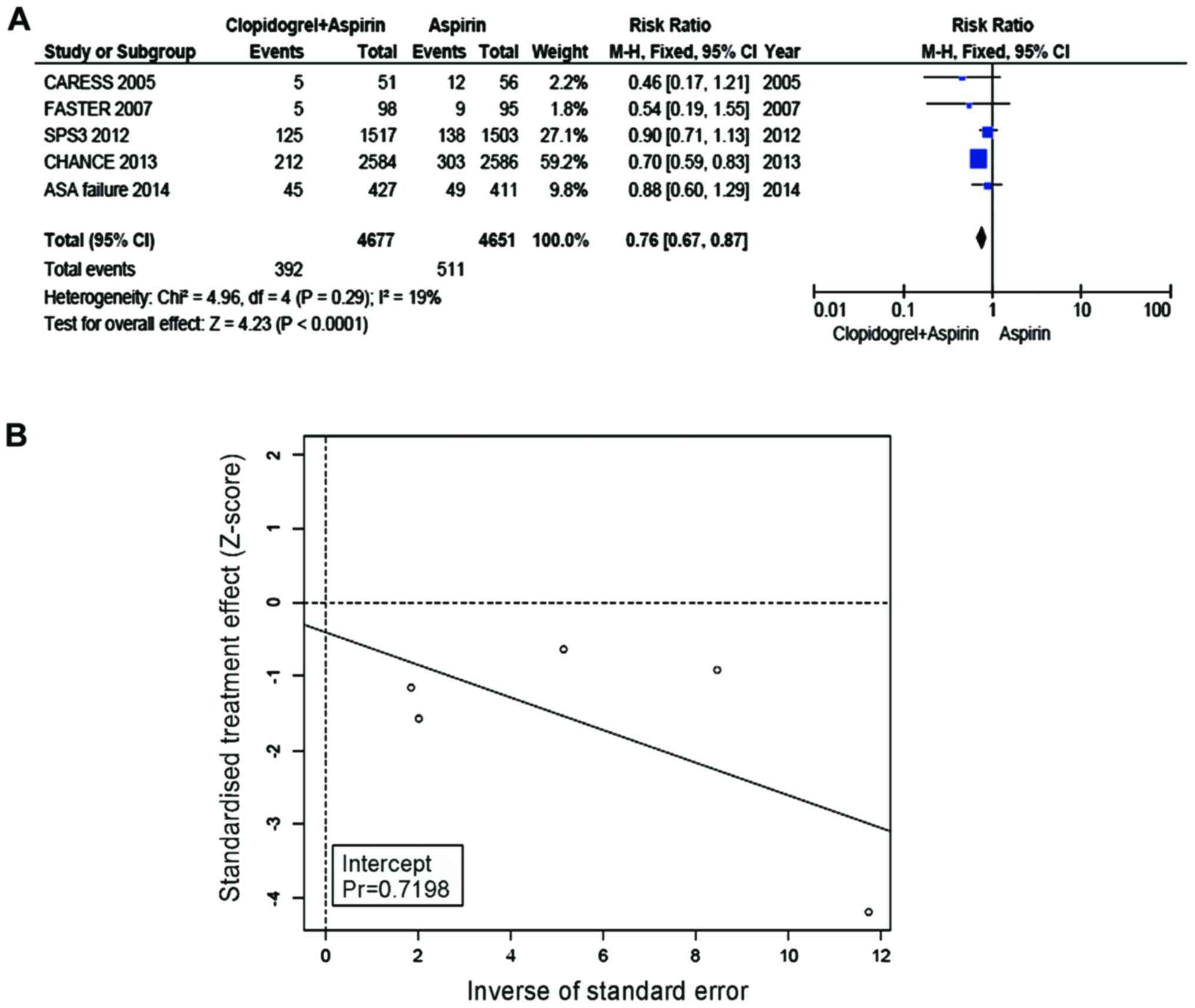
Major outcome: recurrent stroke
The incidence of recurrent stroke in the group of
Clop + ASA was 8.38%, while the incidence in the ASA group was
10.99%. Compared with ASA, Clop + ASA resulted in a significantly
lower incidence of recurrent stroke of the group with TIA/minor
stroke (RR=0.76, 95% CI=0.67–0.87, P<0.0001, Fig. 3A). No heterogeneity was present
between the trial and control groups (I2=19%). Eggers regression
line (Fig. 3B) was made, and a null
hypothesis was tested whether the regression line intercepted 0 or
not. No bias was suggested with the use of Eggers test (P for bias
turned out 0.72).
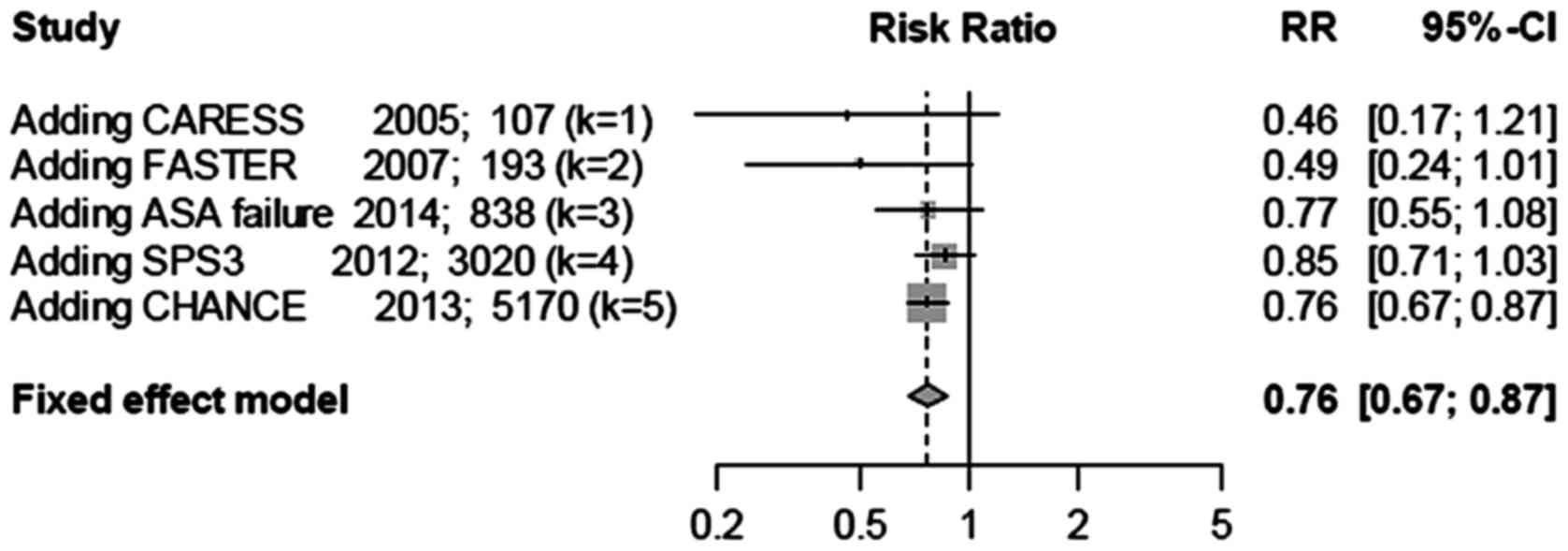
Cumulative meta-analysis was performed based on
sample sizes (Fig. 4). The treatment
effect was initially large, although after adding studies with
large sample sizes, the confidence interval narrowed, and the
treatment effect became smaller and stable, albeit the treatment
effect was still highly significant (P<0.0001). This analysis
indicated that studies with small sample sizes may overestimate the
effect of Clop + ASA in lowering recurrent stroke.
Secondary outcome: myocardial
infarction and vascular mortality
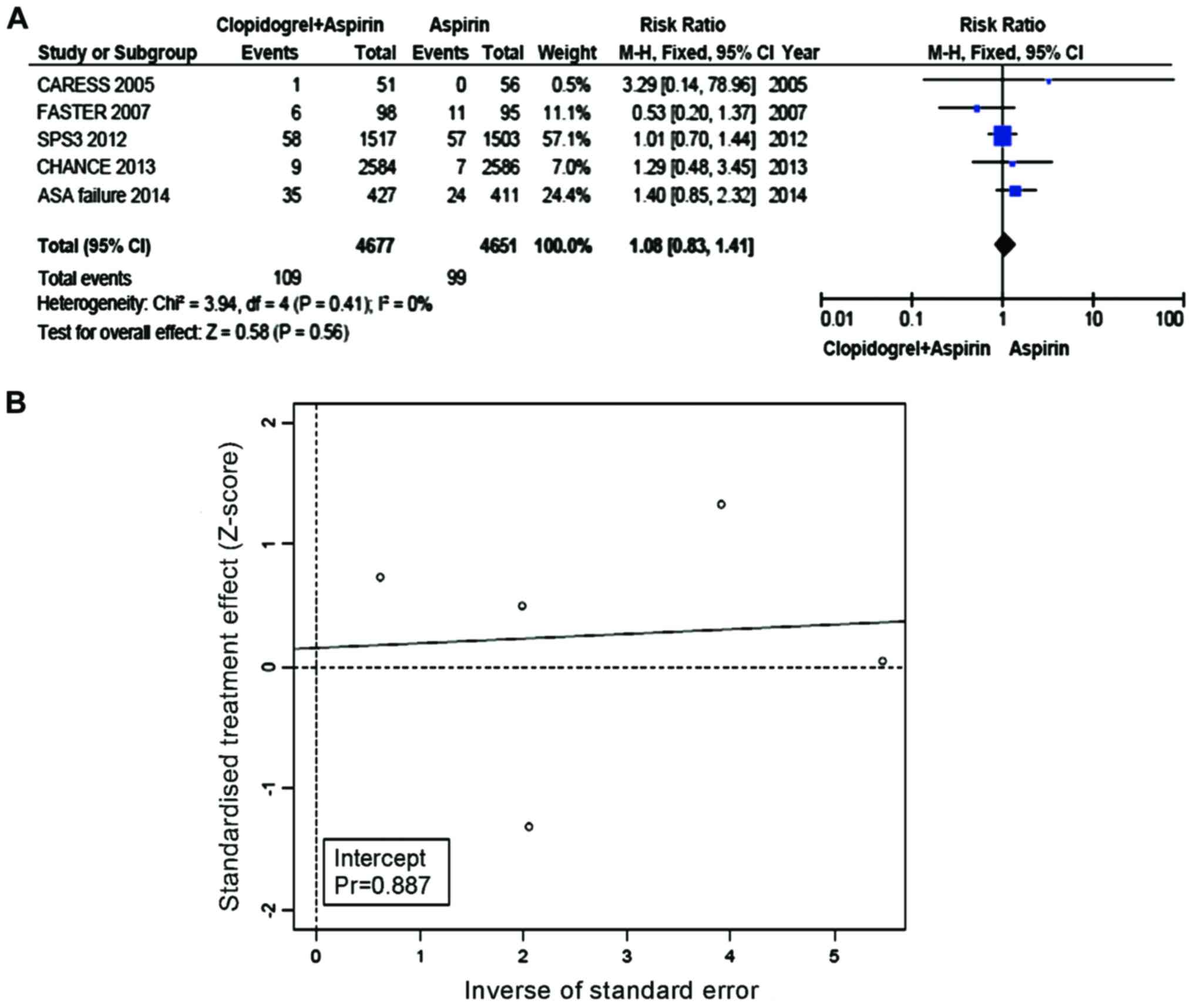
The incidence of MI and vascular mortalities was
2.33% in the Clop + ASA group and 2.13% in the ASA group. Compared
with ASA, the incidence of MI as well as vascular mortalities was
not significantly increased in the group with Clop + ASA (RR=1.08,
95% CI=0.83–1.41, P=0.56, Fig. 5A).
No heterogeneity was present between the trial and control groups
(I2=0%). Eggers regression line (Fig.
5B) was constructed and did not show evidence of any
publication bias with the use of Eggers test (P-value for bias was
0.887).
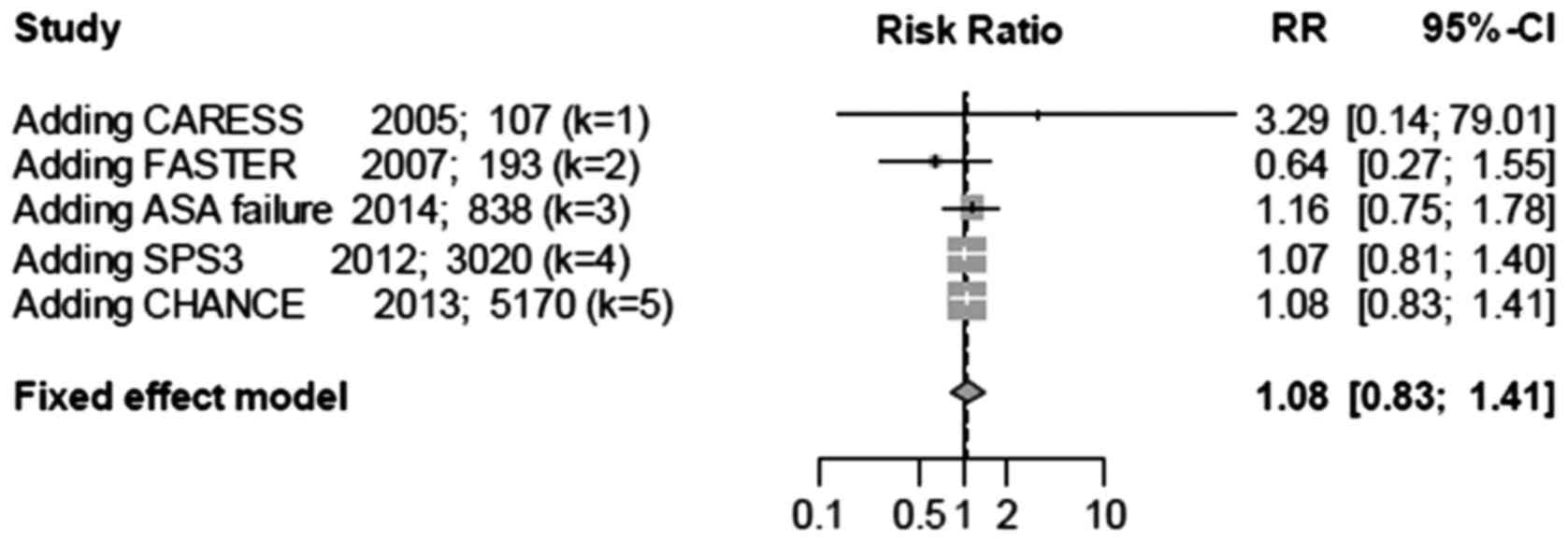
Cumulative meta-analysis was performed based on
sample sizes (Fig. 6). The total RR
was initially unstable as it fluctuated between values of >1.0
and <1.0. However, after accumulating additional studies, the
total RR became stable and the confidence interval became narrow.
This analysis suggested that combination therapy does not increase
the incidence of vascular mortalities.
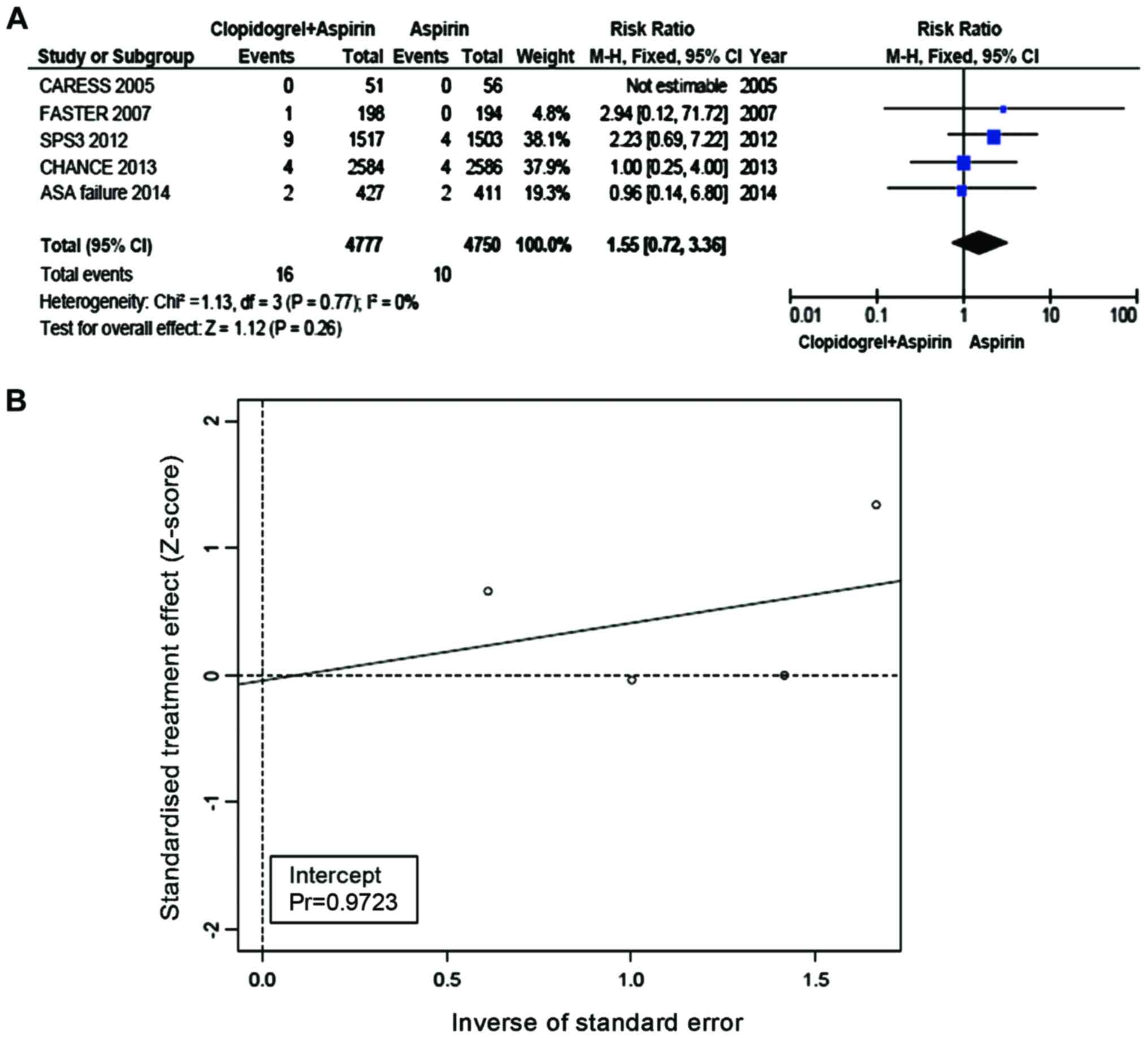
Safety outcome
The incidence of major hemorrhagic events was 0.33%
in the Clop + ASA group and 0.21% in the ASA group. Compared with
ASA, the incidence of hemorrhagic events was not increased in the
group of Clop + ASA (RR=1.55, 95% CI=0.72–3.36, P=0.26, Fig. 7A). No heterogeneity was present
between the trial and control groups (I2=0%). Eggers regression
line (Fig. 7B) was plotted and the
Eggers test did not suggest any publication bias (P-value for bias
was 0.9723).
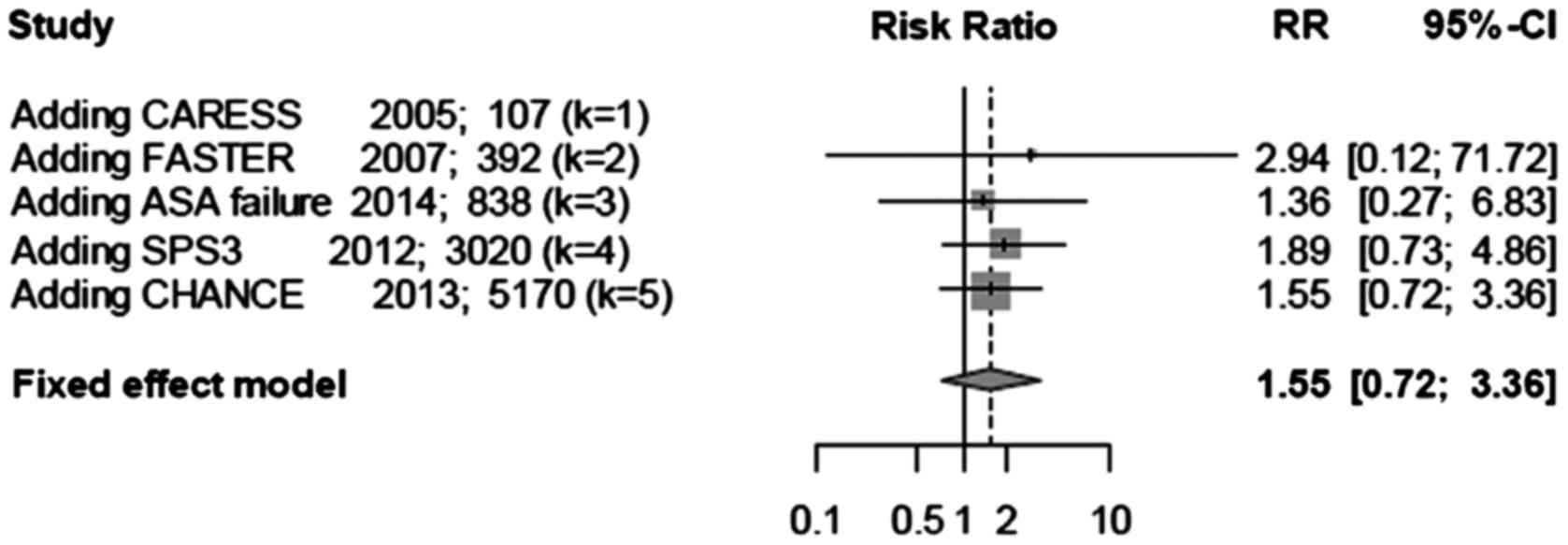
Cumulative meta-analysis was performed based on
sample sizes (Fig. 8). The treatment
effect was large at the beginning, although after adding studies
with large sample sizes, the confidence interval narrowed, and the
treatment effect became smaller and stable. This analysis suggested
that combination therapy does not increase the incidence of major
hemorrhagic events.
Discussion
Of two relevant meta-analyses published in 2012, one
showed that dual antiplatelet therapy may lower the incidence of
recurrent stroke and vascular events along with total mortalities,
without increasing the risk of hemorrhage when compared with
mono-antiplatelet therapy for stroke/TIA patients (19). However, another meta-analysis showed
the opposite result, namely patients with vascular disease taking
Clop + ASA may experience an increased risk of fatal hemorrhage
(20). Therefore, the effects of the
two antiplatelet drugs when used in combination require further
study. Since a significant amount of research results have been
reported in recent years, we evaluated the safety and efficacy of
treatment with Clop + ASA compared with ASA alone for TIA/minor
stroke patients.
The primary and secondary outcomes of this study
show that the incidence of recurrent stroke can be significantly
decreased with the treatment of Clop + ASA (RR=0.76, 95%
CI=0.67–0.87, P<0.0001), and MI and vascular mortalities is not
significantly changed by such treatment (RR=1.08, 95% CI=0.83–1.41,
P=0.56). These findings are inconsistent with the results of
previous meta-analyses (19,20). Transcranial Doppler ultrasound
recordings provided supportive evidence in these studies as the
number of microembolic signals is proportional to recurrent stroke
and risk of other vascular events. Both CARESS (16) and Clop + ASA for infarction reduction
(CLAIR) (21) demonstrate that
compared with ASA alone, combination therapy of Clop + ASA
significantly reduces microembolic signals. Therefore, the risks of
recurrent stroke as well as other vascular events are reduced with
combination therapy as well.
The safety outcomes show that Clop + ASA does not
result in an increase in the risk of major hemorrhagic events
(RR=1.55, 95% CI=0.72–3.36, P=0.26). Possible reasons for the
inconsistency with the results from other studies include: ⅰ)
selection of patients: the population in this study were TIA/minor
stroke patients who suffered from a high risk of recurrent ischemic
events at an early stage and were at low risk of hemorrhages.
However, several other studies included other types of stroke
patients or patients with other vascular pathologies. Thus, the
potential risks of bleeding in patients from these studies were
inherently higher. ⅱ) Drug dosage and duration of treatment: in the
SPS3 study, the drug dosage was 325 mg/day and treatment duration
was 3.5 years (18). These treatment
parameters are greater than those from most other studies, thus
possibly leading to an increase in major hemorrhagic events in the
results.
Combining Clop + ASA is a potent antiplatelet
therapy. Therefore, it is necessary to balance their therapeutic
effects with potential hemorrhagic risks to optimize the benefits
for patients. The results suggest that taking Clop + ASA early
appears to be a safe and effective therapy for TIA/minor stroke
patients. We believe that the ongoing POINT study (22) in the United States and other similar
studies may provide evidence to support our hypothesis in the
future.
This study has the following limitations: ⅰ) because
of the limited amount of research in this field, this meta-analysis
only included five studies, therefore, it is difficult to make
reliable conclusions; ⅱ) only TIA/minor stroke patients and Clop +
ASA vs. ASA therapy are included, and as a result, the narrow scope
may have an impact on the conclusion from extrapolation; ⅲ) among
patients in the included studies, between the onset of symptoms to
inclusion in their respective studies occurred at various time
points. Start time for treatment ranged from 24 h to 3 months after
initial symptoms. Early treatment can lead to improved prognosis.
Thus, the differences in start time of treatment between the
studies may affect the final treatment effect; and ⅳ) the dosages
of Clop + ASA and treatment times in the included studies were
highly variable. Since higher doses of Clop + ASA given for
prolonged periods of time increase the risk of bleeding
complications, the wide range may contribute to differences in
results between our study and others.
In conclusion, future research should focus on
determining: ⅰ) whether Clop + ASA therapy is also suitable for
other types of ischemic stroke; ⅱ) an appropriate treatment window
for TIA/minor stroke patients with Clop + ASA; and ⅲ) the optimal
treatment dosage and duration of Clop + ASA for patients with a
recent TIA/minor stroke. Future large-scale clinical trials may
provide answers to these questions and guide evidence-based
approaches for the treatment of this disease.
Acknowledgements
This study was supported by Research and Development
Project of Xiangyang in 2013 (no. 201302) and Scientific Research
Project of Hubei Provincial Department of Education in 2014
(B2014051).
References
|
1
|
Johnston SC, Fayad PB, Gorelick PB, Hanley
DF, Shwayder P, van Husen D and and Weiskopf T: Prevalence and
knowledge of transient ischemic attack among US adults. Neurology.
60:1429–1434. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wang YL, Wu D, Liao X, Zhang W, Zhao X and
Wang YJ: Burden of stroke in China. Int J Stroke. 2:211–213. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhao D, Liu J, Wang W, Zeng Z, Cheng J,
Liu J, Sun J and Wu Z: Epidemiological transition of stroke in
China: Twenty-one-year observational study from the
Sino-MONICA-Beijing Project. Stroke. 39:1668–1674. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Edlow JA, Kim S, Pelletier AJ and Camargo
CA Jr.: National study on emergency department visits for transient
ischemic attack, 1992–2001. Acad Emerg Med. 13:666–672. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rothwell PM, Giles MF, Chandratheva A,
Marquardt L, Geraghty O, Redgrave JN, Lovelock CE, Binney LE, Bull
LM, Cuthbertson FC, et al: Effect of urgent treatment of transient
ischaemic attack and minor stroke on early recurrent stroke
(EXPRESS study): A prospective population-based sequential
comparison. Lancet. 370:1432–1442. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Luengo-Fernandez R, Gray AM and Rothwell
PM: Effect of urgent treatment for transient ischaemic attack and
minor stroke on disability and hospital costs (EXPRESS study): A
prospective population-based sequential comparison. Lancet Neurol.
8:235–243. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jauch EC, Saver JL, Adams HP Jr, Bruno A,
Connors JJ, Demaerschalk BM, Khatri P, McMullan PW Jr, Qureshi AI,
Rosenfield K, et al American Heart Association Stroke Council;
Council on Cardiovascular Nursing; Council on Peripheral Vascular
Disease; Council on Clinical Cardiology, : Guidelines for the early
management of patients with acute ischemic stroke: A guideline for
healthcare professionals from the American Heart
Association/American Stroke Association. Stroke. 44:870–947. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hankey GJ and Eikelboom JW: Antithrombotic
drugs for patients with ischaemic stroke and transient ischaemic
attack to prevent recurrent major vascular events. Lancet Neurol.
9:273–284. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Moher D, Liberati A, Tetzlaff J and Altman
DG; PRISMA Group, : Preferred reporting items for systematic
reviews and meta-analyses: The PRISMA statement. BMJ.
339:b25352009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Higgins JPT and Green S: Cochrane Handbook
for Systematic Reviews of Interventions Version 5.1.0. [updated
March 2011]. The Cochrane Collaboration; 2011
|
|
11
|
Guyatt GH, Oxman AD, Vist GE, Kunz R,
Falck-Ytter Y, Alonso-Coello P and Schünemann HJ; GRADE Working
Group, : GRADE: An emerging consensus on rating quality of evidence
and strength of recommendations. BMJ. 336:924–926. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Higgins JP and Thompson SG: Quantifying
heterogeneity in a meta-analysis. Stat Med. 21:1539–1558. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Egger M, Smith G Davey, Schneider M and
Minder C: Bias in meta-analysis detected by a simple, graphical
test. BMJ. 315:629–634. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang Y, Wang Y, Zhao X, Liu L, Wang D,
Wang C, Wang C, Li H, Meng X, Cui L, et al CHANCE investigators, :
Clopidogrel with aspirin in acute minor stroke or transient
ischemic attack. N Engl J Med. 369:11–19. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kennedy J, Hill MD, Ryckborst KJ, Eliasziw
M, Demchuk AM and Buchan AM; FASTER Investigators, : Fast
assessment of stroke and transient ischaemic attack to prevent
early recurrence (FASTER): A randomised controlled pilot trial.
Lancet Neurol. 6:961–969. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Markus HS, Droste DW, Kaps M, Larrue V,
Lees KR, Siebler M and Ringelstein EB: Dual antiplatelet therapy
with clopidogrel and aspirin in symptomatic carotid stenosis
evaluated using doppler embolic signal detection: The Clopidogrel
and Aspirin for Reduction of Emboli in Symptomatic Carotid Stenosis
(CARESS) trial. Circulation. 111:2233–2240. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Côté R, Zhang Y, Hart RG, McClure LA,
Anderson DC, Talbert RL and Benavente OR: ASA failure: Does the
combination ASA/clopidogrel confer better long-term vascular
protection? Neurology. 82:382–389. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Benavente OR, Hart RG, McClure LA,
Szychowski JM, Coffey CS and Pearce LA; SPS3 Investigators: Effects
of clopidogrel added to aspirin in patients with recent lacunar
stroke. N Engl J Med. 367:817–825. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Geeganage CM, Diener HC, Algra A, Chen C,
Topol EJ, Dengler R, Markus HS, Bath MW and Bath PM; Acute
Antiplatelet Stroke Trialists Collaboration, : Dual or mono
antiplatelet therapy for patients with acute ischemic stroke or
transient ischemic attack: Systematic review and meta-analysis of
randomized controlled trials. Stroke. 43:1058–1066. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Palacio S, Hart RG, Pearce LA and
Benavente OR: Effect of addition of clopidogrel to aspirin on
mortality: Systematic review of randomized trials. Stroke.
43:2157–2162. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wong KS, Chen C, Fu J, Chang HM, Suwanwela
NC, Huang YN, Han Z, Tan KS, Ratanakorn D, Chollate P, et al CLAIR
study investigators, : Clopidogrel plus aspirin versus aspirin
alone for reducing embolisation in patients with acute symptomatic
cerebral or carotid artery stenosis (CLAIR study): A randomised,
open-label, blinded-endpoint trial. Lancet Neurol. 9:489–497. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Johnston SC, Easton JD, Farrant M, Barsan
W, Battenhouse H, Conwit R, Dillon C, Elm J, Lindblad A,
Morgenstern L, et al: Platelet-oriented inhibition in new TIA and
minor ischemic stroke (POINT) trial: Rationale and design. Int J
Stroke. 8:479–483. 2013. View Article : Google Scholar : PubMed/NCBI
|