Introduction
Postoperative cognitive dysfunction (POCD) is an
illness characterized by cognitive decline in patients who have had
to undergo surgery. Patients with POCD may have attention defects,
poor concentration, language impairments or spatial memory loss
(1,2). Accumulative clinical evidence indicates
that patients undergoing surgery in the absence of general
anesthesia are also at risk of developing POCD (3,4). Due to
the fact that no influence from anesthesia has been identified in
association with POCD, attention has shifted to focus on the
association of the surgical intervention itself with POCD.
Nevertheless, the pathogenesis of POCD remains poorly understood
and there is a lack of effective management for this disorder.
Previous studies have demonstrated that surgery may
lead to the development of POCD by inducing neuroinflammation. One
study identified increased production of proinflammatory cytokines,
such as TNF-α, after surgery (5). In
addition, major surgery results in the activation of microglia in
the brain (6). Despite the fact that
surgical intervention is commonly accompanied by inflammation, only
a minor proportion of the patients receiving major surgery suffer
from POCD (7). The association
between neuroinflammation and cognitive decline in patients after
major surgery remains largely unclear.
Endoplasmic reticulum stress (ERS) is involved in
various cellular processes, such as cell growth, differentiation
and apoptosis. Upon stimulation, the initiation of ERS results in
impaired protein synthesis and the upregulation of molecular
chaperones that promote correct protein folding and cell survival
under harmful conditions (8);
however, sustained ERS contributes to the activation of the
apoptotic cascade (9). It is
understood that ERS is capable of inducing mitochondria- and death
receptor-independent apoptosis (10). In these cases, the upregulation of
ERS-related transcription factors, including glucose-regulated
protein (GRP)78 and CCAAT-enhancer-binding homologous protein
(CHOP) have been identified (11,12). The
involvement of ERS has been widely observed in the brain
pathogenesis of neurological disorders and neurodegenerative
diseases, including cerebral ischemia, Alzheimer's disease,
Parkinson's disease and amyotrophic lateral sclerosis (9,13,14).
Nonetheless, to the best of our knowledge, the regulatory role of
ERS in the brain damage of POCD has not been described.
Edaravone, a free radical scavenger, exerts
neuroprotective effects (15). It
has been reported that edaravone is able to prevent hypoxia- and
ischemia-induced ERS and improve neurological status in mice
(16). The present study aimed to
investigate whether treatment with edaravone attenuates
surgery-induced cognitive decline and to determine the involvement
of ERS in apoptotic neuronal injury in mice after surgery.
Materials and methods
Ethics statement
All animal experiments were approved by the Animal
Care and Use Committee of the China Medical University (Shenyang,
China) and were conducted according to the guidelines for care and
use of laboratory animals outlined by the Chinese Academy of
Science (Beijing, China).
Animals
A total of 96 14-month old C57BL/6 female mice (38±2
g) were used in the present study. Mice were housed in a
pathogen-free environment under a 12-h light-dark cycle at 23°C and
40–70% humidity with free access to food and water.
Surgical procedures and treatment
protocol
C57BL/6 mice were randomly assigned to three groups,
with 32 mice in each group. The groups included a control group
(group C), a surgery group (group S) and an edaravone group (group
E). Half of the mice in each group (n=16) were used in Morris water
maze (MVM) and T-maze tests, while the remaining mice were used for
pathological examinations. In the surgery group, mice received
local anesthesia by subcutaneous injection of 0.5% bupivacaine (0.1
ml; Shanghai Fuxing Chaohui Pharmaceutical Co., Ltd., Shanghai,
China) into the abdominal area. A 2.5-cm incision was subsequently
made in the middle of the abdomen, and the abdominal cavity was
opened and subsequently closed back up. The entire procedure lasted
~5 min. The day on which mice received surgery was defined as day
0. On postoperative days 1 and 2, mice were administered with 2.5%
lidocaine (Shanghai Fuxing Chaohui Pharmaceutical Co., Ltd.,) to
relieve pain. In the control group, mice received the same
anesthetic treatment as the surgery group; however, the incision
intervention was not performed. In the edaravone group, mice
underwent abdominal surgery followed by daily administration of 3
mg/kg edaravone (Xiansheng Pharmaceutical Corp., Nanjing, China)
continued until the end of the experiment.
MWM test
In order to elucidate the effects of edaravone on
the spatial learning ability of mice, the MWM test was performed
(17). Mice were tested daily, with
three trials per day, for 7 consecutive days after surgery. After
each trial of the MWM test, the wound of each mouse was immediately
dried to avoid infection, as described previously (18). In each trial, the animal was placed
in a different starting quadrant and allowed to swim. The overall
swimming distance, swimming speed and escape latency to the hidden
platform were recorded by a video camera. Data were analyzed using
HVS image water maze 2020 software (HVS Image Software Ltd,
Buckingham, UK). Additionally, probe trials were conducted on
postoperative days 1, 3 and 7, in order to evaluate the retention
memory of the mice. In the probe trials, the platform was removed
from the water and the time spent in the target quadrant that
previously contained the submerged platform was recorded during a
time period of 90 sec.
T-maze tests
The T-maze test is a widely-used behavioral test
that enables the evaluation of the cognitive ability of rodents
(19). During testing, mice were
subjected to a restricted feeding schedule of 85% of their
free-feeding weight. Mice were placed into a T-shaped maze and were
left to collect reward foods (small sugar cubes). Each test trial
consisted of a sample run and a choice run with an interval of 10
sec between the sample and choice runs. In the sample run, animals
were placed into the starting arm and were forced to visit one
particular arm to get a reward, while the other arm was blocked. In
the choice run, the blocked door was removed and the mice were
allowed a free choice of either arm. If mice entered the previously
non-visited arm, they were rewarded. The time interval between the
sample and choice runs was further increased to 90 and 180 sec.
Each daily session included five trials, and mice participated in
one trial at a time with an intra-trial interval of 10 min.
Pathological examination
On postoperative day 3, the hippocampus was removed
from 16 mice in each group and tissues were fixed in 10% formalin
solution at room temperature for 24 h. Samples were subsequently
embedded in paraffin and sectioned. Sections of 6 µm in thickness
were used for Nissl staining and the number of survived hippocampal
neurons per 1 mm was counted under a Nikon Eclipse E800 microscope
(Nikon Corporation, Tokyo, Japan). The average number was
calculated from three sections of bilateral hippocampal slices.
Immunohistochemistry
For immunohistochemistry, 5-µm thick hippocampal
sections were blocked in 2% normal goat serum (Beijing Solarbio
Science & Technology Co., Ltd. Beijing, China). Following
blockage, samples were incubated with primary rabbit monoclonal
anti-GRP78 (sc:376878; 1:20) or polyclonal anti-CHOP antibodies
(15204-1-AP; 1:100; both from Santa Cruz Biotechnology, Inc.,
Dallas, TX, USA) overnight at 4°C. Following three washes with
phosphate-buffered saline (PBS), sections were probed with a
biotin-conjugated anti-rabbit secondary antibody (SE205; 1:100) at
37°C for 30 min. Negative control samples were incubated with PBS
in the absence of a primary antibody. Following three washes with
PBS, the reaction product was visualized using diaminobenzidine
(DAB). The integrated optical density (OD) of GRP78- or
CHOP-positive staining in the hippocampal region was evaluated
using a MetaMorph 2.0 software system (Molecular Devices, LLC,
Sunnyvale, CA, USA).
Terminal deoxynucleotidyl transferase
(TdT) dUTP nick-end labeling (TUNEL) staining
To determine cell apoptosis in the hippocampus,
TUNEL staining was conducted according to the manufacturer's
instructions of a TUNEL assay kit (KGA702; Kaiji, Nanjing, China).
In brief, the hippocampal sections from the different experimental
groups were exposed to the TUNEL reaction mixture at 37°C for 60
min. Following this, samples were incubated with horseradish
peroxidase-conjugated antibody (1:100; KGA702; Kaiji, Nanjing,
China) at 37°C for 30 min, followed by a 10-min incubation with DAB
solution at room temperature. Nuclei were counterstained with
hematoxylin. Samples were examined using an Olympus BX51 optical
microscope (Olympus Corporation, Tokyo, Japan; magnification,
×400). Cells exhibiting brown nuclei under DAB staining were
considered to be apoptotic. The mean integrated OD of
TUNEL-positive staining in the hippocampal samples was
determined.
Statistical analysis
SPSS 16.0 software (SPSS, Inc., Chicago, IL, USA)
was used for statistical analysis. Comparison between groups was
made using one-way analysis of variance, followed by the
Student-Newman-Keuls test. Data were presented as the mean ±
standard error of the mean. P<0.05 was considered to indicate a
statistically significant difference.
Results
Edaravone attenuates surgery-induced
cognitive impairment
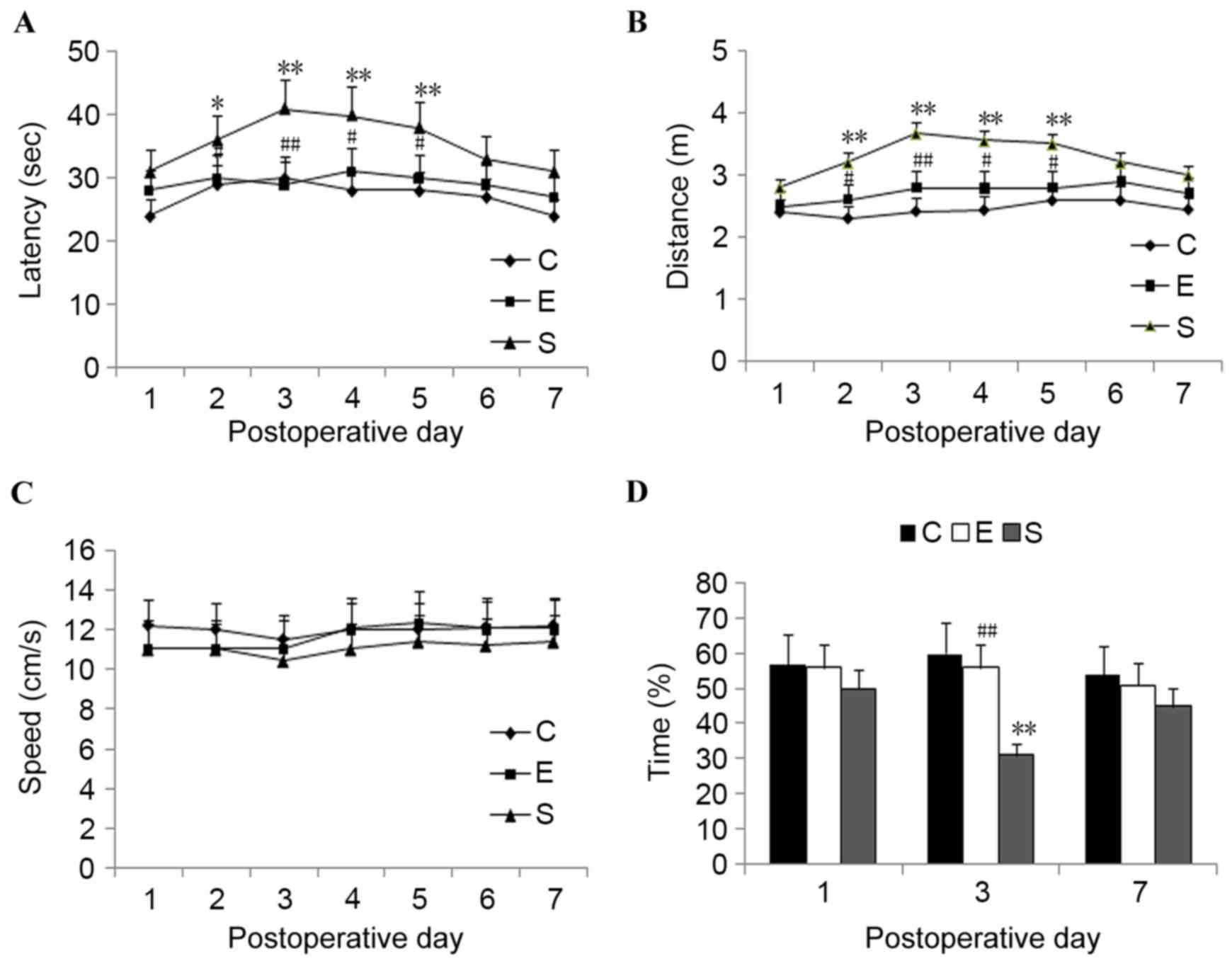
Results from the MWM tests revealed that, compared
with those in the surgery group, the escape latency was
significantly reduced in mice treated with edaravone on
postoperative day 2 (P<0.05) and postoperative days 3 to 5
(P<0.01; Fig. 1A). The swimming
distance was also significantly reduced in the edaravone group from
postoperative days 2 to 5 compared with the surgery group
(P<0.05: Fig. 1B). No significant
difference was detected in the escape latency or swimming distance
between the edaravone group and the control group (P>0.05).
There was no significant difference in the swimming speed among the
three experimental groups (P>0.05; Fig. 1C).
In the probe trial, it was demonstrated that the
mice treated with edaravone spent a significantly longer time in
the target quadrant compared with mice in the surgery group on
postoperative day 3 (P<0.01; Fig.
1D). However, comparison of the cumulative time spent in the
target quadrant demonstrated no significant difference between the
edaravone and control groups on postoperative days 1, 3 and 7.
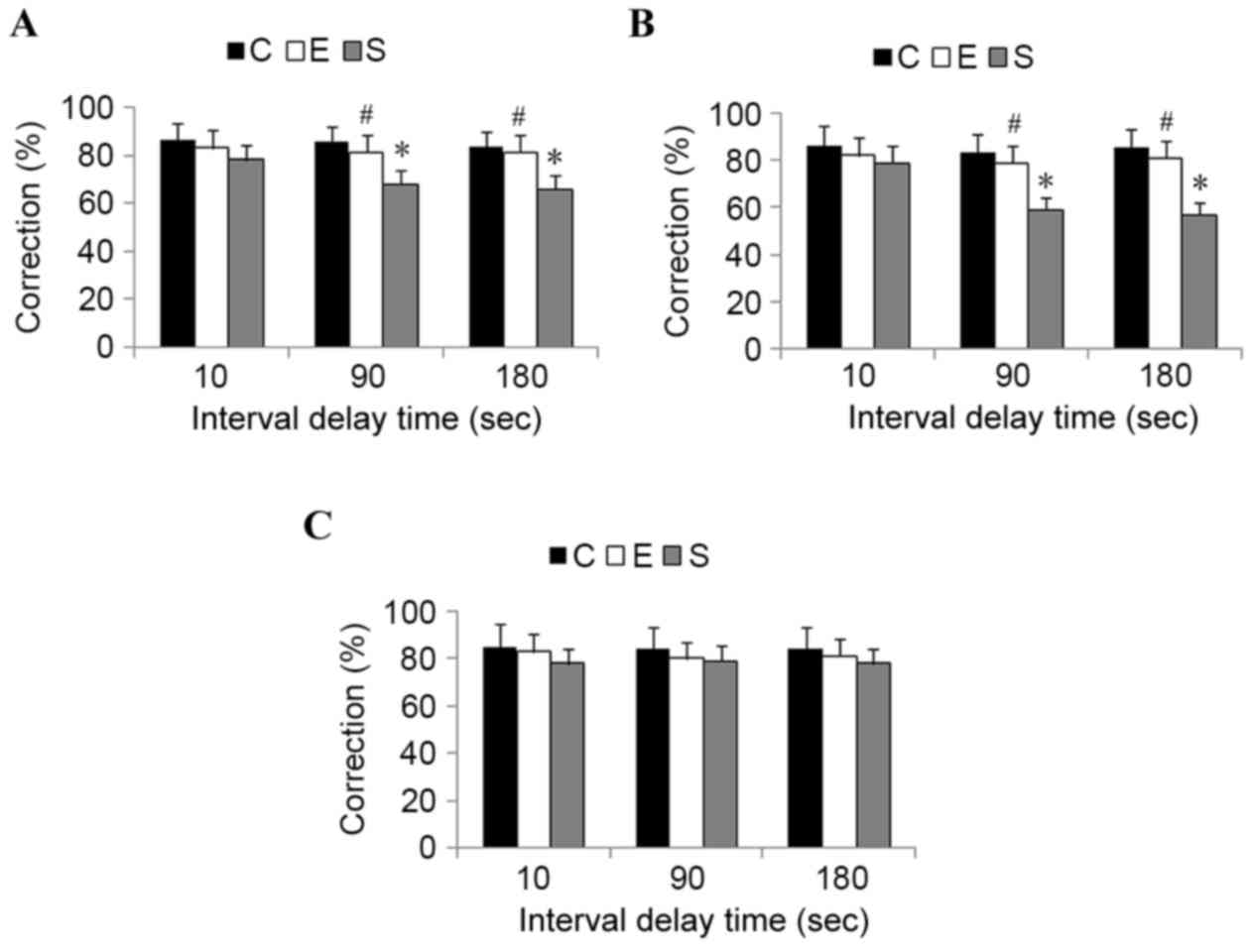
In order to evaluate spatial working memory
function, T-maze tests were conducted. On postoperative days 1, 3
and 7, a similar learning curve was detected between control mice
and mice from the edaravone group (P>0.05). However, compared
with mice in the surgery group, a significantly superior
performance was demonstrated in the edaravone group when increasing
the interval between the sample and choice runs to 90 and 180 sec
on postoperative days 1 and 3 (P<0.05; Fig. 2). These results indicate that
edaravone administration significantly attenuates surgery-induced
cognitive impairment in mice.
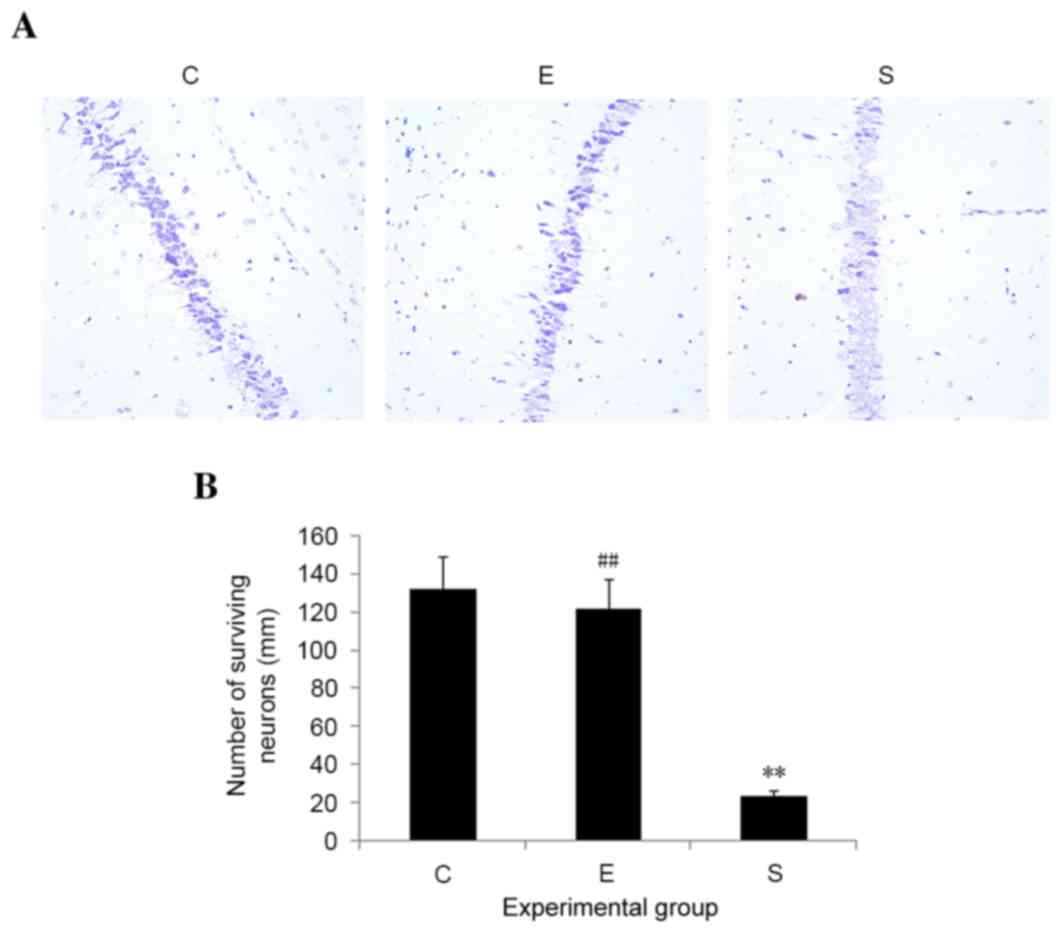
Edaravone reduces neuronal loss in
mice after surgery
Neuronal loss, particularly in the hippocampal
region, commonly occurs during stress injury. In order to examine
whether edaravone inhibited neuronal loss as a result of surgery,
histological changes in the hippocampus on postoperative day 3 were
examined. No signs of histopathological abnormalities were observed
in the hippocampal samples from the control mice; however, mice in
the surgery group exhibited severe neuronal damage in the
hippocampus. Edaravone significantly reduced neuronal loss in the
hippocampus, as compared with those in surgery group (P<0.01;
Fig. 3). These results suggest that
edaravone may inhibit hippocampal neuronal death induced by
surgery.
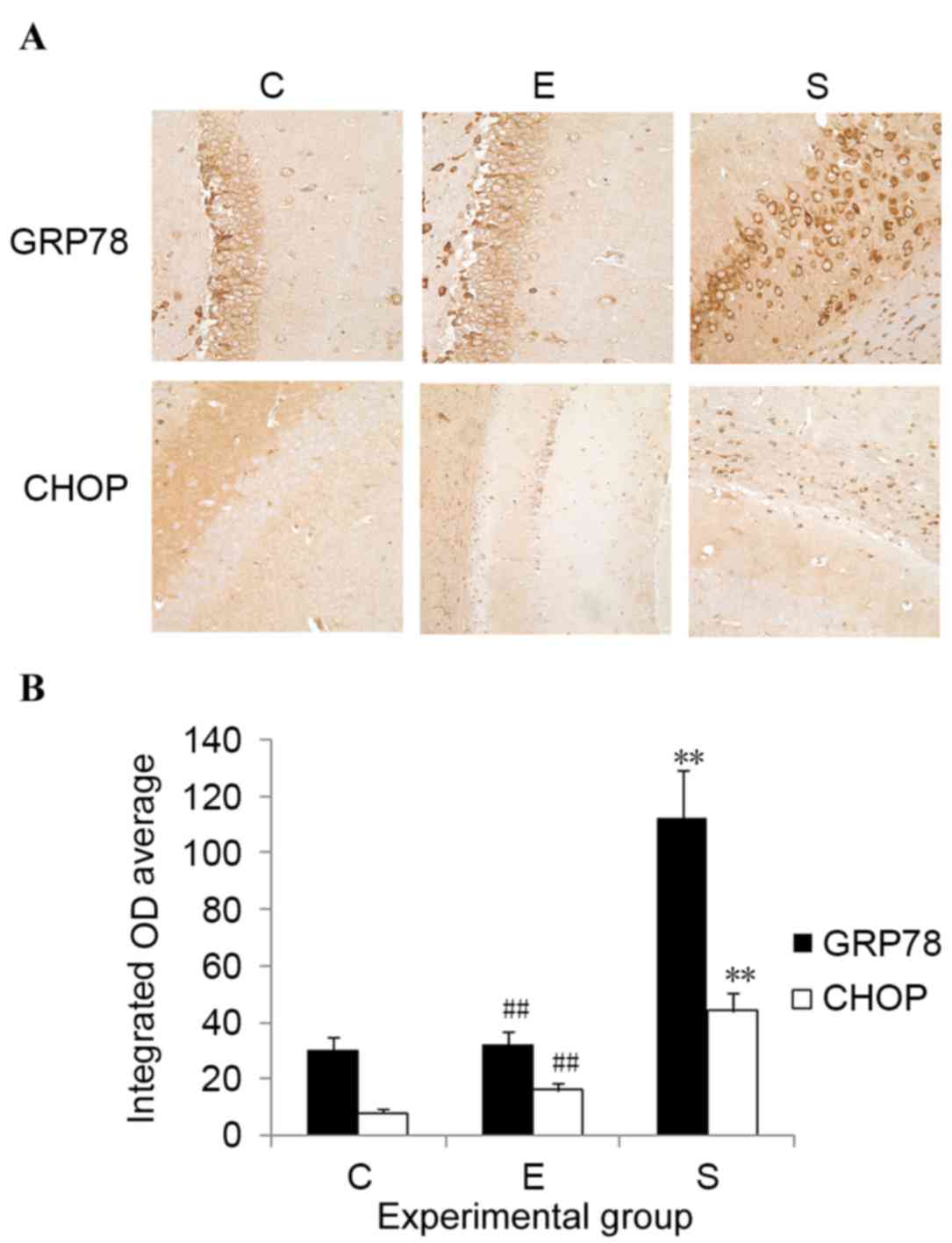
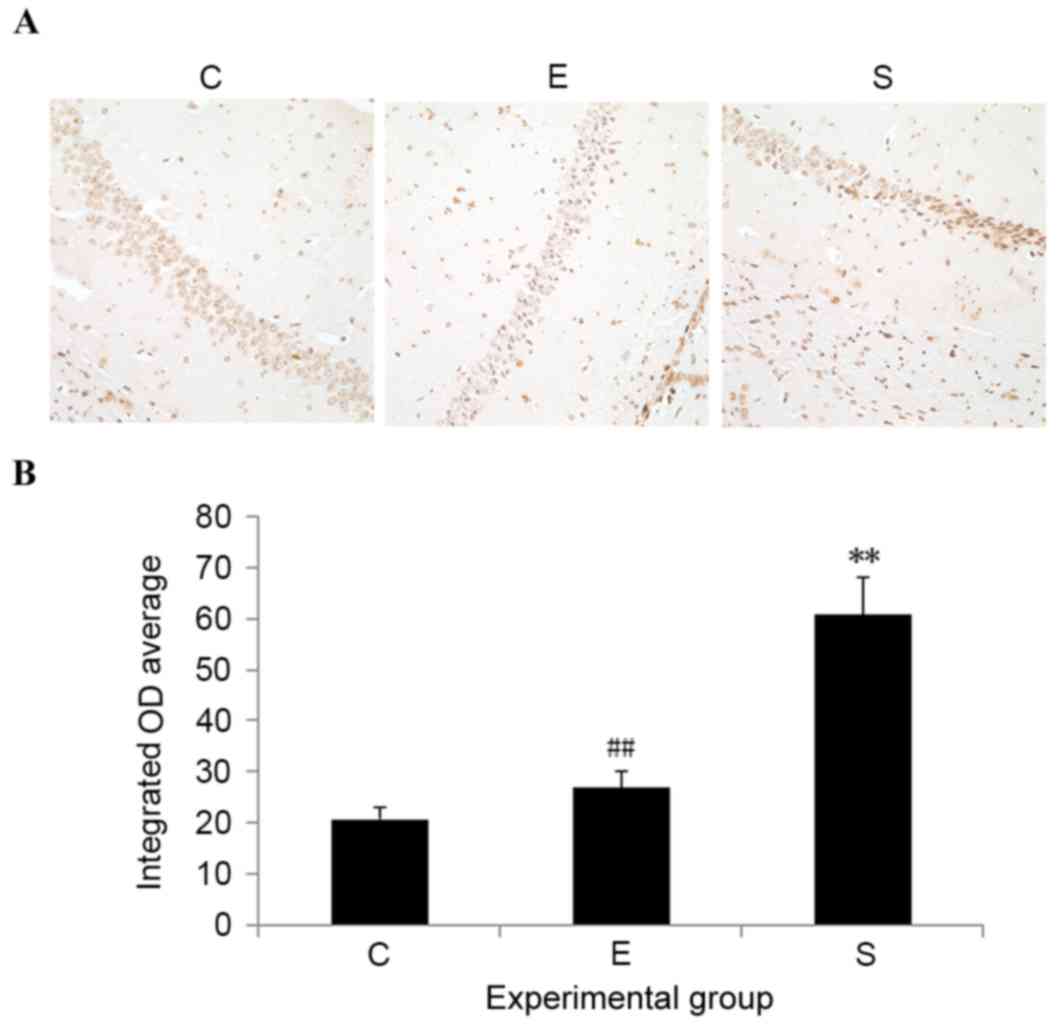
Edaravone downregulates GRP78 and CHOP
expression levels in the hippocampus after surgery
To determine the potential involvement of ERS in
edaravone-mediated neuroprotection, the expression levels of
ERS-related proteins, including GRP78 and CHOP, were measured.
Results indicated that, compared with control mice, the protein
expression levels of both GRP78 and CHOP were significantly
upregulated in the hippocampus on postoperative day 3 in the
surgery group (P<0.01; Fig. 4).
Administration of edaravone significantly reduced the upregulation
of GRP78 and CHOP expression levels in the hippocampus after
surgery (P<0.01; Fig. 4).
Compared with the control mice, no significant difference was
demonstrated in the GRP78 and CHOP expression levels of mice
treated with edaravone. These results indicate that the protective
role of edaravone in mice after surgery may be associated with its
capability to inhibit ERS.
Edaravone inhibits hippocampal neuron
apoptosis after surgery
To evaluate cell apoptosis, TUNEL staining was
performed. On postoperative day 3, mice that only received
abdominal surgery exhibited significant cell apoptosis in the
hippocampus, with an increased number of TUNEL-positive cells, when
compared with control mice (P<0.01; Fig. 5). Edaravone application significantly
prevented cell apoptosis in the hippocampus induced by surgery
(P<0.01; Fig. 5). No significant
difference was detected in the number of TUNEL-positive cells
between the edaravone and control groups. These results suggest
that edaravone may exert neuroprotection in mice after surgery
through inhibiting hippocampal neuron apoptosis.
Discussion
General anesthesia has been demonstrated to have an
important role in the pathogenesis of POCD (20,21);
however, other studies have indicated that there is no causative
relationship between general anesthesia and POCD (3,22). To
rule out the potential influences of general anesthesia on
cognitive impairment, the present study established a murine model
of abdominal surgery under local anesthesia. Results demonstrated
that, under local anesthesia, the abdominal surgical intervention
resulted in cognitive impairment in mice; however, treatment with
edaravone was able to attenuate cognitive decline induced by
surgery. The present study indicated that the therapeutic effect of
edaravone on POCD may be related to its efficacy in inhibiting
ERS-induced apoptosis in mice after surgery.
By using MWM tests, the present study compared the
spatial memory in the three experimental groups. The results
demonstrated that the escape latency, as well as the swimming
distance, of mice that underwent surgery were longer, and the time
spent in the target quadrant was significantly shorter than those
demonstrated by mice in the control and edaravone groups. These
results suggest that abdominal surgery resulted in spatial memory
defects in mice, which were able to be effectively prevented by the
administration of edaravone. Furthermore, the present study
evaluated the working memory in the three experimental groups of
mice using T-maze tests, the difficulty of which was manipulated by
systematically varying the interval between the sample and choice
run trials. Consistent with the MWM test results, edaravone was
able to prevent the impairments of the working memory induced by
surgery in mice. These findings suggest that edaravone prevented
the cognitive decline in mice following surgery.
In addition, the present study demonstrated that
surgery resulted in neuronal apoptosis in the hippocampus of mice,
with the presence of an increased number of TUNEL-positive cells in
the hippocampal region of the brain. Apoptosis is a complex
intracellular cascade, with multiple signals and pathways involved
in the initiation of cellular apoptosis. However, the upstream
mechanism of surgery-induced neuronal apoptosis remains
unknown.
It is understood that stress may result in cognitive
dysfunction (23). In the present
study, mice were given peripheral surgery under local anesthesia.
Therefore, it is not possible to completely exclude the potential
influences of stress on postoperative cognitive decline. A previous
study demonstrated that cellular stress results in ERS by enhancing
the phosphorylation of eukaryotic translation initiation factor 2A
(24).
Emerging evidence suggests that neuronal cell
apoptosis induced by ERS is a predominant pathological issue in
several neurological disorders (25,26).
ERS-driven apoptosis is accompanied by the increased expression of
ERS indicators, such as CHOP and GRP78 (27). CHOP is a transcription factor, the
physiological level of which is relatively low; however, the
protein expression of CHOP may be strongly induced in response to
ERS under diverse pathological conditions (28). Similarly, upregulated expression of
GRP78 is often regarded as an indicator of ERS (29).
In the present study, significant upregulation of
CHOP and GRP78 expression levels were found in the hippocampus of
mice following surgery. Furthermore, the number of TUNEL-positive
cells was significantly increased in the hippocampal region of mice
that received abdominal surgery. It is likely that surgery results
in hippocampal cell apoptosis by inducing ERS, ultimately
contributing to neuronal loss and cognitive impairment in mice.
Notably, treatment with edaravone significantly suppressed the
protein expression levels of CHOP and GRP78 and reduced the number
of TUNEL-positive cells in the hippocampus of mice following
surgery. Thus, it was hypothesized that edaravone yielded a
neuroprotective effect against surgery-induced ERS and hippocampal
apoptosis, and therefore improved spatial learning and working
memory in mice following surgery.
In conclusion, the findings of the present study
demonstrated that ERS-mediated neuronal apoptosis in the
hippocampus has an important role in POCD. The edaravone-induced
amelioration of cognition may be partly attributed to its potency
of inhibiting ERS-induced neuronal apoptosis in the hippocampus
after surgery.
Acknowledgements
The present study was supported by grants from the
Liaoning Province Nature Science Foundation of China (grant nos.
2012408002, 2012225021-73 and 2015010471-301).
Glossary
Abbreviations
Abbreviations:
|
POCD
|
postoperative cognitive
dysfunction
|
|
GRP
|
glucose-regulated proteins
|
|
CHOP
|
CCAAT-enhancer-binding homologous
protein
|
|
ERS
|
endoplasmic reticulum stress
|
|
MWM
|
Morris water maze
|
References
|
1
|
Hansen MV: Chronobiology, cognitive
function and depressive symptoms in surgical patients. Dan Med.
61:B49142014.
|
|
2
|
Steinmetz J, Christensen KB, Lund T, Lohse
N and Rasmussen LS; ISPOCD Group, : Long-term consequences of
postoperative cognitive dysfunction. Anesthesiology. 110:548–555.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Newman S, Stygall J, Hirani S, Shaefi S
and Maze M: Postoperative cognitive dysfunction after noncardiac
surgery: A systematic review. Anesthesiology. 106:572–590. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sauër AM, Kalkman C and van Dijk D:
Postoperative cognitive decline. J Anesth. 23:256–259. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Terrando N, Monaco C, Ma D, Foxwell BM,
Feldmann M and Maze M: Tumor necrosis factor-alpha triggers a
cytokine cascade yielding postoperative cognitive decline. Proc
Natl Acad Sci USA. 107:pp. 20518–20522. 2010; View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wan Y, Xu J, Meng F, Bao Y, Ge Y, Lobo N,
Vizcaychipi MP, Zhang D, Gentleman SM, Maze M and Ma D: Cognitive
decline following major surgery is associated with gliosis,
β-amyloid accumulation, and Τ phosphorylation in old mice. Crit
Care Med. 38:2190–2198. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Evered L, Scott DA, Silbert B and Maruff
P: Postoperative cognitive dysfunction is independent of type of
surgery and anesthetic. Anesth Analg. 112:1179–1185. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Matus S, Glimcher LH and Hetz C: Protein
folding stress in neurodegenerative diseases: A glimpse into the
ER. Curr Opin Cell Biol. 23:239–252. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Oakes SA and Papa FR: The role of
endoplasmic reticulum stress in human pathology. Annu Rev Pathol.
10:173–194. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nakagawa T, Zhu H, Morishima N, Li E, Xu
J, Yankner BA and Yuan J: Caspase-12 mediates
endoplasmic-reticulum-specific apoptosis and cytotoxicity by
amyloid-beta. Nature. 403:98–103. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fradejas N, Pastor MD, Burgos M, Beyaert
R, Tranque P and Calvo S: Caspase-11 mediates ischemia-induced
astrocyte death: Involvement of endoplasmic reticulum stress and
C/EBP homologous protein. J Neurosci Res. 88:1094–1105.
2010.PubMed/NCBI
|
|
12
|
Zhao Y, Yan Y, Zhao Z, Li S and Yin J: The
dynamic changes of endoplasmic reticulum stress pathway markers
GRP78 and CHOP in the hippocampus of diabetic mice. Brain Res Bull.
111:27–35. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Su Y and Li F: Endoplasmic reticulum
stress in brain ischemia. Int J Neurosci. 126:681–691. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Shah SZ, Zhao D, Khan SH and Yang L:
Unfolded protein response pathways in neurodegenerative diseases. J
Mol Neurosci. 57:529–537. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tabrizchi R: Edaravone Mitsubishi-Tokyo.
Curr Opin Investig Drugs. 1:347–354. 2000.PubMed/NCBI
|
|
16
|
Qi X, Okuma Y, Hosoi T and Nomura Y:
Edaravone protects against hypoxia/ischemia induced endoplasmic
reticulum dysfunction. J Pharmacol Exp Ther. 311:388–393. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Vorhees CV and Williams MT: Morris water
maze: Procedures for assessing spatial and related forms of
learning and memory. Nat Protoc. 1:848–858. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Saab BJ, Maclean AJ, Kanisek M, Zurek AA,
Martin LJ, Roder JC and Orser BA: Short-term memory impairment
after isoflurane in mice is prevented by the α5 γ-aminobutyric acid
type A receptor inverse agonist L-655,708. Anesthesiology.
113:1061–1071. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Deacon RM and Rawlins JN: T-maze
alternation in the rodent. Nat Protoc. 1:7–12. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sanders RD, Xu J, Shu Y, Januszewski A,
Halder S, Fidalgo A, Sun P, Hossain M, Ma D and Maze M:
Dexmedetomidine attenuates isoflurane-induced neurocognitive
impairment in neonatal rats. Anesthesiology. 110:1077–1085. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li Y, Zeng M, Chen W, Liu C, Wang F, Han
X, Zuo Z and Peng S: Dexmedetomidine reduces isoflurane-induced
neuroapoptosis partly by preserving PI3K/Akt pathway in the
hippocampus of neonatal rats. PLoS One. 9:e936392014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Rasmussen LS, Johnson T, Kuipers HM,
Kristensen D, Siersma VD, Vila P, Jolles J, Papaioannou A,
Abildstrom H, Silverstein JH, et al: Does anesthesia cause
postoperative cognitive dysfunction? A randomized study of regional
versus general anesthesia in 438 elderly patients. Acta
Anaesthesiol Scand. 47:260–266. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Marcos B, Aisa B and Ramirez MJ:
Functional interaction between 5-HT(6) receptors and
hypothalamic-pituitary-adrenal axis: Cognitive implications.
Neuropharmacology. 54:708–714. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Xu Z, Dong Y, Wang H, Culley DJ,
Marcantonio ER, Crosby G, Tanzi RE, Zhang Y and Xie Z:
Age-dependent postoperative cognitive impairment and
Alzheimer-related neuropathology in mice. Sci Rep. 4:37662014.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Salminen A, Kauppinen A, Suuronen T,
Kaarniranta K and Ojala J: ER stress in Alzheimer's disease: A
novel neuronal trigger for inflammation and Alzheimer's pathology.
J Neuroinflammation. 26:6–41. 2009.
|
|
26
|
Prentice H, Modi JP and Wu JY: Mechanisms
of neuronal protection against excitotoxicity, endoplasmic
reticulum stress, and mitochondrial dysfunction in stroke and
neurodegenerative diseases. Oxid Med Cell Longev. 2015:9645182015.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Schönthal AH: Endoplasmic reticulum
stress: Its role in disease and novel prospects for therapy.
Scientifica (Cairo). 2012:8575162012.PubMed/NCBI
|
|
28
|
Han J, Back SH, Hur J, Lin YH,
Gildersleeve R, Shan J, Yuan CL, Krokowski D, Wang S, Hatzoglou M,
et al: ER-stress-induced transcriptional regulation increases
protein synthesis leading to cell death. Nat Cell Biol. 15:481–490.
2013. View
Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhang LH and Zhang X: Roles of GRP78 in
physiology and cancer. J Cell Biochem. 110:1299–1305. 2010.
View Article : Google Scholar : PubMed/NCBI
|