Introduction
Preeclampsia, a pregnancy-specific disorder
affecting ~5% of all pregnancies, is a leading cause of maternal
and neonatal morbidity and mortality during pregnancy (1,2). It is
characterized by hypertension and proteinuria after 20 weeks of
gestation (3). The exact
pathogenesis of preeclampsia remains unclear, and it is reported
that the renin-angiotensin system (RAS), a major blood pressure
regulating system, plays a critical role in the development of
preeclampsia (4).
As a key signaling cascade, the circulating RAS is
classically described in the kidney. Renin is an enzyme synthesized
and released by juxtaglomerular cells of the afferent renal
arterioles. It is involved in blood pressure and sodium chloride
regulation (4). In RAS, renin can
cleave angiotensinogen (AGT) to produce angiotensin I (Ang I) and
is a rate-limiting factor in the RAS cascade (4). Ang I is then cleaved by
angiotensin-converting enzyme (ACE) to produce the effector
molecule angiotensin II (Ang II), which could eventually affect the
function of vascular smooth muscle cells and adrenal glands
(5). According to previous reports
(6,7), decreased levels of renin, Ang I and Ang
II and increased levels of ACE and Ang II sensitivity are observed
in preeclampsia.
In addition to RAS, endothelial dysfunction caused
by abnormal placentation is reported to be closely related to the
development of preeclampsia (8).
Transforming growth factor beta (TGF-β) is a major cytokine
produced abundantly in vascular endothelial cells and trophoblasts.
It plays a key role in various physiological processes, including
embryonic growth and development, inflammation repair and
angiogenesis (9–12). TGF-β1, one of three isoforms of
TGF-β, is a key mediator in vascular endothelial cell apoptosis and
proliferation, immunosuppression and cellular matrix synthesis
(13–15). Moreover, TGF-β1 participates in
successful placentation through trophoblast invasion regulation
(16–18) and its levels are higher in pregnant
women than in non-pregnant women (19). Ayatollahi et al demonstrated
that TGF-β1 is a regulatory factor in fetal allograft survival
during pregnancy (19).
As the source and target cells of urinary TGF-β1,
renal cells could be regulated by TGF-β1. Murakami et al
(20) demonstrated that
significantly higher levels of urinary TGF-β are found in patients
with IgA nephritis and focal glomerulosclerosis, compared with
patients with other types of glomerular diseases and healthy
controls. In patients with proliferative-type diseases, urinary
TGF-β was significantly correlated with the grade of mesangial
matrix increase and the magnitude of proteinuria. Their results
indicated that urinary TGF-β could reflect the grade of
interstitial fibrosis in glomerular diseases and the mesangial
matrix increased in proliferative-type glomerulonephritis (20). Measuring TGF-β levels in the urine
might be helpful in monitoring patients with renal disease.
Previous results have also suggested that renal TGFβ1 is associated
with proteinuria in pregnancy-induced hypertension (21).
The current study aimed to analyze serum Ang II,
urinary AGT and urinary TGFβ1 levels in relation to the clinical
manifestation of preeclampsia, and to explore the effects of
circulating RAS on preeclampsia and proteinuria development.
Materials and methods
Patients and controls
A total of 83 pregnant women were recruited between
December 2007 and March 2010 and assigned to one of three groups:
Group A (n=33), preeclampsia; Group B (n=19), pregnancy-induced
hypertension; or Group C (n=31), normotensive pregnancy. All of the
participants were of Chinese origin and were pregnant with a single
fetus. The protocol was approved by the Ethics Committee of The
Fifth People's Hospital of Shanghai, Fudan University (Shanghai,
China). Written informed consent was obtained from all
participants.
Preeclampsia was defined as follows: i) Sustained
systolic blood pressure of >140 mmHg or a sustained diastolic
blood pressure of >90 mmHg on two separate readings; ii)
proteinuria measurement of ≥1+ or 24-h urine protein collection of
>300 mg. Pregnancy-induced hypertension was defined as
hypertension (>140/90 mmHg) during the pregnancy period that was
resolved 12 weeks later, with no proteinuria. Normal pregnancy was
defined as normal blood pressure (<140/90 mmHg) during the
pregnancy period with no proteinuria or obstetric and medical
complications.
Blood and urinary sampling
Blood samples were collected into tubes containing
EDTA as an anticoagulant. Plasma samples were obtained by
centrifugation at 3,000 × g for 10 min. Aliquots of samples were
prepared, stored at −80°C and used within 12 weeks. Morning spot
urine samples were collected from all subjects for laboratory
analysis. Collected 24-h urine was used for quantitation of daily
urinary protein excretion.
Laboratory analysis
Serum alanine aminotransaminase, albumin (Alb),
creatinine (Scr), urea nitrogen (BUN) uric acid (UA) and
albumin/creatinine ratio (ACR), as well as estimated glomerular
filtration rate (eGFR, calculated using the Modification of Diet in
Renal Disease formula) and 24-h urine protein quantification were
determined using an Automatic Biochemistry Analyzer (Roche Modular
P800; Roche Diagnostics GmbH, Mannheim, Germany).
Serum Ang II, urinary AGT and urinary
TGFβ1 determination
Levels of Ang II, AGT and TGFβ1 were determined
using a commercially available enzyme-linked immunosorbent assay
(ELISA; R&D Systems, Inc., Minneapolis, MN, USA), according to
the manufacturer's instructions. All samples were run in duplicate
on the assay plate. If >10% variation existed between
duplicates, the assay was repeated and the average was
reported.
Statistical analysis
All statistical analyses were performed using SPSS
17.0 (SPSS, Inc., Chicago, IL, USA). Measurement and enumeration
data are expressed as the mean ± standard deviation. Mann-Whitney U
and χ2 tests were employed to compare variables between
two groups. Multiple group comparisons were performed by analysis
of variance and further comparisons between two groups were
performed with post-hoc tests. Correlations were calculated using
Spearman's rank correlation. Receiver operating characteristic
(ROC) curve analysis was used to assess the optimal cut-off value
of one or two combined factors for preeclampsia prediction. Overall
accuracy was estimated using area under the curve (AUC). MedCalc
statistical software version 13.0 (MedCalc Software bvba, Ostend,
Belgium) was used to compare the AUCs of different ROC curves.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Patient demographic data
The patient demographic data are shown in Table I. Significantly decreased Alb and
eGFR were found in preeclampsia patients compared with group B
(both P<0.05) and significantly increased Scr, BUN, UA and ACR
(all P<0.05) were found in preeclampsia patients compared with
group C.
 | Table I.Demographic data of included
subjects. |
Table I.
Demographic data of included
subjects.
| Characteristics | Group A (n=33) | Group B (n=19) | Group C (n=31) |
|---|
| Age (years) | 28 (24–32) | 28 (23.5–35) | 27 (25–29) |
| SBP (mmHg) | 150 (145–160) | 140 (140–150) | 120 (110–120) |
| DBP (mmHg) | 100 (95–110) | 100 (90–100) | 75 (70–80) |
| ALT (U/l) | 9 (8–14) | 10 (7–13) | 11 (10–15) |
| Alb (g/l) | 30.4
(26.5–34.5)a | 36.4 (31.8–39.1) | 38.0 (35.8–39.1) |
| Scr (µmol/l) | 48
(43–55)b | 47 (43–52) | 45 (40–48) |
| BUN (mmol/l) | 3.8
(3.0–5.1)b | 3.5 (3.0–3.9) | 2.9 (2.6–3.3) |
| UA (µmol/l) | 315
(254–420)b | 260 (247–297) | 265 (221–296) |
| eGFR (ml/min) | 134.08
(105.66–160.19)a | 148.55
(140.05–161.53) | 167.41
(154.55–184.88) |
| ACR (mg/mmol) | 31.05
(12.00–121.10)b | 3.70 (1.11–5.94) | 1.98 (0.89–4.44) |
Comparison of serum Ang II, urinary
AGT and urinary TGFβ1
Serum Ang II, urinary AGT and urinary TGFβ1 levels
were evaluated using ELISA. The results showed a significantly
decreased level of AGT in preeclampsia patients as compared with
pregnancy-induced hypertension (P<0.05) and normotensive
pregnancy patients (P<0.05; Table
II). Moreover, an increased TGF-β1 level was observed in
preeclampsia patients, although this result was not significant
when compared with pregnancy-induced hypertension or normotensive
pregnancy patients.
 | Table II.Serum Ang II, urinary TGFβ1 and
urinary AGT levels among different groups of subjects. |
Table II.
Serum Ang II, urinary TGFβ1 and
urinary AGT levels among different groups of subjects.
| Characteristics | Group A (n=33) | Group B (n=19) | Group C (n=31) |
|---|
| Serum Ang II
(ng/l) | 70.81±16.68 | 70.29±11.97 | 68.08±11.85 |
| Urinary TGFβ1
(ng/l) | 433.25±139.77 | 403.09±63.87 | 401.79±106.39 |
| Urinary AGT
(ng/l) |
185.72±30.43a,b | 201.65±17.60 | 205.11±22.25 |
Correlation analysis
Correlation analysis was performed for all
participants between urinary AGT and ACR, systolic and diastolic
blood pressure (SBP and DBP, respectively), serum Ang II and
urinary TGFβ1 (Fig. 1). A negative
correlation was found between AGT and ACR (r=−0.302, P=0.004); AGT
and blood pressure (rSBP=−0.275, P=0.009; rDBP=−0.279, P=0.008); a
positive correlation was found between AGT and Ang II (r=0.255,
P=0.015); a positive correlation was found between AGT and TGFβ1
(r=0.386, P<0.001).
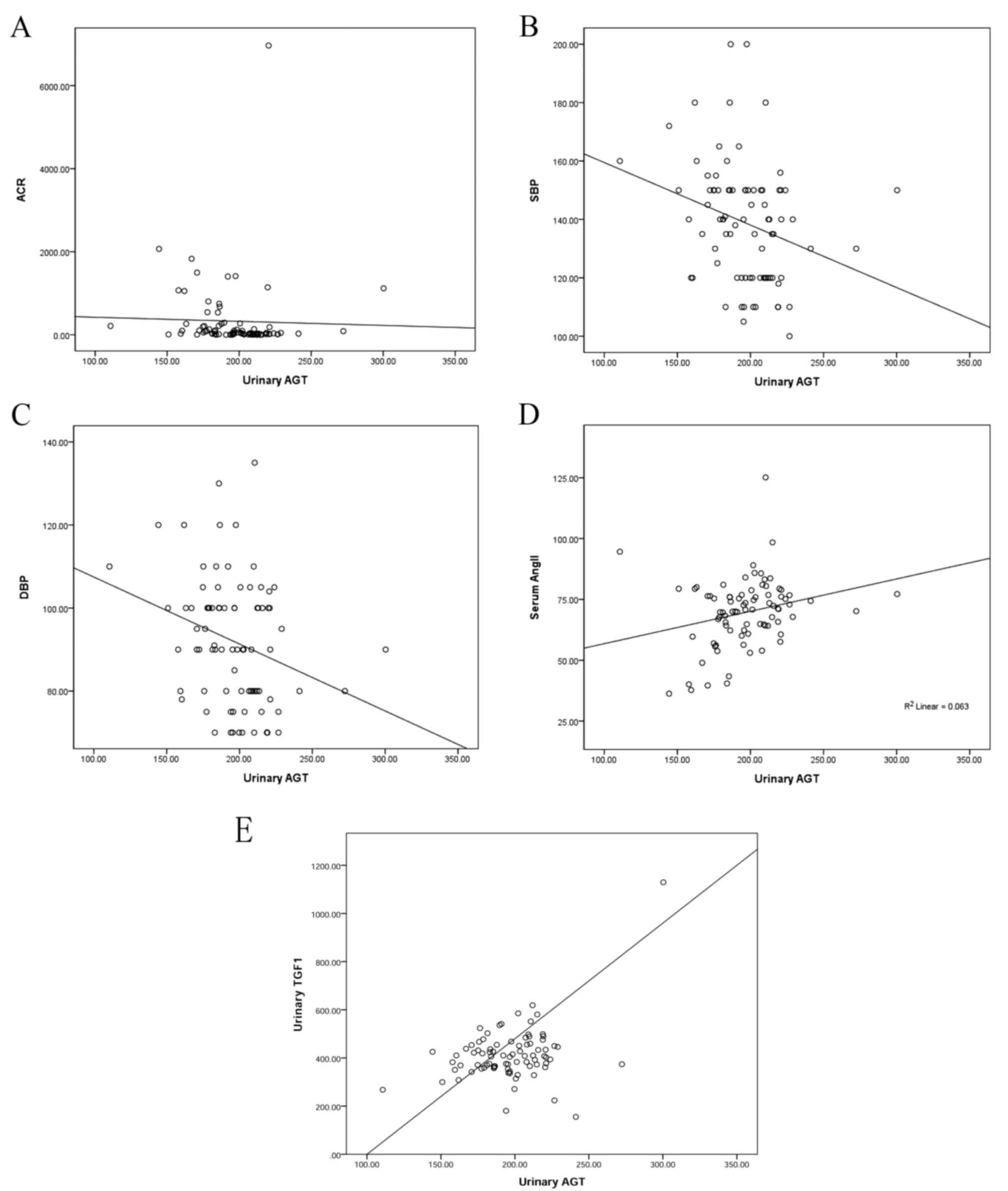 | Figure 1.Correlation analysis between urinary
AGT and ACR, SBP, DBP, serum Ang II and urinary TGFβ1. (A)
Correlation between AGT and ACR (r=−0.302, P=0.004). (B)
Correlation between AGT and SBP (r=−0.275, P=0.009). (C)
Correlation between AGT and DBP (r=−0.279, P=0.008). (D)
Correlation between AGT and serum Ang II (r=0.255, P=0.015). (E)
Correlation between AGT and TGFβ1 (r=0.386, P=0.000). AGT,
angiotensinogen; ACR, albumin/creatinine ratio; SBP, systolic blood
pressure; DBP, diastolic blood pressure; Ang II, angiotensin II;
TGFβ, transforming growth factor β1. |
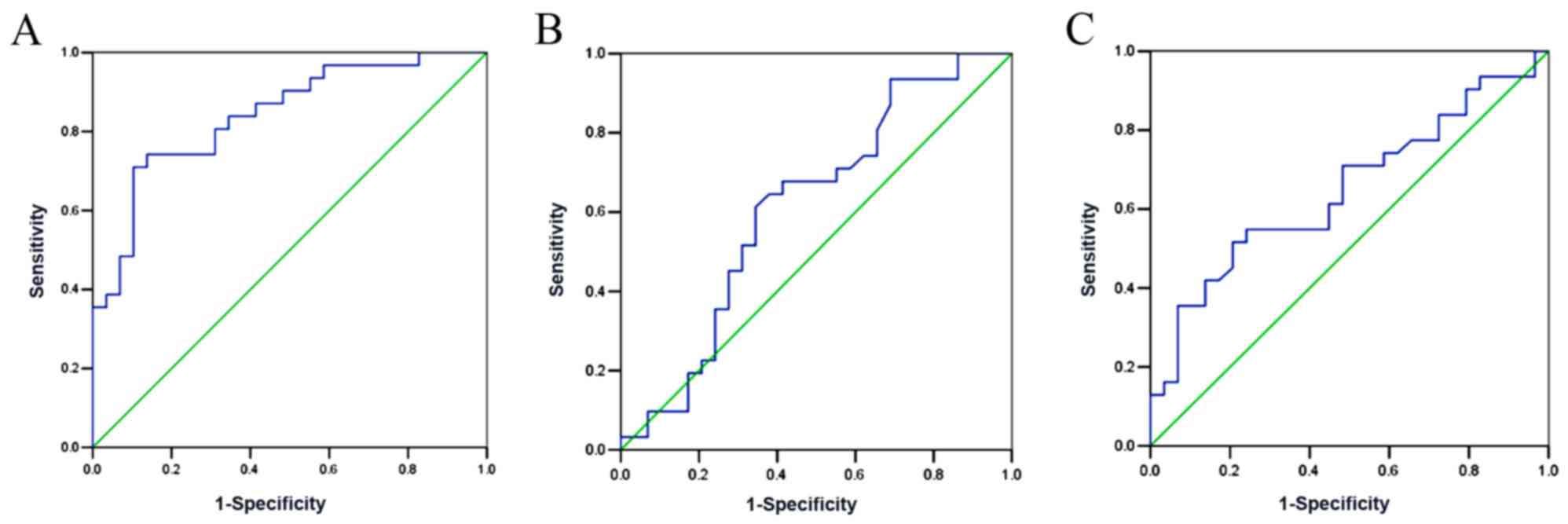
Utility of serum Ang II and/or urinary
TGFβ1 and AGT in predicting preeclampsia
The performance of serum Ang II, urinary TGFβ1 and
AGT in preeclampsia prediction were used to construct ROC curves
(Fig. 2). The results showed that
the AUC of urinary AGT was 0.841 (95% CI: 0.742–0.940, P<0.001),
which was significantly higher than that of urinary TGFβ1
(AUC=0.613, 95% CI: 0.467–0.759, P=0.133) and serum Ang II
(AUC=0.647, 95% CI: 0.507–0.787, P=0.05). Moreover, the optimal
cut-off value of urinary AGT was determined. The results showed
that urinary AGT=193 ng/l exhibited the highest sensitivity and
specificity in preeclampsia diagnosis (Tables III–V).
 | Table III.Performance of different cut-off
values of urinary angiotensinogen for diagnosis of
preeclampsia. |
Table III.
Performance of different cut-off
values of urinary angiotensinogen for diagnosis of
preeclampsia.
| Cut-off value
(ng/l) | Sensitivity
(%) | Specificity
(%) |
|---|
| 175 |
35.5 | 100 |
| 180 |
45.2 |
93.1 |
| 193 |
74.2 |
86.2 |
| 200 |
80.6 |
65.5 |
| 210 |
93.5 |
44.8 |
| 220 | 100 |
17.2 |
 | Table V.Performance of different cut-off
values of serum angiotensin II for diagnosis of preeclampsia. |
Table V.
Performance of different cut-off
values of serum angiotensin II for diagnosis of preeclampsia.
| Cut-off value
(ng/l) | Sensitivity
(%) | Specificity
(%) |
|---|
| 40 | 100 |
3.4 |
| 60 |
83.9 |
27.6 |
| 65 |
71.0 |
51.7 |
| 75 |
54.8 |
75.9 |
| 80 |
19.4 |
93.1 |
| 85 |
12.9 | 100 |
In order to improve the sensitivity and specificity
of preeclampsia prediction, ROC curves were also constructed for
combinations of urinary AGT, urinary TGFβ1 and serum Ang II
(Fig. 3, Table VI). The results showed that the AUC
for urinary AGT + urinary TGFβ1 was 0.806 (95% CI: 0.777–0.959,
P<0.001); the AUC for urinary AGT + serum Ang II was 0.901 (95%
CI: 0.822–0.980, P<0.001); the AUC for serum Ang II+urinary
TGFβ1 was 0.684 (95% CI: 0.549–0.819, P=0.014); and the AUC for
urinary AGT + serum Ang II + urinary TGFβ1 was 0.918 (95% CI:
0.845–0.990, P<0.001).
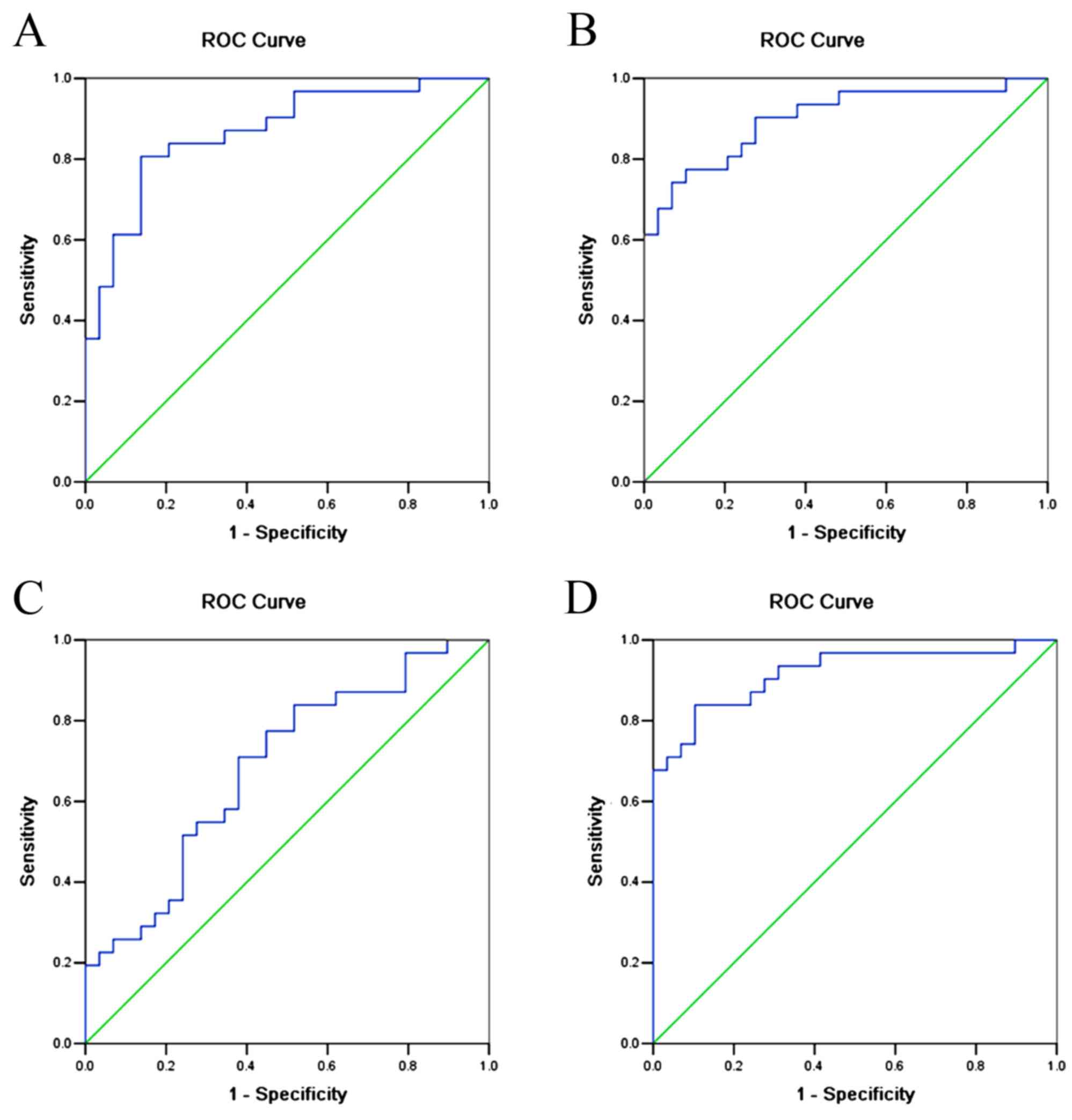 | Figure 3.ROC curves for combining urinary AGT,
TGFβ1 and serum Ang II as diagnostic indicators of preeclampsia.
(A) Combined urinary AGT and urinary TGFβ1. AUC=0.806 (95% CI:
0.777–0.959, P<0.001). (B) Combined urinary AGT and serum Ang
II. AUC=0.901 (95% CI: 0.822–0.980, P<0.001). (C) Combined
urinary TGFβ1 and serum Ang II. AUC=0.684 (95% CI: 0.549–0.819,
P=0.014). (D) Combined urinary AGT, serum Ang II and urinary TGFβ1.
AUC=0.918 (95% CI: 0.845–0.990, P<0.001). AGT, angiotensinogen;
Ang II, angiotensin II; TGFβ, transforming growth factor β1; AUC,
area under the curve; CI, confidence interval; ROC, receiver
operating characteristic. |
 | Table VI.Performance of combination tests for
diagnosis of preeclampsia. |
Table VI.
Performance of combination tests for
diagnosis of preeclampsia.
| Combination of
biomarkers | Sensitivity
(%) | Specificity
(%) |
|---|
| Urinary AGT +
urinary TGFβ1 | 80.6 | 86.2 |
| Urinary AGT + serum
Ang II | 71.0 | 62.1 |
| Serum Ang II +
urinary TGFβ1 | 80.6 | 79.3 |
| Urinary AGT + serum
Ang II + urinary TGFβ1 | 83.9 | 89.7 |
Discussion
To the best of our knowledge, this was the first
study to explore the expression of RAS in the kidney in patients
with preeclampsia and the value of urinary AGT in preeclampsia
diagnosis. A decreased level of urinary AGT was found in
preeclampsia patients and this was associated with hypertension and
proteinuria. It was proposed that a high value of preeclampsia
diagnosis could be achieved using urinary AGT or a combination of
urinary AGT, serum Ang II and urinary TGFβ1.
Senatorski et al (22) showed that higher levels of urinary
TGF-β1 could be found in membranous glomerulonephritis patients
compared with a control group. In the current study, elevated
urinary TGFβ1 was observed in preeclampsia patients compared with
normotensive pregnancy patients, but this result was not considered
to be significant. Moreover, no linear correlation was found
between urinary TGFβ1 and ACR. In addition, an ROC curve indicated
that urinary TGFβ1 had a lower diagnostic value in preeclampsia
compared with urinary AGT. This discrepancy with previous results
could be attributed to a smaller sample size. Furthermore, Blush
et al (23) demonstrated that
estradiol administration to Alb/TGF-β transgenic mice (which
overexpress TGF-β) could ameliorate progressive renal injury.
Estradiol was able to reverse the pro-fibrotic effects of TGF-β,
which could help to explain the sexual dimorphism in renal disease
progression observed in humans (23). Furthermore, Potier et al
(24) showed that 17beta-estradiol
increased both matrix metalloproteinase 9 (MMP9) mRNA and MMP-9
activity in mesangial cells. Therefore, the protective effect
exerted by the hormone during pregnancy may be the reason for
decreased renal fibrosis. However, further study should be
conducted to elucidate the role of TGFβ1 in patients with
pregnancy-induced hypertension.
All the components of RAS are known to be present in
the uterine placenta, as previous studies have confirmed the
expression of the renin gene in the human placenta, villus and
uterus (25,26), and observed higher levels of GFR,
plasma renin (PRC) and plasma aldosterone during normal pregnancy
compared with non-pregnancy (27).
Inhibition of ACE was able to effectively control the conversion of
Ang I to Ang II, reduce the inactivation of bradykinin (BK) and
stimulate the production of prostaglandin I2 and estradiol.
Therefore, decreasing the sensitivity of vessel walls to Ang II
could assist in maintaining a balance of blood pressure (28). Previously, decreased PRC and
increased ACR activity was observed in normal pregnancy (29). Increased activity of serum ACE could
result in BK inactivation and inhibition of NO release, thereby
leading to endothelial damage.
Alexander et al (30) observed no significant difference in
the efficacy of oral captopril treatment for lowering mean arterial
blood pressure between normal pregnancy and long-term uterus
hypoperfusion pregnancy in rats, indicating that RAS did not play a
major role in mediating the hypertension produced by chronic
uterine perfusion pressure in pregnancy. However, RAS was highly
likely to be involved in the pathophysiological process of
preeclampsia, as activation of local RAS in the uterine placenta
could result in preeclampsia (30).
In the current study, a higher level of serum Ang II was observed
in preeclampsia pregnancy patients than in pregnancy-induced
hypertension or normal pregnancy patients, but this result was not
considered to be significant. However, no correlation was found
between serum Ang II and 24-h urinary protein quantification or
ACR. In addition, the ROC curve indicated a lower diagnostic value
of urinary serum Ang II in compared with urinary AGT. This
discrepancy with previous results could be attributed to a smaller
sample size.
Kobori et al (31) reported that Ang II-dependent
hypertension could result in elevated intrarenal Ang II and AGT
levels, reflected by increased urinary AGT, but that this did not
occur in an Ang II-independent hypertensive model. In the current
study, a significantly decreased level of urinary AGT was observed
in preeclampsia patients compared with normal pregnancy or
pregnancy-induced hypertension patients. A negative correlation was
also found between urinary AGT and blood pressure in pregnant
women, which indicated that inhibition of local renal RAS was
associated with the development of hypertension and proteinuria in
patients with preeclampsia. It is proposed that preeclampsia might
be similar to the two kidney-one clip model in hypertensive animal
models (32). In normal pregnancy,
reduced blood flow to the placenta may result in the activation of
RAS, while in preeclampsia, blood flow may not be reduced and
therefore RAS activity may be inhibited. The ROC curve also
indicated a high sensitivity and specificity of urinary AGT in the
diagnosis of preeclampsia. The combined use of urinary AGT, serum
Ang II and urinary TGFβ1 further improves the diagnostic value for
preeclampsia. Due to the relative ease of collecting urine from
patients, urinary AGT might be readily used as an indicator for
preeclampsia diagnosis in clinical practice.
The current study had a number of limitations.
First, it is a single center study with a relatively small sample
size. Further studies with larger samples in multiple centers
should be performed in order to confirm the current results.
Second, a cross-sectional design in the second trimester could have
resulted in patient selection bias.
In conclusion, the current results help to elucidate
the mechanisms of preeclampsia, kidney injury and proteinuria.
Subsequent studies are needed to investigate the relationship
between local placental RAS and circulating and local renal RAS in
preeclampsia and the expression of local renal RAS components. A
systemic analysis of RAS components in animal model and human
subjects is also required.
Acknowledgements
This study was funded by the Shanghai Municipal
Science and Technology Project (grant no. 124119a7802), the Fudan
University Project 985 key discipline construction project (grant
no. 2012FDYSXK02), the Shanghai City Health Bureau Project (grant
no. 20114311), the Major State Basic Research Development Program
of China (973 Program; grant no. 2012CB517700), Minhang District
Natural Fund (grant no. 2013MHZ015), Phase III of the Nephropathy
Discipline Construction of 211 Project of State Education
Commission and the Minhang District key discipline construction
project and the Hospital Youth Fund (grant no. 2010QJ02).
References
|
1
|
Redman CW and Sargent IL: Latest advances
in understanding preeclampsia. Science. 308:1592–1594. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sibai B, Dekker G and Kupferminc M:
Pre-eclampsia. Lancet. 365:785–799. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Noris M, Perico N and Remuzzi G:
Mechanisms of disease: Pre-eclampsia. Nat Clin Pract Nephrol.
1:98–114; quiz 120. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Irani RA and Xia Y: The functional role of
the renin-angiotensin system in pregnancy and preeclampsia.
Placenta. 29:763–771. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chung O, Kuhl H, Stoll M and Unger T:
Physiological and pharmacological implications of AT1 versus AT2
receptors. Kidney Int Suppl. 67:S95–S99. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Langer B, Grima M, Coquard C, Bader AM,
Schlaeder G and Imbs JL: Plasma active renin, angiotensin I, and
angiotensin II during pregnancy and in preeclampsia. Obstet
Gynecol. 91:196–202. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Brown MA, Zammit VC, Mitar DA and
Whitworth JA: Renin-aldosterone relationships in pregnancy-induced
hypertension. Am J Hypertens. 5:366–371. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sanchez-Aranguren LC, Prada CE,
Riano-Medina CE and Lopez M: Endothelial dysfunction and
preeclampsia: Role of oxidative stress. Front Physiol. 5:3722014.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Roberts AB and Sporn MB: Transforming
growth factor-beta: Potential common mechanisms mediating its
effects on embryogenesis, inflammation-repair, and carcinogenesis.
Int J Rad Appl Instrum B. 14:435–439. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Akhurst RJ, FitzPatrick DR, Gatherer D,
Lehnert SA and Millan FA: Transforming growth factor betas in
mammalian embryogenesis. Prog Growth Factor Res. 2:153–168. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bertolino P, Deckers M, Lebrin F and ten
Dijke P: Transforming growth factor-beta signal transduction in
angiogenesis and vascular disorders. Chest. 128 6 Suppl:585S–590S.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lebrin F, Deckers M, Bertolino P and Ten
Dijke P: TGF-beta receptor function in the endothelium. Cardiovasc
Res. 65:599–608. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ferrari G, Pintucci G, Seghezzi G, Hyman
K, Galloway AC and Mignatti P: VEGF, a prosurvival factor, acts in
concert with TGF-beta1 to induce endothelial cell apoptosis. Proc
Natl Acad Sci USA. 103:pp. 17260–17265. 2006; View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sales VL, Engelmayr GC Jr, Mettler BA,
Johnson JA Jr, Sacks MS and Mayer JE Jr: Transforming growth
factor-beta1 modulates extracellular matrix production,
proliferation, and apoptosis of endothelial progenitor cells in
tissue-engineering scaffolds. Circulation. 114 1 Suppl:I193–I199.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bommireddy R and Doetschman T: TGFbeta1
and Treg cells: Alliance for tolerance. Trends Mol Med. 13:492–501.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Karmakar S and Das C: Regulation of
trophoblast invasion by IL-1beta and TGF-beta1. Am J Reprod
Immunol. 48:210–219. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Power LL, Popplewell EJ, Holloway JA,
Diaper ND, Warner JO and Jones CA: Immunoregulatory molecules
during pregnancy and at birth. J Reprod Immunol. 56:19–28. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhao MR, Qiu W, Li YX, Zhang ZB, Li D and
Wang YL: Dual effect of transforming growth factor beta1 on cell
adhesion and invasion in human placenta trophoblast cells.
Reproduction. 132:333–341. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ayatollahi M, Geramizadeh B, Yazdani M and
Azarpira N: Effect of the immunoregulatory cytokines on successful
pregnancy depends upon the control of graft rejection mechanisms.
Transplant Proc. 39:pp. 244–245. 2007; View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Murakami K, Takemura T, Hino S and
Yoshioka K: Urinary transforming growth factor-beta in patients
with glomerular diseases. Pediatr Nephrol. 11:334–336. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Powe CE, Levine RJ and Karumanchi SA:
Preeclampsia, a disease of the maternal endothelium: The role of
antiangiogenic factors and implications for later cardiovascular
disease. Circulation. 123:2856–2869. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Senatorski G, Paczek L, Sułowicz W,
Gradowska L and Bartłomiejczyk I: Urine activity of cathepsin B,
collagenase and urine excretion of TGF-beta 1 and fibronectin in
membranous glomerulonephritis. Res Exp Med (Berl). 198:199–206.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Blush J, Lei J, Ju W, Silbiger S, Pullman
J and Neugarten J: Estradiol reverses renal injury in Alb/TGF-beta1
transgenic mice. Kidney Int. 66:2148–2154. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Potier M, Elliot SJ, Tack I, Lenz O,
Striker GE, Striker LJ and Karl M: Expression and regulation of
estrogen receptors in mesangial cells: Influence on matrix
metalloproteinase-9. J Am Soc Nephrol. 12:241–251. 2001.PubMed/NCBI
|
|
25
|
Hodari AA, Smeby R and Bumpus FM: A
renin-like substance in the human placenta. Obstet Gynecol.
29:313–317. 1967.PubMed/NCBI
|
|
26
|
Symonds EM, Stanley MA and Skinner SL:
Production of renin by in vitro cultures of human chorion and
uterine muscle. Nature. 217:1152–1153. 1968. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Cheung KL and Lafayette RA: Renal
physiology of pregnancy. Adv Chronic Kidney Dis. 20:209–214. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Barreras A and Gurk-Turner C: Angiotensin
II receptor blockers. Proc (Bayl Univ Med Cent). 16:pp. 123–126.
2003; PubMed/NCBI
|
|
29
|
Gilbert JS, Ryan MJ, LaMarca BB, Sedeek M,
Murphy SR and Granger JP: Pathophysiology of hypertension during
preeclampsia: Linking placental ischemia with endothelial
dysfunction. Am J Physiol Heart Circ Physiol. 294:H541–H550. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Alexander BT, Cockrell K, Cline FD, Llinas
MT, Sedeek M and Granger JP: Effect of angiotensin II synthesis
blockade on the hypertensive response to chronic reductions in
uterine perfusion pressure in pregnant rats. Hypertension.
38:742–745. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kobori H, Nishiyama A, Harrison-Bernard LM
and Navar LG: Urinary angiotensinogen as an indicator of intrarenal
Angiotensin status in hypertension. Hypertension. 41:42–49. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wiesel P, Mazzolai L, Nussberger J and
Pedrazzini T: Two-kidney, one clip and one-kidney, one clip
hypertension in mice. Hypertension. 29:1025–1030. 1997. View Article : Google Scholar : PubMed/NCBI
|