Introduction
Coronary artery disease (CAD) is associated with
changes in coronary artery circulation due to atherosclerosis. An
imbalance between the vascular supply and myocardial demand for
blood could result in myocardial injury (1). The pathophysiological basis of CAD is
an imbalance between myocardial microcirculation and blood flow
regulation in the coronary artery (1,2).
Although coronary computed tomography angiography (CCTA) has shown
good diagnostic accuracy for the detection of CAD, the
physiological significance of numerous lesions can be uncertain
(3). Moreover, the presence of
calcified atherosclerotic plaque reduces the ability to
differentiate significant stenosis from non-obstructive plaque
(4). CT perfusion (CTP) imaging does
not only provide accurate anatomical information regarding the
coronary artery and heart, but also information regarding
myocardial perfusion and cardiac functions (5). In addition, dynamic enhanced CTP
requires separate imaging of the coronary artery and myocardial
perfusion that usually implies a high effective radiation dose
(3,6–12). The
dual-energy computed tomography (DECT) has two sets of X-ray tube
as well as two sets of detectors, which allows dual energy CT
imaging and can differentiate different tissues or organs in the
body according to the different intravascular iodine spectrum
signals by various levels of X-ray penetration. DECT has been
suggested as a potential approach to provide a one-stop source of
medical information regarding the coronary artery morphology and
myocardial blood supply (13–18). As
an improvement over first-generation DECT, second-generation DECT
enables a larger field of view (FOV) and less image noise in the
energy spectrum due to the installation of a spectrum photon shield
(SPS) on the CT tube (19,20). To the best of our knowledge, few
reports have been published concerning the application of the
detection of suspected myocardial infarction (MI) using DECT. The
aim of this study was to evaluate the diagnostic accuracy of DECT
myocardial perfusion imaging for the detection of myocardial
infarction in patients with suspected MI.
Materials and methods
Patient population
A total of 56 patients suspected to have MI who were
presented to Baotou Central Hospital with a symptom of chest pain
but without an abnormal Q wave or ST-segment elevation on an
electrocardiogram (ECG), or patients with an abnormal Q wave but
without typical chest pain in our hospital between August 2010 and
October 2013 were included in the present study. The present study
was approved by the institutional review board (IRB) of Baotou
Central Hospital and informed consent to review medical records was
obtained from each patient. Amongst these patients, 16 were
excluded (including 10 cases without follow-up records and 6 cases
unwilling to receive coronary artery angiography). A total of 40
cases were finally recruited (complete data included coronary CT
angiography, increased troponin I and dynamic ECG). In addition,
there were 23 males and 17 females, aged between 36 and 76 years
(average age, 55±10 years), and with body mass indexes (BMIs)
between 19.1 and 27.3 kg/m2. Patients were excluded if any of the
following conditions were present: Past history of MI or having an
already confirmed MI, having received stent implantation or
coronary artery bypass grafting and having arrhythmia or a BMI of
≥30 kg/m2. Cases were excluded from CTA if any of the following
conditions occurred: Contrast agent allergy, liver or kidney
dysfunction, cardiogenic shock, acute MI, decompensated cardiac
insufficiency and other symptoms not suitable for enhanced CT.
DECT scan protocol and image
reconstruction
All patients were examined using a 64-section DSCT
system (SOMATON Definition FLASH; Siemens AG, Forchheim, Germany)
with DE mode. The following acquisition parameters were used:
Detector collimation, 2×64×0.6 mm and slice acquisition, 2×128×0.6
mm by means of a z-flying focal spot, with a gantry rotation time
of 280 msec. The tube voltage and tube current were set as follows:
Pitch 1.2, tube current 104–158/91–158 per rotation and tube
voltage Sn140/100 Kv.
The contrast agent (Omnipaque 350 mgI/ml; GE
Healthcare Bio-Sciences, Pittsburgh, PA, USA) was intravenously
injected through the antecubital vein using a power injector
(SCT-210; Medrad, Inc., Indianola, PA, USA) with a 20-gauge needle
(Weihai Jierui Medical Products Co., Ltd, Weihai, China).
Contrast-agent application was controlled by the bolus-tracking
technique in the ascending aorta (signal attenuation threshold, 100
HU). Data acquisition was initiated after threshold was reached in
the ascending aorta, with a mean delay of 8 sec. A total of 80 ml
contrast agent was used, followed by 60 ml saline solution at flow
rates of 5 ml/sec.
A single detector image at 100 kV and with 140 msec
temporal resolution protocol raw data for reconstruction was used
for coronary artery evaluation. CT angiographic images were
reconstructed in a standard way using retrospective ECG-gating and
single-segment reconstruction in the best diastolic phase when
heart rate (HR) ≤70 bpm or best systolic when HR >70 bpm
(BestPhase; Siemens AG) with a slice thickness of 0.75 mm with an
increment of 0.4 mm, as well as a medium-soft convolution kernel
(B26f). For the evaluation of myocardial iodine content, images
from the same raw data set were then automatically reconstructed
into three image datasets (100 and 140 kVp, and an average weighted
image set, M_0.3) merging 70% of the 100 kVp and 30% of the 140 kVp
information. For the evaluation of myocardial iodine distribution
with dual-energy software, additional images from the same raw data
set were reconstructed with 1.0-mm thick slices, at 0.75-mm
increments, using a dedicated dual-energy convolution D30f kernel
(21). Further post-processing was
performed on a commercially available workstation (Syngo MMWP,
VA36; Siemens AG) using the dedicated DE heart-PBV application to
obtain myocardial ‘iodine maps’ in the short and long axes, and
four chamber views. These iodine maps, which represent the
myocardial blood pool, were 16-bit color-coded and then
superimposed onto the grayscale anatomic multiplanar reformations
of the myocardium. The resulting color-coded iodine maps were then
superimposed on gray-scale multiplanar reformats of the virtual
non-enhanced data sets of the myocardium in short and long axes
views of the left ventricle. Color coding was performed using
shades of green (‘PET’ template) with bright green coding for high
iodine content and black coding for a complete lack of iodine. For
image analysis, the iodine maps were superimposed as a 60% overlay
onto the grayscale multiplanar reformats with a slice thickness of
1.5 mm. Curved planar reformatting (thickness, 8.0 mm), maximum
intensity projection (thickness, 10.0 mm), multiplanar reformatting
and volume rendering were used to evaluate the coronary
arteries.
Image interpretation
Two additional radiologists who were experienced in
the field of cardiac imaging (3 and 5 years of experience)
independently analyzed all three reconstructed DECT series in a
random order. Coronary segments were defined according to the
American Heart Association (AHA) standards (22): i) Segments 1–4 included the right
coronary artery; ii) segment 5 included the left main artery; iii)
segments 6–10 included the left anterior descending artery; iv)
segments 11–15 included the left circumflex artery; and v) segment
16 included the intermediary artery. The quality of images of the
coronary were rated on a four-point scale (23) as follows: 1=Excellent, no artifacts;
2=good, mild artifacts, unrestricted evaluation; 3=evaluable,
moderate artifacts but still diagnostic combined with axial images;
and 4=unevaluable, severe artifacts leading to non-diagnostic
images on a per-segment basis (22).
Coronary artery stenosis was graded by visually observing the
diameter: Stenosis <50% was defined as mild; stenosis ≥50 and
<75% was defined as moderate; stenosis ≥75% was considered
severe and 100% stenosis was considered to be vessel occlusion.
The myocardium of the left ventricle was segmented
using a 17-segment model proposed by the AHA. DECT myocardial
perfusion images were evaluated as follows: Green color coding
indicated maximum blood perfusion; blue color coding indicated low
blood perfusion; and blood flow deficiency was indicated by the
absence of any color coding.
Effective radiation dose: The dose length product
(DLP) was calculated automatically by the scanner system. Effective
dose=k × DLP (the k value was determined according to the EU
guidelines on quality criteria for CT; k=0.014 mSv/mGycm for the
chest scan) (24).
Invasive angiography
Invasive coronary angiography was performed via a
transradial or transfemoral approach; 5- or 6-F diagnostic
catheters were used, and 0.2 mg nitroglycerin was injected into the
left and right coronary arteries, as described previously (25,26). At
least five projections were acquired for the left coronary artery
and at least two for the right coronary artery. Additional
projections were acquired as required. The presence of luminal
stenosis with a diameter reduction of ≥50% was assessed on a
per-segment, per-vessel, and per-patient level using visual
estimation by an independent observer blinded to CT findings.
Sensitivity, specificity, positive predictive value
(PPV), negative predictive value (NPV) of CTA, iodine map and CTA +
iodine map for detection of MI were calculated for each imaging
modality on a segment basis using the follow-up data, combining
invasive angiography findings, troponin I lever and dynamic ECG as
the gold standard. The mismatch between the iodine map and CTA was
defined as low perfusion in an iodine map whereas there was no
coronary occlusion or normal perfusion in the iodine map at the
occlusive coronary dominated region with CTA.
Statistical analysis
All statistical analyses were performed using SPSS
software, version 19.0 (IBM SPSS, Armonk, NY, USA). Continuous
variables are expressed as the mean ± standard deviation and
categorical variables are expressed as frequencies or percentages.
Sensitivity, specificity, PPV and NPV were calculated from χ2 tests
of contingency. Non-evaluative segments were considered as positive
findings. Moreover, statistics for diagnostic accuracy of coronary
CTA, iodine map and CTA + iodine map for detection of MI were
calculated on a per-segment, per-vessel and on a per-patient basis.
P<0.05 was used to indicate a statistically significant
difference.
Inter-observer agreements regarding the image
quality read-out and assessment of significant artery stenosis with
CTA were evaluated using Cohen's kappa statistics (kappa >0.81:
excellent agreement; kappa=0.61–0.80: good agreement;
kappa=0.41–0.60: moderate agreement; kappa=0.21–0.40: fair
agreement and kappa <0.20: poor agreement).
Results
Patient characteristics
All examinations were performed without
complications during or after the procedure. The average heart rate
was 65±7 bpm (range, 50–80 bpm). During follow-up, two patients
experienced a dynamic evolution of their MI, and three cases had a
pathological Q wave. The troponin I levels indicated eight patients
being positive and 32 being negative.
Diagnostic accuracy of CCTA
CCTA demonstrated that patients had the following:
12 patients had complete occlusion (five patients with anterior
descending branch, five in the right coronary artery, one with
anterior descending branch and one in the anterior descending
branch and circumflex). In total, two patients had severe stenosis,
four had mild stenosis, one patient had myocardial bridging and one
patient was suspected with anterior descending branch occlusion.
Moreover, 20 patients had normal coronary arteries. For 40 cases,
120 segments of the coronary artery were evaluated. Combining the
follow-up data (increased troponin I and dynamic ECG), there were
18 segments that had a true positive occlusion of the coronary
artery, nine had a false positive occlusion, 91 had a true negative
occlusion and two had a false negative.
Diagnostic accuracy of myocardial
iodine map with DECT
A total of 120 segments were evaluated by DECT
myocardial perfusion imaging. In total 24 segments were poorly
perfused regions with black coding indicating a complete lack of
iodine, while 96 were normal segments with bright green coding
indicating high iodine content. Combining invasive angiography
findings, troponin I lever and dynamic ECG as the gold standard, 17
segments were confirmed as infracted areas, while seven had a false
positive and three had a false negative. An inter-rater consistency
test demonstrated a kappa of 0.84.
Diagnostic accuracy of combining CCTA
and iodine map with DECT
There were 19 cases that had a true positive for MI,
while three were a false negative, 97 were a true negative and one
was a false negative. Invasive angiography failed to visualize the
distal segment of the left coronary artery in one case suspected of
having an occlusion. Right coronary artery angiography revealed
that the collateral circulation supplied the middle and distal
segments of the anterior descending branch. However, no infarct
region was observed on the myocardial iodine-distribution map with
DECT. Considering the normal troponin level, with no evident chest
pain or ECG results indicative of MI, this case was finally
diagnosed as having a congenital variation of the anterior
descending branch. The middle and distal segments were small, and
the right coronary artery supplied blood to this region. Moreover,
the inter-rater consistency test demonstrated a kappa value of
0.86. The sensitivity, specificity, PPV, and NPV of DECT myocardial
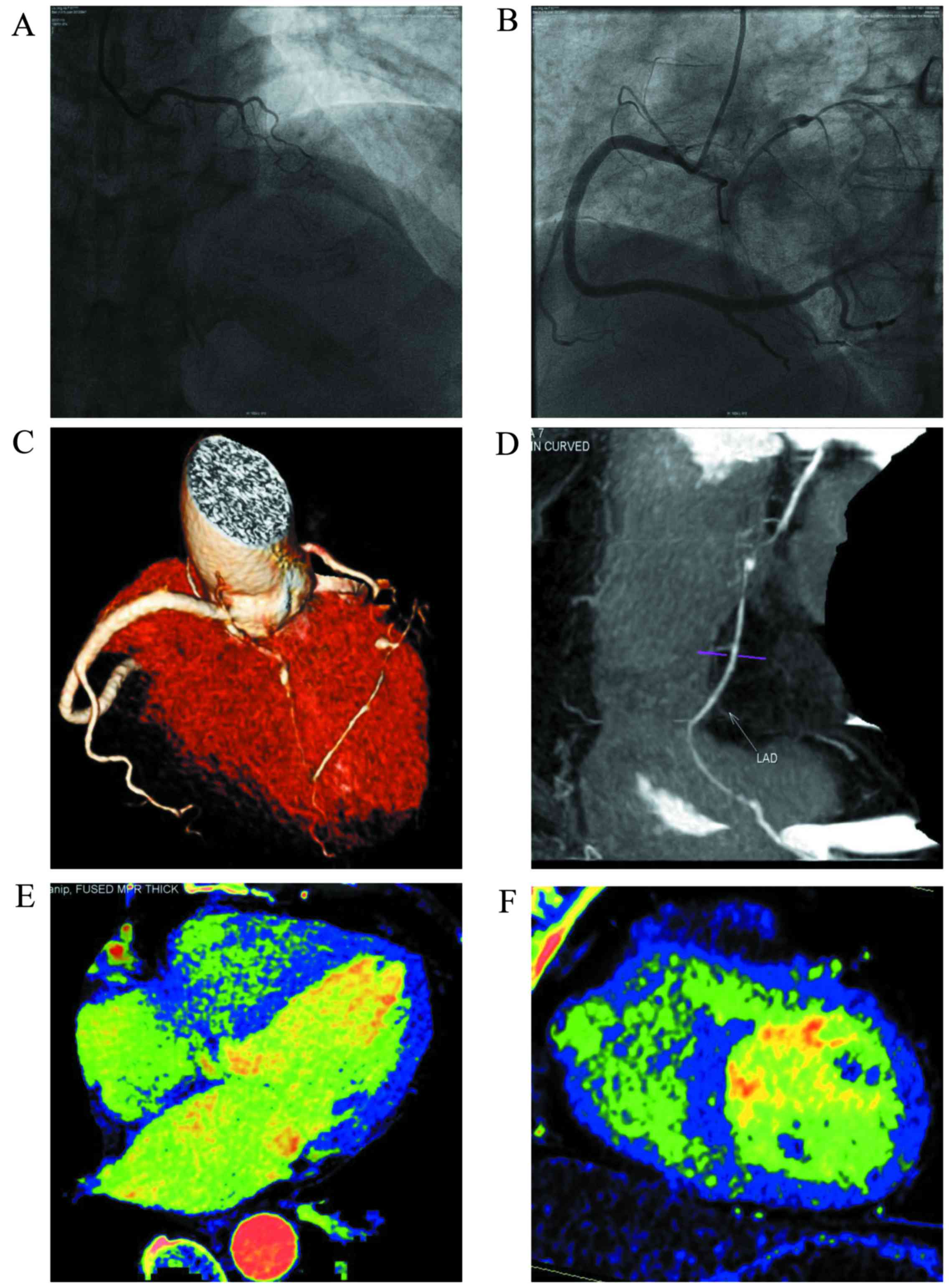
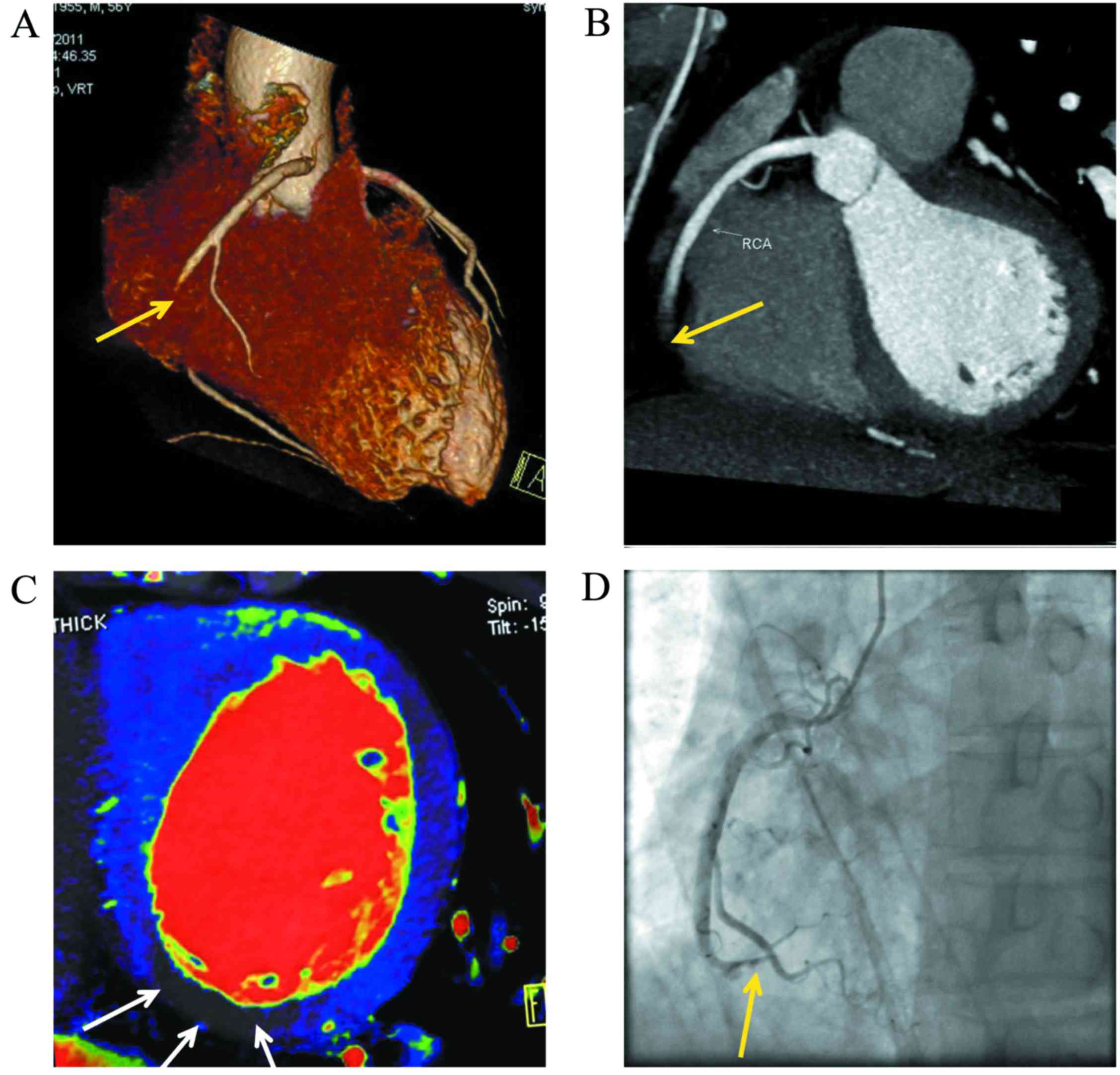
perfusion imaging for the detection of MI are shown in Table I. For typical cases see Figs. 1 and 2.
 | Table I.Diagnostic accuracy of dual-energy
computed tomography for myocardial infarction based on
cardiovascular evaluation. |
Table I.
Diagnostic accuracy of dual-energy
computed tomography for myocardial infarction based on
cardiovascular evaluation.
| Parameter | CTA only | Iodine only | CTA + iodine |
|---|
| True positive | 18 | 17 | 19 |
| False positive | 9 | 7 | 3 |
| True negative | 91 | 93 | 97 |
| False negative | 2 | 3 | 1 |
| Sensitivity (%) | 90.0 (95% CI,
68.2–98.4%) | 85.0 (95% CI,
62.1–96.6%) | 95.0 (95% CI,
75.1–99.2%) |
| Specificity (%) | 91.0 (95% CI,
83.6–98.4%) | 93 (95% CI,
86.1–97.1%) | 97.0 (95% CI,
91.5–99.3%) |
| Positive predictive
value (%) | 66.7 (95% CI,
46.1–83.4%) | 70.8 (95% CI,
48.9–87.3%) | 86.4 (95% CI,
65.1–96.9%) |
| Negative predictive
value (%) | 97.8 (95% CI,
92.4–99.6%) | 96.8 (95% CI,
91.1–99.3%) | 98.9 (95% CI,
94.4–99.8%) |
Qualitative analysis
In each patient, the subjective image quality
assessment of coronary was conducted based on the analysis of
segments. In total, 1,127 coronary segments were evaluated. The
inter-observer agreement for image quality rating was good
(kappa=0.63). In addition, diagnostic image quality of coronary
artery was observed in 99% (1,122/1,127) of all segments. Overall,
three patients had non-diagnostic images due to motion artifacts
because of coronary artery movement.
In addition, quality analysis of coronary artery
angiograms was performed. Out of 40 cases, one had artifacts with
poor middle and distal segments of the right coronary artery and
left circumflex that could not be evaluated. In another case, there
was a motion artifact in the middle and distal segments of the
right coronary artery because of serious heart rate variability.
However, the images were of excellent quality for the other cases.
In cases where the DECT scan failed, a retrospective ECG-gated
helical scan was performed. The quality scores for the coronary
artery images are shown in Table
II.
 | Table II.Qualitative analysis of image
quality. |
Table II.
Qualitative analysis of image
quality.
| Coronary artery |
| RCA |
|
| LM |
|
| LAD |
|
|
|
| CX |
|
| IA |
|---|
| Segment | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 |
| Evaluated
segments | 40 | 40 | 35 | 35 | 40 | 40 | 40 | 34 | 34 | 34 | 40 | 38 | 38 | 32 | 23 | 28 |
| Score 1 | 29 | 25 | 23 | 31 | 40 | 40 | 40 | 33 | 33 | 34 | 40 | 38 | 31 | 30 | 20 | 28 |
| Score 2 | 6 | 9 | 9 | 3 | 0 | 1 | 1 | 1 | 0 | 0 | 3 | 0 | 4 | 0 | 0 | 0 |
| Score 3 | 3 | 4 | 2 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| Score 4 | 2 | 2 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 0 |
The effective radiation dose was also calculated,
and the average DLP was 436±107.8 mGy.cm (219–779 mGy.cm), and the
average effective radiation dose was 6.1±1.5 mSv (3.1–10.9
mSv).
Discussion
The results of the present study demonstrate a
sensitivity of 95% and a specificity of 97% for detecting acute MI
using the DE single contrast-enhanced myocardial perfusion imaging
with the DECT. This ‘one-step’ DECT combined coronary CT
angiography and myocardial perfusion protocol provides a
comprehensive image quality of the coronary artery.
Ruzsics et al (27) initially reported the satisfactory
diagnostic accuracy of DECT for MI using myocardial perfusion
imaging in 2008. According to previous studies, the sensitivity of
the first generation DECT for the detection of MI/infarction using
myocardial perfusion is 72–93%, and its specificity is 72–94%
(13–18,27).
Compared with the first generation DECT, the tube rotational speed
in second generation DECT increases from 165 to 140 msec/cycle.
With a larger FOV and the installation of an SPS on the tube,
second generation DECT can achieve a more accurate iodine
concentration measurement and better quality of coronary artery
imaging.
Koonce et al (25) demonstrated the diagnostic accuracy of
DECT scans using a human model. Their results indicated that the
DECT provided a stable myocardial iodine-concentration measurement
and an accurate iodine concentration measurement for patients with
various BMIs. In the present study, the sensitivity of CCTA
combined with the myocardial iodine map detected by DECT was 95.0%
for MI, and the specificity was 97.0%. Both values were higher than
those for CCTA alone (sensitivity, 90.0%; specificity, 91.0%) and
the iodine map (specificity, 85.0%; specificity, 93%). The reasons
for the false positive and false negative of CCTA include the
following: Acute MI induced by an angiospasm accompanied by typical
chest pain symptoms, increased cardiac enzyme levels and a
perfusion defect indicated by DECT. The reasons for the false
positive and false negative results with the iodine maps are as
follows: A false positive may be caused by a heart beat-related
artifact and beam artifact secondary to an excess iodine
concentration in the left ventricle. A false positive is probably
attributed to a coronary spasm or patchy occlusion-induced
infarction of the myocardium. Following revascularization, no
cardiovascular abnormalities were observed, although the infracted
region had a perfusion defect. There are also other reasons for low
myocardial perfusion, including myocarditis. The results of the
present study indicated that coronary artery CTA combined with the
iodine map from DECT could further improve the diagnostic accuracy
for suspected MI. Quality evaluation of the coronary artery images
was also performed.
Previously published literature regarding DECT
imaging for myocardial perfusion mostly focused on the accuracy and
rarely on image quality. In previous studies, it was not possible
to obtain diagnostic coronary CT angiograms, even with the
intravenous administration of pharmacological agents to reduce
heart rate (14,18). In the present study, good coronary
artery image quality was obtained using the second generation DECT.
One possible reason may be the increase in temporal resolution of
the CT tube in the second-generation DECT scanner (an increase in
rotation speed from 165 to 140 msec/cycle). Moreover, the effective
radiation dose was also examined. Ruzsics et al (27) reported that the effective radiation
dose for DECT imaging of perfused myocardium was 4±5 mSv. However,
Kerl et al (15) proposed
that the effective radiation dose was 5.4±0.8 mSv during an animal
experiment. In the present study, the average effective radiation
dose was lower than what was reported for first generation DECT.
Moreover, a second generation DECT scan is a one-stop myocardial
and coronary artery examination that integrates myocardial
perfusion imaging and coronary artery CTA.
A number of limitations of the present study have to
be addressed. Firstly, there was a lack of inclusion of
single-photon emission CT or magnetic resonance imaging for
parallel comparison. However, future studies are warranted to
further investigate this area. Secondly, the small patient
population used in the present study and the lack of a reference
standard for the assessment of coronary artery stenosis
necessitates future studies using a larger population. Thirdly, the
results using DECT were focused on the detection of healed
infarction from a single-phase scan.
In conclusion, combined iodine maps and coronary CTA
using the one-step DECT myocardial perfusion imaging provide a high
diagnostic accuracy for detecting MI with lower radiation exposure
in patients with suspected MI.
References
|
1
|
Lanza GA and Crea F: Primary coronary
microvascular dysfunction: Clinical presentation, pathophysiology,
and management. Circulation. 121:2317–2325. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Osto E, Fallo F, Pelizzo MR, Maddalozzo A,
Sorgato N, Corbetti F, Montisci R, Famoso G, Bellu R, Lüscher TF,
et al: Coronary microvascular dysfunction induced by primary
hyperparathyroidism is restored after parathyroidectomy.
Circulation. 126:1031–1039. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Blankstein R, Shturman LD, Rogers IS,
Rocha-Filho JA, Okada DR, Sarwar A, Soni AV, Bezerra H, Ghoshhajra
BB, Petranovic M, et al: Adenosine-induced stress myocardial
perfusion imaging using dual-source cardiac computed tomography. J
Am Coll Cardiol. 54:1072–1084. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
King M, Rodgers Z, Giger ML, Bardo DM and
Patel AR: Computerized method for evaluating diagnostic image
quality of calcified plaque images in cardiac CT: Validation on a
physical dynamic cardiac phantom. Med Phys. 37:5777–5786. 2010.
View Article : Google Scholar
|
|
5
|
Kim SM, Choi JH, Chang SA and Choe YH:
Detection of ischaemic myocardial lesions with coronary CT
angiography and adenosine-stress dynamic perfusion imaging using a
128-slice dual-source CT: Diagnostic performance in comparison with
cardiac MRI. Br J Radiol. 86:201304812013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kurata A, Mochizuki T, Koyama Y, Haraikawa
T, Suzuki J, Shigematsu Y and Higaki J: Myocardial perfusion
imaging using adenosine triphosphate stress multi-slice spiral
computed tomography: Alternative to stress myocardial perfusion
scintigraphy. Circ J. 69:550–557. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cury RC, Magalhães TA, Borges AC, Shiozaki
AA, Lemos PA, Júnior JS, Meneghetti JC, Cury RC and Rochitte CE:
Dipyridamole stress and rest myocardial perfusion by 64-detector
row computed tomography in patients with suspected coronary artery
disease. Am J Cardiol. 106:310–315. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Okada DR, Ghoshhajra BB, Blankstein R,
Rocha-Filho JA, Shturman LD, Rogers IS, Bezerra HG, Sarwar A,
Gewirtz H, Hoffmann U, et al: Direct comparison of rest and
adenosine stress myocardial perfusion CT with rest and stress
SPECT. J Nucl Cardiol. 17:27–37. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rocha-Filho JA, Blankstein R, Shturman LD,
Bezerra HG, Okada DR, Rogers IS, Ghoshhajra B, Hoffmann U,
Feuchtner G, Mamuya WS, et al: Incremental value of
adenosine-induced stress myocardial perfusion imaging with
dual-source CT at cardiac CT angiography. Radiology. 254:410–419.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tamarappoo BK, Dey D, Nakazato R,
Shmilovich H, Smith T, Cheng VY, Thomson LE, Hayes SW, Friedman JD,
Germano G, et al: Comparison of the extent and severity of
myocardial perfusion defects measured by CT coronary angiography
and SPECT myocardial perfusion imaging. JACC Cardiovasc Imaging.
3:1010–1019. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Feuchtner GM, Plank F, Pena C, Battle J,
Min J, Leipsic J, Labounty T, Janowitz W, Katzen B, Ziffer J and
Cury RC: Evaluation of myocardial CT perfusion in patients
presenting with acute chest pain to the emergency department:
Comparison with SPECT-myocardial perfusion imaging. Heart.
98:1510–1517. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ko SM, Choi JW, Hwang HK, Song MG, Shin JK
and Chee HK: Diagnostic performance of combined noninvasive
anatomic and functional assessment with dual-source CT and
adenosine-induced stress dual-energy CT for detection of
significant coronary stenosis. AJR Am J Roentgenol. 198:512–520.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ruzsics B, Schwarz F, Schoepf UJ, Lee YS,
Bastarrika G, Chiaramida SA, Costello P and Zwerner PL: Comparison
of dual-energy computed tomography of the heart with single photon
emission computed tomography for assessment of coronary artery
stenosis and of the myocardial blood supply. Am J Cardiol.
104:318–326. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang LJ, Peng J, Wu SY, Yeh BM, Zhou CS
and Lu GM: Dual source dual-energy computed tomography of acute
myocardial infarction: Correlation with histopathologic findings in
a canine model. Invest Radiol. 45:290–297. 2010.PubMed/NCBI
|
|
15
|
Kerl JM, Deseive S, Tandi C, Kaiser C,
Kettner M, Korkusuz H, Lehmann R, Herzog C, Schoepf UJ, Vogl TJ and
Bauer RW: Dual energy CT for the assessment of reperfused chronic
infarction-a feasibility study in a porcine model. Acta Radiol.
52:834–839. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ko SM, Choi JW, Song MG, Shin JK, Chee HK,
Chung HW and Kim DH: Myocardial perfusion imaging using
adenosine-induced stress dual-energy computed tomography of the
heart: Comparison with cardiac magnetic resonance imaging and
conventional coronary angiography. Eur Radiol. 21:26–35. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang R, Yu W, Wang Y, He Y, Yang L, Bi T,
Jiao J, Wang Q, Chi L, Yu Y and Zhang Z: Incremental value of
dual-energy CT to coronary CT angiography for the detection of
significant coronary stenosis: Comparison with quantitative
coronary angiography and single photon emission computed
tomography. Int J Cardiovasc Imaging. 27:647–656. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Peng J, Zhang LJ, Schoepf UJ, Gibbs KP, Ji
HS, Yang GF, Zhu H and Lu GM: Acute myocardial infarct detection
with dual energy CT: Correlation with single photon emission
computed tomography myocardial scintigraphy in a canine model. Acta
Radiol. 54:259–266. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Petersilka M, Bruder H, Krauss B,
Stierstorfer K and Flohr TG: Technical principles of dual source
CT. Eur J Radiol. 68:362–368. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kang DK, Schoepf UJ, Bastarrika G, Nance
JW Jr, Abro JA and Ruzsics B: Dual-energy computed tomography for
integrative imaging of coronary artery disease: Principles and
clinical applications. Semin Ultrasound CT MR. 31:276–291. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Delgado S, ánchez-Gracián C, Oca Pernas R,
López C Trinidad, Armentia E Santos, de Liste A Vaamon, Vázquez
Caamaño M and Tardáguila de la Fuente G: Quantitative myocardial
perfusion with stress dual-energy CT: Iodine concentration
differences between normal and ischemic or necrotic myocardium.
Initial experience. Eur Radiol. 26:3199–3207. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Austen WG, Edwards JE, Frye RL, Gensini
GG, Gott VL, Griffith LS, McGoon DC, Murphy ML and Roe BB: A
reporting system on patients evaluated for coronary artery disease.
Report of the ad hoc committee for grading of coronary artery
disease, council on cardiovascular surgery, American heart
association. Circulation. 51 4 Suppl:S5–S40. 1975. View Article : Google Scholar
|
|
23
|
Ghadri JR, Küest SM, Goetti R, Fiechter M,
Pazhenkottil AP, Nkoulou RN, Kuhn FP, Pietsch C, von Schulthess P,
Gaemperli O, et al: Image quality and radiation dose comparison of
prospectively triggered low-dose CCTA: 128-slice dual-source
high-pitch spiral versus 64-slice single-source sequential
acquisition. Int J Cardiovasc Imaging. 28:1217–1225. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Korn A, Fenchel M, Bender B, Danz S,
Thomas C, Ketelsen D, Claussen CD, Moonis G, Krauss B, Heuschmid M,
et al: High-pitch dual-source CT angiography of supra-aortic
arteries: Assessment of image quality and radiation dose.
Neuroradiology. 55:423–430. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Koonce JD, Vliegenthart R, Schoepf UJ,
Schmidt B, Wahlquist AE, Nietert PJ, Bastarrika G, Flohr TG and
Meinel FG: Accuracy of dual-energy computed tomography for the
measurement of iodine concentration using cardiac CT protocols:
Validation in a phantom model. Eur Radiol. 24:512–518. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Budoff MJ, Dowe D, Jollis JG, Gitter M,
Sutherland J, Halamert E, Scherer M, Bellinger R, Martin A, Benton
R, et al: Diagnostic performance of 64-multidetector row coronary
computed tomographic angiography for evaluation of coronary artery
stenosis in individuals without known coronary artery disease:
Results from the prospective multicenter ACCURACY (Assessment by
Coronary Computed Tomographic Angiography of Individuals Undergoing
Invasive Coronary Angiography) trial. J Am Coll Cardiol.
52:1724–1732. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ruzsics B, Lee H, Zwerner PL,
Gebregziabher M, Costello P and Schoepf UJ: Dual-energy CT of the
heart for diagnosing coronary artery stenosis and myocardial
ischemia-initial experience. Eur Radiol. 18:2414–2424. 2008.
View Article : Google Scholar : PubMed/NCBI
|